Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.11-12 Pretoria Nov./Dec. 2020
http://dx.doi.org/10.17159/sajs.2020/7860
RESEARCH ARTICLE
Application of plant extracts and Trichoderma harzianum for the management of tomato seedling damping-off caused by Rhizoctonia solani
Mapula T.R Hlokwe; Mapotso A. Kena; David N. Mamphiswana
Department of Plant Production, Soil Science and Agricultural Engineering, University of Limpopo, Rolokwane, South Africa
ABSTRACT
Seedling production under smallholder farming systems can be negatively affected by both abiotic and biotic factors. Seedling damping-off caused by Rhizoctonia solani is one of the major biotic factors which causes significant yield reduction. Management is mainly based on the application of synthetic fungicides and cultural practices. However, both methods have limitations which result in their inefficiency. Several studies have reported on the use of plant extracts and biological control to manage plant diseases. The aim of this study was to formulate an effective and practical approach to manage tomato seedling damping-off using extracts of Monsonia burkeana and Moringa oleifera and a biological control agent Trichoderma harzianum. The efficacy of both extracts was investigated under laboratory conditions to determine the most suppressive concentration to R. solani growth. Methanolic extracts from both plants significantly suppressed pathogen growth at different concentrations. M. burkeana significantly reduced R. solani growth at 8 g/mL (71%) relative to control whilst Moringa oleifera extract reduced pathogen growth by 60% at a concentration of 6 g/mL. The highest suppressive concentrations were further evaluated under greenhouse conditions to test their efficacy on seedling damping-off. In damping-off treatments, both plant extracts and T. harzianum also significantly reduced (p=0.5) pre- and post-emergence damping-off incidence. M. burkeana recorded the highest suppression at 78%, followed by M. oleifera at 64%. Trichoderma harzianum reduced incidence of damping-off by 60% and this was higher than both plant extract treatments.
SIGNIFICANCE:
• The use of M. burkeana and M. oleifera extracts and T. harzianum effectively suppressed pathogen growth and disease incidence and can be used to reduce the use of synthetic pesticides that are harmful to the environment and human health.
• Application of plant extracts and biological control agents as possible alternatives to synthetic fungicides is considered a sustainable and affordable practice for smallholder farmers.
Keywords: biocontrol, Monsonia burkeana, Moringa oleifera, soil-borne diseases, seed treatment
Introduction
Vegetable production is a major farming activity for smallholder farmers in the Limpopo Province of South Africa as it contributes to food security and improved livelihoods for rural communities. Production can however be negatively impacted by both biotic and abiotic factors, with diseases causing major yield losses. High incidences of soil-borne diseases such as damping-off and root rot that occur during seedling stage can cause crop losses of 60-90%.1Rhizoctonia solani Kuhn is an important soil-borne fungal pathogen that is capable of causing diseases on a wide range of plants under favourable environmental conditions.1 It is a facultative parasite that is very competitive against other soil-borne organisms.2 Its survival in infected soils is mainly due to the formation of sclerotia for long-term survival without a host.3 Germination of sclerotia which takes place in the presence of a susceptible host, results in the infection and spread of disease.1 The tomato plant is highly susceptible to R. solani during different growth stages, with seedlings being particularly susceptible to attack by this pathogen.4,5 At seedling stage, plants are more susceptible to R. solani infection due to reduced resistance mechanisms which normally emerge at the adult stage of plant growth.3 Seedling blight, root and hypocotyl rots are typical symptoms of R. solani infection in highly susceptible plants, especially when planted under suitable environmental conditions.2
Management of damping-off is mainly through growth media treatment with chemicals or heat6, seed treatment, use of cultural practices and planting of resistant cultivars7. However, all control measures have limitations which result in their inability to provide significant disease control. For example, the detrimental effect of soil fumigants on the environment and human health has resulted in their ban in agricultural production systems.8 Also, development of resistant cultivars against damping-off has proven difficult due to the diversity of soil pathogens involved in seedling infection.9 Seed treatment, on the other hand, is more efficient during seed germination7 and this is normally lost during seedling stage10. For these reasons, there is a growing need to identify and develop new approaches for the control of R. solani damping-off based on the sustainable management of crops and application of environmentally friendly compounds, especially for smallholder farmers.
In recent years, alternative control measures such as plant-based bioactive compounds in the form of extracts have been studied and have provided promising results, especially against soil- and seed-borne diseases.11-13 Plants produce compounds which have been shown to inhibit the growth and development of diseases caused by bacteria, fungi and other disease-causing organisms.14,15 Rlant extracts have the ability to induce defences in plants, resulting in an effective tolerance against pathogen attack.16 Therefore, the presence of these antimicrobial compounds in plants provides an opportunity for their use in the management of pests and diseases as environmentally safe alternatives to synthetic pesticides.17
In previous studies, Monsonia burkeana, also known as special tea, and Moringa oleifera extracts displayed strong antimicrobial activity against Fusarium wilt of tomato and its causal agent Fusarium oxysporum f. sp. lycopersici.11,18 Reports further show that both plants have high contents of secondary metabolites such as alkaloids, flavonoids, glycosides and many more which are responsible for pathogen suppression.19-21 Despite promising application in the management of other soil-borne plant diseases, reports are still lacking on the application of both plants in the management of tomato seedling damping-off caused by R. solani. Both plants are also easily available and accessible to smallholder farmers in most rural communities in Limpopo Province.
Application of biological control agents such as Trichoderma spp. and Bacillus spp. have also been used successfully in the management of various plant diseases, especially those caused by soil-borne pathogens.22,23 For example, Trichoderma spp. has been used as a seed treatment and soil inoculant to prevent pathogen establishment and suppress disease development in various crops24, whilst Bacillus spp. has been used to control both soil-borne and foliage diseases25. Biological control agents employ various modes of action in the suppression and control of plant pathogens and these can include competition, antibiosis, hyperparasitism and many more.24
The objective of this study was to evaluate the efficacy of M. burkeana and M. oleifera extracts and the biocontrol agent T. harzianum in their ability to suppress R. solani induced seedling damping-off in tomato plants. Both plant extracts were applied as seed treatment to control pre-emergence damping-off and soil drenching to suppress post-emergence damping-off.
Materials and methods
Study location
The in-vitro and greenhouse experiments were conducted in the Plant Pathology Laboratory and the Green Biotechnologies Research Centre greenhouse of the University of Limpopo, South Africa, respectively. The average maximum/minimum temperatures in the greenhouse were 28/21 °C in summer, whereas in winter the average temperatures were 24/16 °C.
Fungal isolates preparation
Isolates of R. solani (PPRI 13845) and Trichoderma harzianum (PPRI 8230) used in this study were provided by the Mycology Division of the Agricultural Research Council - Plant Protection Research Institute (Pretoria, South Africa). R. solani was isolated from a diseased maize seedling showing characteristic symptoms of damping-off and the biological control agent T. harzianum used in this study was isolated from cabbage roots. Fungal isolates were maintained on potato dextrose agar (PDA; Lab-M Neogen Company) and stored at 4 °C. Before use, both fungal isolates were grown on PDA and incubated at ±25 °C for 7-8 days.
Plant collection and extract preparation
Healthy whole plants of M. burkeana (including roots, leaves, flowers, stems) and M. oleifera leaves were collected from the University of Limpopo experimental farm in Mankweng, Limpopo Province, South Africa (23°53'10" S, 29°44'15" E). A representative specimen for each plant was taken to the University of Limpopo herbarium for confirmation of identification before use. Collected plant materials were dried under shade for 7 days and then milled into a fine powder with a laboratory mill (Model FZ-102, Zhongxingweiye Instrument Ltd, China). M. burkeana and M. oleifera powders (100 g) were added separately to 700 mL methanol and placed on a rotary shaker for 24 h. Methanol was then evaporated on a rotary evaporator under reduced pressure at 64 °C. The obtained extract was oven dried for 21 days at 35 °C to constant weight, yielding a green solid suspension. Prepared plant extracts were kept at 4 °C until further use.
Effect of M. burkeana and M. oleifera extracts on mycelial growth of R. solani
Amounts of 0, 2, 4, 6, 8 and 10 g of the resultant suspension of each plant extract were separately dissolved in 10 mL sterile distilled water and thoroughly mixed before being added to 200 mL bottles and content containing PDA. Bottles were then autoclaved at 121 °C for 15 min. In the previous studies11, heat was found to have no effect on the ability of both extracts to suppress pathogen growth. The extract amended PDA was poured into 80-mm Petri plates and left to solidify overnight. Disks of 5 mm in diameter were cut from 7-day-old actively growing R. solani cultures and were placed at the centre of extract-amended PDA Petri plates. Inoculated PDA Petri plates were then incubated at 25 °C under aseptic conditions for 7 days. Non-amended PDA plates served as control treatments. Pathogen colony growth diameter was measured using a transparent ruler17 after ±7 days. Mycelia growth inhibition was calculated using the formula:
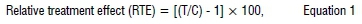
where C is the average diameter of the fungal colony in control plates and T is the average diameter of the fungal colony in extracts-amended plates.
Greenhouse experiment
Fungal inoculation and treatment preparation
Rhizoctonia solani inoculum was prepared by soaking 240 g clean quartz in 75 mL of sterile, distilled water for 24 h in 500-mL Erlenmeyer flasks. Thereafter, 6.0 g yellow maize meal and 75 mL tomato juice were added to the flasks and autoclaved twice for two consecutive days. The autoclaved mixture was then inoculated with 20 discs of 7-day-old pure R. solani culture and incubated for 14 days at 25 °C. After incubation, the inoculum was oven dried at 30 °C for 14 days.
Tomato (cv. Money maker) was used as a test plant against R. solani seedling damping-off. Plant extracts were first tested for their effect on seed germination by soaking surface-sterilised tomato seeds in different concentrations of M. burkeana and M. olifera extract solutions used in the laboratory and then determining the number of germinated seeds. The plant extracts were then confirmed to have no effect on seed germination and were further used for the greenhouse experiment.
Plastic pots (250 mm in diameter) were filled with pasteurised sand and Hygromix in a 3:1 (v/v) ratio. Four holes, 80 mm deep and 50 mm wide, were made and the media was artificially inoculated with 20 g R. solani inoculum in each hole. Inoculated growth media was moistened with 200 mL sterile distilled water and left to stand for 7 days before planting to allow the pathogen to establish in the soil. After 7 days, five surface sterilised seeds were planted in each pot, followed with soil drenching with plant extract concentrations. Concentrations of plant extracts that displayed pathogen growth suppression6 under in-vitro evaluation were used. These were 0.4, 0.6 and 0.8 g/mL for M. burkeana and 0.2, 0.4 and 0.6 g/mL for M. oleifera. Soil treatment with each plant extract solution was done once after 7 days of inoculation. Irrigation with tap water was applied once a week for 4 weeks. Each treatment was replicated four times and damping-off was determined by counting the number of non-geminated seeds and dead seedlings for pre- and post-emergence damping-off, respectively. Pathogen re-isolation was done from dead seedlings to confirm the presence of R. solani.
Biocontrol inoculum and treatment preparation
Trichoderma harzianum inoculum was prepared following the same procedure used for R. solani and received the same amount of dried biocontrol treatment 7 days after R. solani was established in the soil. Pots treated with T. harzianum and control treatment were irrigated with 100 mL tap water once every 2 days. Pre- and post-emergence damping-off were assessed and recorded as described:
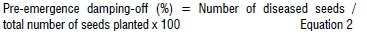
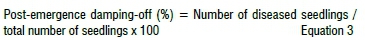
Efficacy of plant extracts of each treatment was evaluated as:
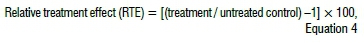
where reduction was expressed with a negative sign and stimulation or increase was expressed by a positive sign.
Data analysis
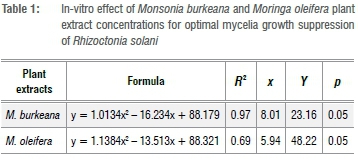
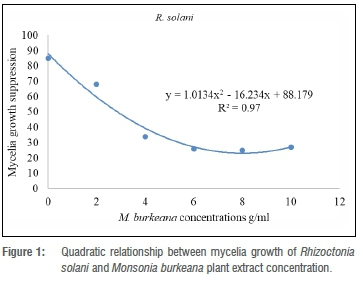
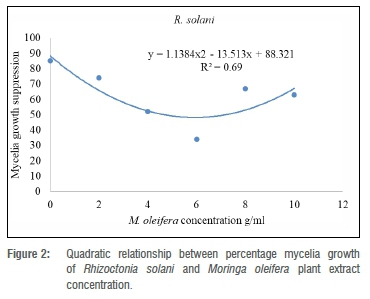
The experiment was laid down in a completely randomised design with six treatments and four replicates. Data were subjected to a partial ANOVA using SAS statistical program. Mean separation was achieved by using Fisher's least significant difference at a probability level of 5%. Mean suppression level (/-axis) and M. burkeana or M. oleifera concentration levels (x-axes) were subjected to the lines of the best fit using MS Excel v. 2016. The responses of mean suppression to increasing M. burkeana or M. oleifera concentration level were modelled by the regression curve estimations resulting in a quadratic equation: Y=b2 x2+b1x+a, where Y is mean suppression level and x is M. burkeana or M. oleifera concentration level using x=-b1 / 2b2 relation for the saturation point for each extract.
Results
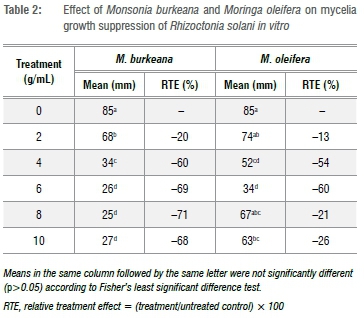
Effect of M. burkeana and M. oleifera extracts on mycelial growth of R. solani
All tested concentrations of both M. burkeana and M. oleifera reduced mycelial growth of R. solani when compared to control (Table 1). M. burkeana displayed the highest mycelia growth inhibition at a concentration of 8 g/mL (Table 2; Figure 1). With M. oleifera treatments, the highest pathogen growth suppression was obtained at 6 g/mL (Table 2; Figure 2). The maximum growth inhibition was measured at 71% and 60% for both M. burkeana and M. oleifera, respectively (Table 2).
Greenhouse experiment
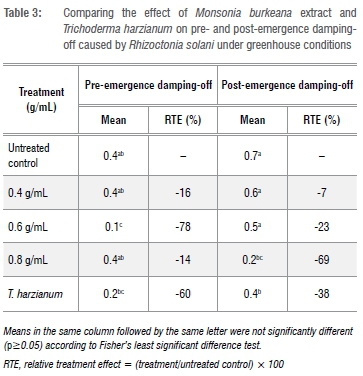
Amendment of R. solani inoculated soil with different concentrations of M. burkeana and M. oleifera had a varying effect on damping-off incidences with treatments displaying low, moderate and high suppressive effect (Table 3 and Table 4). The same trend was also observed for T. harzianum treatments, in which the levels of suppression were different for both pre- and post-emergence damping-off. However, despite these variations, suppression was still higher than in the inoculated non-amended control. For example, at 0.6 g/mL, M. burkeana significantly reduced pre-emergence damping-off (78%), whilst the highest post-emergence damping-off reduction of 69% was recorded for 0.8 g/mL treatments. At a concentration of 0.4 g/mL, M. burkeana had no effect on damping-off incidence, resulting in high incidences of both pre- and post-emergence damping-off. Treating R. solani inoculated soil with T. harzianum also resulted in a relative reduction of pre-emergence (60%) and post-emergence damping-off (38%) (Table 3). However, reduction was lower when compared to M. burkeana treatment.
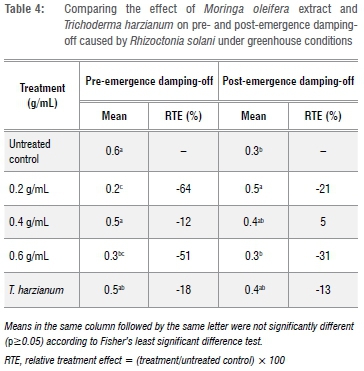
The three tested M. oleifera concentrations displayed a significant difference in their ability to reduce both pre- and post-emergence damping-off (Table 4). Treatment of infected soil with 0.2 g/mL of M. oleifera extract solution resulted in a significant reduction in both pre-and post-emergence damping-off incidence whilst 0.6 g/mL was only effective in reducing pre-emergence damping-off. Soil treatment with 0.4 g/mL had no effect on either pre- or post-emergence damping-off of tomato as there was no significant difference for this concentration compared with the non-amended control. A significant reduction in damping-off was also recorded where R. solani inoculated soil was amended with T. harzianum, resulting in 60% and 39% pre- and post-emergence damping-off reduction, respectively. To confirm differences between treatments, a relative treatment effect was also carried out against the untreated control (Table 4). The results show that there was a significant difference amongst the concentrations of M. oleifera and biocontrol T. harzianum and their ability to reduce damping-off incidences. For example, although damping-off incidence in T. harzianum treated pots was slightly reduced with RtEs of 18% and 13% for pre- and post-emergence damping-off, respectively, these were still significantly higher than that for the control treatment. The RTE for M. oleifera treatments further shows that pre-emergence damping-off was reduced by 64% with a concentration of 0.2 g/mL, whilst post-emergence damping-off was reduced by 31% in 0.6 g/mL relative to untreated control (Table 4).
Discussion
Monsonia burkeana and M. oleifera plant extracts separately reduced the incidence of damping-off under greenhouse conditions. T. harzianum as a biological control agent was also found to be effective in reducing damping-off in vivo. The efficacy of the treatments corroborates previous studies which demonstrated the ability of M. burkeana21, M. oleifera and T. harzianum26to reduce the disease severity and disease incidence of fungal soil-borne diseases. Besides reducing disease severity, plant extracts have also been shown to increase shoot and root mass.27
Most medicinal plants - including M. burkeana and M. oleifera - contain a number of phytochemicals that exhibit antimicrobial activity.1217,23 Most of these phytochemicals include secondary metabolites and compounds such as flavonoids and tannins19,28, which are the main antifungal components associated with disease suppression. Furthermore, these secondary metabolites form complexes with the polysaccharides and proteins associated with the external layer of fungal cells that might result in possible death of the pathogen.10 However, additional work is necessary to determine the mode of action exhibited by the plant extracts on R. solani.
The effectiveness of T. harzianum might be due to a number of factors including competition, production of antifungal metabolites with fungicidal capabilities, toxic antibiotics and mycoparasitism.26 The degree of reduction of damping-off by T. harzianum is possibly attributed to the secretion of antibiotics by the antagonist10 or other inhibitory substances produced by the antagonistic chemical compounds such as geodin, terricin and terric acids.29 For example, certain Trichoderma species colonise and penetrate plant root tissues and initiate a series of morphological and biochemical changes in the plant, which are considered to be part of the plant defence responses, which eventually lead to an induced systemic resistance in the entire plant.26
Although the main focus of the current study was on the effects of plant extracts and T. harzianum on disease incidence, it was also observed that the level of suppression differed between extracts. For example, M. burkeana extract was more effective in suppressing both pre- and post-emergence damping-off incidence than the extract obtained from M. oleifera. Despite their ability to suppress pathogen growth to reduce disease incidence, many reports have shown that this occurs to varying degrees and is mainly dependent on the plant species and its interaction with the pathogen at physiological and molecular levels. For example, a report by Hassanein et al.30 indicated greater efficacy of neem (Azadirachta indica) extracts when compared to other extracts, probably due to different chemical compounds in neem that had greater antifungal activities. This phenomenon also applies to biocontrol agents, with reports showing that their degree of effectiveness varies according to the nature, quality and quantity of antibiotics or inhibitory substances secreted.6
Conclusion
The current findings demonstrate the effectiveness of both M. burkeana and M. oleifera extracts and T. harzianum in the management of soil-borne diseases in seedling production. The tested plant extracts are easily accessible to smallholder farmers, they are easy to process and are environmentally friendly; they can therefore be used as possible parts of an integrated control measure against seedling damping-off. In this study, both plant extracts and T. harzianum were applied separately; further studies on their combined application are recommended to determine their synergistic relationship. Further research is also recommended to determine the impact of plant extracts on the soil and rhizosphere microbiome, especially on beneficial microorganisms.
Acknowledgements
Financial assistance to carry out this study was provided by the South African National Research Foundation (grant no. 75909).
Competing interests
We declare that there are no competing interests.
Authors' contributions
M.T.PH.: Methodology; data collection and analysis; sample analysis; validation; writing. M.A.K.: Project leader; conceptualisation; student supervision; funding acquisition; assistance with data collection and analysis; writing and correction; final approval for submission. D.N.M.: Student co-supervision; data analysis; writing.
References
1. Drizou F, Graham NS, Bruce TJA, Ray RV. Development of high-throughput methods to screen disease caused by Rhizoctonia solani AG 2-1 in oilseed rape. Plant Methods. 2017;13:45. https://doi.org/10.1186/s13007-017-0195-1 [ Links ]
2. Fang L. Biological indicators of compost-mediated disease suppression against the soil-borne plant pathogen Rhizoctonia solani [MS thesis]. Burlington, VT: University of Vermont; 2015; p. 456. https://scholarworks.uvm.edu/graddis/456 [ Links ]
3. Wang L, Wang ZG, Huang SW. Genetic structure and aggressiveness of Rhizoctonia solani AG1-IA, the cause of sheath blight of rice in southern China. J Phytopathol. 2013;161:753-762. https://doi.org/10.1111/jph.12127 [ Links ]
4. Al-Rahmah AN, Mostafa AA, Abdel-Megeed A, Yakout SM, Hussein SA. Fungicidal activities of certain methanolic plant extracts against tomato phytopathogenic fungi. Afr J Microbiol Res. 2013;7:517-524. https://doi.org/10.5897/AJMR12.1902 [ Links ]
5. Zhou J, Wang M, Sun Y, Gu Z, Wan R, Saydin A, et al. Nitrate increased cucumber tolerance to Fusarium wilt by regulating fungal toxin production and distribution. J Toxicol. 2017;9:1-20. https://doi.org/10.3390/toxins9030100 [ Links ]
6. Vidyasagar GM, Tabassum N. Antifungal investigations on plant essential oils: A review. Int J Pharm Pharm Res. 2013;5:19-28. [ Links ]
7. Hossain MT, Khan A, Chung EJ, Rashid MH, Chung YR. Biological control of rice Bakanae by an endophytic Bacillus oryzicola YC7007. Plant Pathol J. 2016;32:228-241. https://doi.org/10.5423/PPJ.OA.10.2015.0218 [ Links ]
8. Gupta S, Singh G, Sharma N. In vitro screening of selected plant extracts against Alternaria alternata. J Exp Biol Agric Sci. 2014;2:344-351. [ Links ]
9. Jendoubi W, Bouhadida M, Boukteb A, Béji M, Kharrat M. Fusarium wilt affecting chickpea crop. Agriculture. 2017;7(3):23. https://doi.org/10.3390/agriculture7030023 [ Links ]
10. Rongai D, Pulcini P Pesce B, Milano F. Antifungal activity of pomegranate peel extract against Fusarium wilt of tomato. Eur J Plant Pathol. 2017;147:229-238. https://doi.org/10.1007/s10658-016-0994-7 [ Links ]
11. Hlokwe MTP Kena MA, Mamphiswana ND. Evaluating crude extracts of Monsonia burkeana and Moringa oleifera against Fusarium wilt of tomato. Acta Agr Scand B-S-P. 2018;68:757-764. https://doi.org/10.1080/09064710.2018.1484946 [ Links ]
12. Mboto CI, Eja ME, Adegoke AA, Iwatt GD, Asikong BE, Takon I, et al. Phytochemical properties and antimicrobial activities of combined effect of extracts of the leaves of Garcinia kola, Vernonia amygdalina and honey on some medically important microorganisms. Afr J Microbiol Res. 2009;3:557-559. [ Links ]
13. Kena MA, Swart WJ. Effects of plant extracts on in vitro suppression of fungal pathogens and control of damping-off of vegetable seedlings in the greenhouse. Bots J Agric Appl Sci. 2011;7:36-44. [ Links ]
14. Kekuda PTR, Akarsh S, Nawaz SAN, Ranjitha MC, Darshini SM, Vidya P. In vitro antifungal activity of some plants against Bipolaris sarokiniana (Sacc.) Shoem. Int J Curr Microbiol App Sci. 2016;5:331-337. https://doi.org/10.20546/ijcmas.2016.506.037 [ Links ]
15. Nefzi A, Abdallah BAR, Jabnoun-Khiareddine H, Saidiana-Medimagh S, Haouala R, Daami-Remadi M. Antifungal activity of aqueous and organic extracts from Withania somnifera L. against Fusarium oxysporum f. sp. radias-lycopersici. J Microb Biochem Technol. 2016;8:144-150. https://doi.org/10.4172/1948-5948.1000277 [ Links ]
16. Mamphiswana ND, Mashele PW, Mdee LK. Distribution of selected essential nutrient elements and secondary metabolites in Monsonia burkeana. Afr J Agric Res. 2011;18:2570-2575. [ Links ]
17. Naswa SMA, Kamal AMA. Evaluation of various plant extracts against the early blight disease of tomato plants under green house and field conditions. Plant Protect Sci. 2012;48:74-79. https://doi.org/10.17221/14/2011-PPS [ Links ]
18. Gopalakrishnan L, Doriya K, Kumar DS. Moringa oleifera: A review on nutritive importance and its medicinal application. Food Sci Human Well. 2016;5:49-56. https://doi.org/10.1016/j.fshw.2016.04.001 [ Links ]
19. Mamphiswana ND, Mashela PW, Mdee LK. Distribution of total phenolic and antioxidant activity in fruit, leaf, stem and root of Monsonia burkeana. Afr J Agric Res. 2010;5:2570-2575. [ Links ]
20. Gour HN, Agrawal S. A wilt toxin from Fusarium oxysporum f. sp. cumini patel and prasad. Curr Sci. 1988;15:849-851. http://www.jstor.org/stable/24091254 [ Links ]
21. Kena MA. Antifungal activities of Monsonia burkeana and Euphorbia ingens extracts against Penicillium digitatum, the causal agent of citrus green mould. Afr Plant Prot. 2016;19:1-3. [ Links ]
22. Jantasorn A, Moungsrimuangdee B, Dethoup T. In vitro antifungal activity evaluation of five plant extracts against five plant pathogenic fungi causing rice and economic crop diseases. J Biopest. 2016;9:1-7. [ Links ]
23. Sesan TE, Enache E, Iacomi M, Oprea M, Oancea F, Iacomi C. Antifungal activity of some plant extract against Botrytis cinerea Pers. in the blackcurrant crop (Ribes nigrum L.). Acta Sci Pol Hortorum Cultus. 2015;14(1):29-43. [ Links ]
24. O'Callaghan M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl Microbiol Biotechnol. 2016;100:5729-5746. https://doi.org/10.1007/s00253-016-7590-9 [ Links ]
25. Shafi J, Tian H, Ji M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol Equip. 2017;31:446-459. https://doi.org/10.1080/13102818.2017.1286950 [ Links ]
26. Adandonon A, Aveling TAS, Labuschagne N, Tamo M. Biocontrol agents in combination with oleifera extract for integrated control of Sclerotium-caused cowpea damping-off and stem rot. Eur J Plant Pathol. 2006;23:97-127. https://doi.org/10.1007/s10658-006-9031-6 [ Links ]
27. Obongoya B, Wagai S, Odhiambo G. Phytotoxic effect of selected crude plant extracts on soil-borne fungi of common bean. Afr Crop Sci J. 2010;18:15-22. https://doi.org/10.4314/acsj.v18i1.54189 [ Links ]
28. Shafighi M, Amjad L, Madaniin M. In vitro antifungal activity of methanolic extract of various parts of Punica granatum (L.). J Sci Eng. 2012;3:1-1. [ Links ]
29. Waing KGD, Abella EA, Kalaw SP Waing FP Galvez CT. Antagonistic interactions among different species of leaf litter fungi of Central Luzon State University. Plant Pathol Quar. 2015;5:122-130. https://doi.org/10.5943/cream/5/3/10 [ Links ]
30. Hassanein NM, Ali MM, Youssef KA. Mahmoud DA. Control of tomato early blight and wilt using aqueous extract of neem leaves. Phytopathol Mediterr. 2010;49:143-151. [ Links ]
 Correspondence:
Correspondence:
Mapotso Kena
Email:Mapotso.Kena@ul.ac.za
Received: 24 Jan. 2020
Revised: 21 Apr. 2020
Accepted: 28 Apr. 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: South African National Research Foundation (grant no. 75909)














