Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.11-12 Pretoria Nov./Dec. 2020
http://dx.doi.org/10.17159/sajs.2020/7914
RESEARCH ARTICLE
Establishment of Beauveria bassiana isolates as endophytes in rice cultivars and their biocontrol efficacy against rice stem borer, Sesamia calamistis
Wonroo B.A. BancoleI; Mark D. LaingI; Kwasi S. YoboI; Abou TogolaII
IDiscipline of Plant Pathology, School of Agricultural, Earth and Environmental Sciences, College of Agriculture, Engineering and Science, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIInternational Institute of Tropical Agriculture (IITA), Kano Station, Kano, Nigeria
ABSTRACT
Possible endophytic colonisation of rice cultivar parts (leaves, stem and roots) by Beauveria bassiana isolates and their potential as biocontrol agents against Sesamia calamistis Hampson (African pink stem borer) were investigated. Five promising B. bassiana isolates were evaluated for their endophytic colonisation, the effectiveness of the inoculation methods and the efficacy of the isolates as biocontrol agents against S. calamistis. The plant part colonised is often dependent on the inoculation method. Colonisation of plant parts was assessed at 30 and 60 days after seed inoculation and foliar spray. For the pathogenicity activity, third instar larvae of S. calamistis were fed with rice stems that were previously inoculated with endophytic isolates of B. bassiana. Both inoculation methods led to the colonisation of the rice cultivar tissues, but were affected by the interactions of cultivars x isolates x inoculation methods. The colonisation of the cultivar plant parts varied over time (30- and 60-day intervals), and was affected by the inoculation method used. For both inoculation methods, highly significant differences were observed in the roots and the leaves over time (p=0.0001). However, with seed treatment, there was no significant difference in levels of colonisation in stems by the isolates x time (p=0.32). The B. bassiana isolates were pathogenic on the third instar larvae of S. calamistis, causing mortalities of more than 50% at 28 days after treatment. However, the virulence of the isolates varied. According to the isolates and the inoculation methods, B. bassiana formed an endophytic relationship with rice plants, and produced various mortality rates.
SIGNIFICANCE:
• Beauveria bassiana could be a potential biocontrol agent of rice stem borer, S. calamistis as there is no report of endophytic isolates of B. bassiana for the control of rice borers.
• Currently there is no commercially registered biocontrol agent against rice borers; hence further studies into B. bassiana could lead to the registration and commercialisation of B. bassiana as a bio-pesticide for rice stem borers.
Keywords: entomopathogenic fungi, cereal grain, colonisation, inoculation
Introduction
Rice (Oryza spp L.) is one of the world's most important crops, providing food for more than half of the world's population.1-3 Rice and wheat (Triticum spp L.) together contribute about 21% of the total energy consumed by humans.4 In West Africa it has become the main source of calories for low-income households.5 Two Oryza spp. are cultivated globally: Asian rice (Oryza sativa L.) and African rice (Oryza glaberrima S.), for which the cultivation is limited to tropical West Africa.6 Rice is now grown and consumed in more than 40 countries on the African continent.7 Its consumption has increased rapidly in Africa, making it the second largest source of carbohydrates in sub-Saharan Africa.4 Imports of rice account for nearly 40% of the total rice consumption of the region.8,9
Losses caused by biotic factors such as pests, diseases and weeds reduce yields of rice worldwide. According to estimates of the Food and Agriculture Organization of the United Nations (FAO), diseases, insects and weeds cause as much as 25% yield losses annually in cereal crops.2 The most serious pests of rice plants worldwide are rice stem borers, which belong to three families (Noctuidae, Pyralidae and Diopsidae). Sesamia calamistis Hampson (Lepidoptera: Noctuidae) is one of the major pests that attacks grain crops including rice, maize (Zea mays L.), pearl millet (Pennisetum sp L.), wheat, sorghum (Sorghum bicolor L.) and sugarcane (Saccharum officinarum L.). The control of this borer by commercial farmers has mainly relied on the application of synthetic insecticides.10-12 However, control of S. calamistis using chemicals is difficult because of a prolonged emergence pattern, multiple generations and a cryptic feeding behaviour.13 A further issue is that, as with many other stem borers, S. calamistis has developed resistance to chemicals. In addition to the insecticides' high costs and their inefficacy against the borers, they may also cause ecological problems, and are usually unaffordable for small-scale farmers.14-17
The need for alternative methods for the control of major pests has driven research to develop biological control products. Indigenous predators, parasites and entomopathogens are the most commonly used biological control agents in tropical Asia and Africa to control stem borers.18 Entomopathogenic fungi are important among biological control agents due to their broad host range, their diverse mechanisms of pathogenicity, and their environmental safeness.19-21 Some strains of the entomopathogen B. bassiana have been introduced into several plant species [maize, banana (Musa spp L.), tomatoes (Solanum lycopersicum L.), sorghum, coffee (Coffea arabica L.), wheat and pumpkins (Cucurbita spp D.)] to control various insects.2225 Various inoculation methods (seed treatments, soil drenches, foliar and flower sprays, and stem injections) have been used for their establishment as endophytes in those crops. The main reason for conducting this study was to determine if endophytic strains of B. bassiana in rice cultivars might provide protection against S. calamistis, the major rice stem borer prevalent in West Africa.
Materials and methods
Five isolates of B. bassiana previously identified as endophytes in sorghum were evaluated for their potential establishment as endophytes in rice cultivars plant tissues. Third larval instars of S. calamistis were used as the test insects.
Production of conidial suspensions of B. bassiana isolates
Five B. bassiana isolates (Bb3, Bb4, Bb10, Bb21 and Bb35) were used for the study. These had been isolated from various soils, including soil samples collected from the rhizosphere of mangoes (Mangifera indica L.), rooibos tea {Aspalathus linearis Burm. f.) and wheat. They were characterised by the Plant Protection Research Institute (Pretoria, South Africa). In prior research, they were selected for their endophytic abilities in sorghum plant tissues (leave, stem and root).18 Conidial suspensions used for the study were prepared following the method of Parsa et al.18 The strains were cultured in 90-mm diameter plastic Petri dishes containing potato dextrose agar (PDA) supplemented with antibiotics (100 mg/L of ampicillin and streptomycin), and incubated at 28 °C. The cultures were allowed to grow for 14-18 days, after which the conidia were harvested. The conidia were harvested under sterile conditions by gently scraping the fungal growth from the surface of the agar with a sterile spatula, and rinsing with sterile distilled water. The resulting suspensions were filtered using sterile cheese cloth to remove mycelia and agar debris. Conidial density was determined using an improved Neubauer haemocytometer, and adjusted to 2x106 conidia/ mL with sterile distilled water containing Tween-80 (1 mL/L). The viability of the conidia for all the experiments was evaluated by taking a 100-uL sample of each strain, spreading it on PDA and incubating at 25 °C. Conidia germination was assessed after 24 h of incubation. The percentage germination of conidia was determined from 100 randomly selected conidia under a light microscope. The germination of conidia was assumed when the hyphae were visible or the germ tube was about twice the length of the conidium. For each strain, the mean of three replicates was used to assess the viability of the conidia. The final inoculum was used for seed treatment and foliar spray experiments.
Production of rice plants for greenhouse studies
Three African rice cultivars (NERICA1, NERICA8 and NERICA-L19)26 were used as the host plants as there may be differential interactions between host plants and endophytic strains. Seeds of each cultivar were separately surface sterilised in 3% sodium hypochlorite for 3 min followed by 70% ethanol for 2 min. They were rinsed three times with sterile distilled water, air dried on a laminar flow bench and then divided into two sets. The first set was used for seed inoculation and the second for foliar spray experiments. The second set of seeds used for foliar spray experiment were sown in Speedling® 24 trays filled with Composted Pine Bark (CPB) seedling mix growing medium. The seeds were watered with tap water and placed under greenhouse conditions at 20-28 °C day and night. Two weeks after germination, the seedlings were transplanted into 30-cm diameter pots filled with CPB seedling mix growing medium and placed under greenhouse conditions at 20-28 °C day and night. The plants were allowed to grow for 7 days before being used in the foliar spray experiment. Plants were irrigated three times a day with irrigation water containing NPK fertiliser [3: 1: 3 (38)] (50%) together with calcium nitrate (50%) and trace elements.
Inoculation of B. bassiana isolates for endophytic colonisation in rice cultivars
Seed treatment
The seeds of the three rice cultivars were surface sterilised as previously described. After surface sterilisation, the seeds for each cultivar were separately soaked in the conidial suspension of each B. bassiana isolate [5 mL of the prepared inoculum (2x106 conidia/mL)], allowed to stand overnight, then removed and air dried on a laminar flow bench. The seeds were then planted in Speedling® 24 trays filled with CPB seedling mix growing medium. The control plants consisted of non-inoculated seeds treated in a similar manner using sterile distilled water. After 2 weeks, the emerging seedlings were transplanted into 30-cm diameter pots filled with CPB seedling mix growing medium and placed under greenhouse conditions at 20-28 °C day and night. Three plants per pot were arranged in the greenhouse in a randomised complete block (RCB) design with three replicates. Plants were irrigated three times a day with irrigation water containing NPK fertiliser [3: 1: 3 (38)] (50%) together with calcium nitrate (50%) and trace elements. The plants were grown for 30 or 60 days before they were harvested, and the roots, stems and leaves were evaluated for evidence of endophytic colonisation.
Foliar spray
The seedlings of the three rice cultivars were sprayed 15 days after transplanting into pots. A hand spray was used to inoculate the rice plant leaves with the inocula of the B. bassiana isolates. A volume of 50 mL inoculum of each B. bassiana isolate was used per plant. Before the leaves were sprayed, the base of each pot was covered with aluminum foil, with a hole to allow the plant to emerge. This was to stop inoculum running off the leaves onto the roots and creating a root drenching situation. Plastic bags were used to cover the entire plant for 24 h to increase humidity. For the control plants, sterile distilled water was applied in a similar manner as described for the B. bassiana treatments. The treated plants (three plants per pot) were then placed in a greenhouse (20-28 °C day and night) using a RCB design with three replicates. Plants were irrigated three times a day with irrigation water containing NPK fertiliser [3: 1: 3 (38)] (50%) together with calcium nitrate (50%) and trace elements. The roots, stems and leaves of each treated plant were harvested after 30 and 60 days, for evaluation for endophytic colonisation.
Evaluation of endophytic colonisation of the B. bassiana isolates
The colonisation of rice plant tissues by B. bassiana was determined 30 and 60 days after inoculation with each B. bassiana isolate. From each rice cultivar x B. bassiana treatment combination, plants were carefully removed from their pots and sampled into leaves, stems and roots. The roots were gently washed with tap water to remove residues of CPB. The plant tissues were surface sterilised by immersing them in 3% sodium hypochlorite for 3 min, followed by 70% ethanol for 2 min. They were rinsed three times with sterile distilled water. The surface sterilised samples were placed on sterile tissue paper under a laminar flow cabinet for air drying. After drying, six pieces of each of the samples (leaves, stem and roots) from each treated plant were randomly taken and plated separately onto a B. bassiana selective medium (39 g/L PDA + 2 g yeast extract + 1.1 g Dodine + 100 mg/L of streptomycin and ampicillin)27 and incubated for 15 days at 25 °C. To confirm that the surface sterilisation was effective, 10 mL of the sterile distilled water used to rinse the samples during the surface-sterilisation procedure was spread onto Petri dishes containing the B. bassiana selective media. The plates were incubated for 10-15 days at 25 °C to count the colony forming units. However, the sterilisation resulted in clean plates. Therefore, any B. bassiana mycelium emerging from surface-sterilised plant tissues was assumed to have originated from within the plant tissues as an endophyte. The plates that contained the plant samples were monitored every 2-3 days for the emergence of fungal mycelia. After 10-15 days, the presence or absence of B. bassiana colonies were recorded. The fungal colonies grown from the samples were confirmed to be B. bassiana based on morphological characteristics.
Mass rearing of S. calamistis larvae
A suitable number of S. calamistis pupae collected from a maize field were placed into cages that contained cotton soaked in sugar/honey water that served as food for the moths once they emerged from the pupae. Sheets of transparent paper were wound around wooden rods (40 cm) to create a slot for the female moths to lay their eggs. These eggs were harvested from the slots by scraping the wooden rods with a sterile spatula. The eggs were placed into plastic containers on a sterile paper towel. The containers were incubated at 26 °C with a relative humidity of 60% (±10%), and a photoperiod of 12-h light and 12-h dark. The containers were monitored daily until the larvae hatched, creating the stage called 'black heads' (first instars). These young larvae were transferred into other transparent plastic containers whose lids were perforated but covered with mosquito netting to ensure permanent ventilation, and were incubated at 26 °C. Fresh maize stalks were harvested and placed into the plastic containers to serve as food for the black head larvae. After 3 days, the maize stalks were dissected and the larvae were extracted and transferred into new containers that contained fresh maize stalks (Figure 1), and kept in an incubator at 26 °C. This procedure was repeated until larvae of the desired third instar larval stage had developed. The number of days for each stage varied from one larval stage to the next. Development from the second to the third instar took 5 days, while development from the third to the fourth instar took 8-10 days.
Production of endophytic stems of rice plants
Seeds of a rice cultivar (NERICA 1) were surface sterilised in 3% sodium hypochlorite for 3 min, followed by 70% ethanol for 2 min. They were then rinsed three times with sterile distilled water and air dried. The surface-sterilised seeds were then dipped separately in a conidial suspension of each of the five B. bassiana isolates [5 mL of the prepared inocula (2x106 conidia/mL)] and left overnight before air drying under a laminar flow cabinet. The seeds were then planted in Speedling® 24 trays filled with CPB seedling mix growing medium. After 2 weeks, the seedlings were transplanted into 30-cm diameter pots filled with CPB seedling mix growing medium and placed under controlled greenhouse conditions set at 20-28 °C day and night. Three plants per pot were arranged in the greenhouse in three replicates per treatment, using a RCB design. Plants were irrigated three times a day with irrigation water containing NPK fertiliser [3: 1: 3 (38)] (50%) together with calcium nitrate (50%) and trace elements. The plants were allowed to grow for 30 days before one plant per treatment from each crop cultivar was harvested and sampled (stems) to confirm their endophytic colonisation by B. bassiana isolates. The stems were separately surface sterilised by immersing them in 3% sodium hypochlorite for 3 min, followed by 70% ethanol for 2 min. They were separately rinsed three times with sterile distilled water and placed on a sterile paper towel in a laminar flow cabinet to air dry. After drying, six pieces of each treated stem were randomly selected and plated separately onto B. bassiana selective medium.27 The inoculated plates were incubated for 15 days at 25 °C. The plates were monitored every 2-3 days for the emergence of fungal mycelia. After colonisation of the stems by B. bassiana isolates was confirmed, the remaining inoculated plants were harvested, washed with tap water, and the stems were then used for pathogenicity testing on the stem borer, S. calamistis.
Efficacy of B. bassiana isolates against S. calamistis
The endophyte positive stems produced as previously described were harvested and washed with distilled water, before being fed to the third instar larvae of S. calamistis. A total of 10 third instar larvae of S. calamistis were placed into plastic containers of 10 g of B. bassiana infected rice stems. The containers were placed in an incubator at 28 °C. Mortality of the larvae was recorded after 7, 14, 21 and 28 days. For the control, larvae were fed with non-inoculated rice stems. Dead larvae were collected at 7, 14, 21 and 28 days and were maintained in plastic containers on Whatman filter paper previously wetted with sterile distilled water. Two to three days after collection, the dead larvae were surface sterilised in 3% sodium hypochlorite for 1 min followed by 70% ethanol for 1 min. They were then rinsed three times with sterile distilled water for 15 s. The surface-sterilised dead larvae were placed on sterile paper towels under a laminar flow cabinet for air drying. The dried dead larvae were plated onto Petri dishes that contained a B. bassiana selective media27 (Figure 2). The plates were kept in an incubator at 26-28 °C and monitored every 2-3 days. Fungi that appeared on the surface-sterilised larvae of S. calamistis were harvested and sub-cultured onto fresh PDA plates for pure culture and identification. After 15 days, the colonies were compared to the endophytic B. bassiana isolates that were initially inoculated onto the rice seed. The experiment was performed three times to confirm the pathogenicity of the B. bassiana isolates.
Data analysis
Colonisation of the rice plant tissues resulting from the various treatment combinations was analysed using SAS (version 9.4). A general linear model was used for the analysis of variance (ANOVA). If the ANOVA F-test was significant (p<0.05), then treatment means were separated using the Duncan Multiple Range Test.
The cumulative percentage of mortality of S. calamistis was recorded, and the area under the mortality progress curve (AUMPC) was calculated. The data collected were analysed using GenStat (18th edition). A two-way ANOVA was run with B. bassiana isolates and time (days) as the main factors.
Results
The rice plants were colonised by B. bassiana isolates using both inoculation methods. The colonisation of each rice plant tissue (root, stem and leaf) was both isolate and inoculation method dependent and varied also with time (30-60 days) (Tables 1 and 2).
Following seed treatment, highly significant differences were observed in the colonisation of the tissues (roots, stem and leaves) x B. bassiana isolates (p=0.0001) and x rice cultivar (p=0.0001). At both 30 and 60 days for all interactions [(isolates x cultivars, isolates x time, cultivars x time and isolates x cultivars x time] there were highly significant differences in the colonisation of the roots and the leaves (p=0.0001). There was no interaction between strain x time (p=0.32) (Table 1).
After foliar sprays of inoculum, highly significant differences were observed in the levels of colonisation of the roots between B. bassiana isolates, rice cultivars, B. bassiana isolates x cultivars, B. bassiana isolates x time, cultivars x time, and B. bassiana isolates x cultivars x time (p=0.0001; Table 2). Highly significant differences were observed in colonisation of the leaves between B. bassiana isolates, cultivars, time (30-60 days), B. bassiana isolates x cultivars, B. bassiana isolates x time, cultivars x time, and B. bassiana isolates x cultivars x time (p=0.0001). In the stem, significant differences were observed between strains (p=0.005), time (p=0.02) and cultivars x time (p=0.0018). The interactions of B. bassiana isolates x cultivars, and B. bassiana isolates x cultivars x time were highly significant (p=0.0001 and p=0.0002, respectively). No colonisation by B. bassiana isolates was observed in the tissues of the control plants, with either inoculation method (Tables 1 and 2).
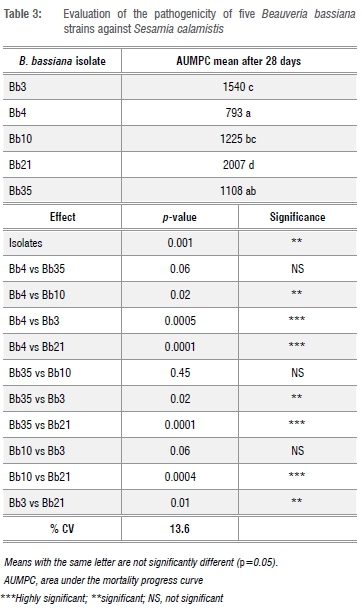
There were highly significant differences between the pathogenicity of the five B. bassiana isolates that were used against the third instar larvae of S. calamistis (p=0.001; Table 3). B. bassiana isolates Bb4 and Bb35 were the most effective strains, killing 93.3% and 76.6% of S. calamistis larvae at 28 days, respectively (Figure 3). The AUMPC data revealed that the B. bassiana isolates Bb4 vs Bb35, Bb35 vs Bb10 and Bb10 vs Bb3 showed similar levels of pathogenicity (Table 3).
Discussion
Beauveria bassiana has been reported to colonise many plants as an endophyte24-30, which supports the results of our study. Colonisation of plants by B. bassiana depends on the inoculation method, fungal isolate and plant species. Some isolates of B. bassiana were able to colonise maize plants via the epidermis, thereafter persisting in the plant throughout the entire growing season, and reducing tunnelling by Ostrinia nubilalis Hubner (Lepidoptera: Pyralidae).31,32 As demonstrated in this study, B. bassiana can become established as an endophyte in rice when seeds or seedlings are inoculated with conidia of B. bassiana strains. Similar results have been demonstrated in other studies.25,33,34 Successful B. bassiana colonisation of coffee leaves21, banana roots35 and maize plants29 has been reported. These studies used inoculation techniques such as leaf injection, seed treatment, root drench and foliar sprays. All these techniques led to successful B. bassiana inoculation and colonisation.
The level of colonisation of the various plant tissues (leaf, root and stem) differed according to the B. bassiana isolates and the rice cultivars used in this study. The inoculation methods used conferred good colonisation of the rice stem by some of the B. bassiana isolates. Our study confirmed that there are several possible pathways to inoculation and recovery of B. bassiana from plant tissues.36 Both inoculation methods (seed treatment and foliar spray) resulted in high levels of leaf and root colonisation. The inoculation method did not appear to favour a specific pattern of local colonisation of the rice cultivars. This is contrary to the results of Posada et al.21 who reported that foliar sprays favoured leaf colonisation, whereas soil drenching favoured root colonisation in coffee. Similar findings were demonstrated for the common bean.25
The systemic spread of each B. bassiana isolate differed over the two sampling time periods (30 and 60 days) used in this study. A reduction in level of colonisation over time may have been caused by a host resistance response to the heterotrophic fungi or because of competition from other endophytes in the rice tissues.23 The colonisation of the rice cultivars by the B. bassiana isolates did not cause any apparent negative effects on the growth of the rice plants, as was reported by Van Bael et al.37
Gurulingappa et al.38 reported that fungal isolates from different insect hosts possess varying degrees of virulence to the different insects. In this study, the five B. bassiana isolates showed differential pathogenicity against the third instar larvae of S. calamistis. Mortalities of 93.3% and 76.6% were achieved on third instar larvae of S. calamistis with two of the five selected B. bassiana strains used in this study. Similarly, a lower frequency of S. calamistis was recorded in B. bassiana treated maize plants compared to non-inoculated maize plants.39
Research from Valda et al.40 and Godonou et al.41 proved the effectiveness of B. bassiana strains on a diamondback moth population, Plutella xylostella L. (Lepidoptera: Plutellidae). Similarly, the survivorship and development of banana weevil larvae, Cosmopolites sordidus Germar (Coleoptera: Curculionidae) were significantly affected by endophytic B. bassiana strains35 as were the adult banana weevils as reported in Ghana.41 Tesfaye et al.42 also isolated different strains of B. bassiana and found that they caused mortalities greater than 75% of adults of Myzus persicae Sulzer (Homoptera: Aphididae). Bing and Lewis31,32 found that 60% of O. nubilalis larvae collected from maize plants inoculated with B. bassiana were controlled by the fungus. A reduction in feeding is one of the reported altered behaviours by insects when infected by B. bassiana. For example, Tefera and Pringle26 showed that there was a significant reduction in feeding by Chilo partellus Swinhoe (Lepidoptera: Pyralidae) as a result of 1-4 days inoculation with B. bassiana. The results reported in this study differ from that previously reported by Cherry et al.39 The difference in the results may be due to the different strains of B. bassiana and inoculation methods used in the two studies. The results from this study therefore indicate that two of the five B. bassiana isolates used in this study have potential as biological control agents against S. calamistis in rice.
Conclusion
This study revealed that rice cultivars could be colonised by strains of B. bassiana. The five B. bassiana isolates tested in this study were endophytic with various degrees of colonisation and pathogenicity against the rice stem borer, S. calamistis. The results of this study indicate that two of the five tested B. bassiana isolates hold promise as biological control agents of rice stem borers. Further studies under field conditions at different sites and seasons are needed to ascertain the potential of these isolates. From this study, seed treatment seems to be the most appropriate and practical way to introduce the best B. bassiana strains during field studies. The field experiments will be implemented where rice is grown on a large scale using an experimental formulation of the best two B. bassiana strains.
Acknowledgements
This study was funded by the Organization for Women in Science for the Developing World (OWSD) and Plant Health Products (KwaZulu-Natal, South Africa).
Competing interests
We declare that there are no competing interests.
Authors' contributions
W.B.A.B. wrote the initial manuscript, collected the samples, isolated the endophytic fungus, and performed all the morphological, in vitro and in vivo bioassays. A.T. provided guidance and the protocol for the rearing of the borer, Sesamia calamistis. M.D.L. and K.S.Y. provided student supervision, project leadership and management, acquired the funding, and edited the manuscript.
References
1. Chapagain A, Hoekstra A. The blue, green and grey water footprint of rice from production and consumption perspectives. Ecol Econ. 2011;70:749-758. https://doi.org/10.1016/j.ecolecon.2010.11.012 [ Links ]
2. Kfir R. Attempts at biological control of the stem borer Chilo partellus Swinhoe (Lepidoptera: Pyralidae) in South Africa. Afr Entomol. 1994;2:67-68. [ Links ]
3. Vfen Nguyen N, Ferrero A. Meeting the challenges of global rice production. Paddy Water Environ. 2006;4:1-9. https://doi.org/10.1007/s10333-005-0031-5 [ Links ]
4. Khush GS. What it will take to feed 5.0 billion rice consumers in 2030. Plant Mol Biol. 2005;59:1-6. https://doi.org/10.1007/s11103-005-2159-5 [ Links ]
5. AfricaRice Center. Boosting Africa's rice sector: A research for development strategy 2011-2020. Cotonou, Benin: AfricaRice; 2011. [ Links ]
6. Bardenas EA, Chang TT. Morphology and varietal characteristics of the rice plant. Los Banos, the Philippines: The International Rice Research Institute; 1965. [ Links ]
7. Bing LA, Lewis LC. Occurrence of the entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes and in Zea mays L. and virulence towards Ostrinia nubilalis (Hubner). Agr Ecosyst Environ. 1993;45:147-156. https://doi.org/10.1016/0167-8809(93)90065-W [ Links ]
8. Seck PA, Tollens E, Wopereis MC, Diagne A, Bamba I. Rising trends and variability of rice prices: Threats and opportunities for sub-Saharan Africa. Food Policy. 2010;35:403-411. https://doi.org/10.1016/j.foodpol.2010.05.003 [ Links ]
9. Somado E, Guei R, Keya S. NERICA: The New Rice for Africa, a compendium. Cotonou, Benin: Africa Rice Center (WARDA); 2008. [ Links ]
10. Quesada-Moraga E, Munoz-Ledesma F, Santiago-Alvarez C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ Entomol. 2009;38:723730. https://doi.org/10.1603/022.038.0324 [ Links ]
11. Van den Berg J, Van Rensburg J. Comparison of various directional insecticide sprays against Busseola fusca Fuller (Lepidoptera: Noctuidae) and Chilo partellus Swinhoe (Lepidoptera: Pyralidae) in sorghum and maize. S Afr J Plant Soil. 1996;13:51-54. https://doi.org/10.1080/02571862.1996.10634375 [ Links ]
12. Warui C, Kuria J. Population incidence and the control of maize stalk borers Chilo partellus Swinhoe, C. orichalcociliellus Strand and Sesamia calamistis Hampson, in Coast Province, Kenya. Int J Trop Ins Sci. 1983;4:11-18. https://doi.org/10.1017/S1742758400003970 [ Links ]
13. Ignoffo CM, PuttlerB, HostetterDL, Dickerson WA. Susceptibility of the cabbage looper, Trichoplusia ni, and the velvet bean caterpillar, Anticarsia gemmatalis, to several isolates of the entomopathogenic fungus Nomuraearileyi. J Invertebr Pathol. 1976;28:259-262. https://doi.org/10.1016/0022-2011(76)90132-4 [ Links ]
14. Deedat YD. Problems associated with the use of pesticides: An overview. Int J Trop Ins Sci. 1994;15:247-251. https://doi.org/10.1017/S1742758400017537 [ Links ]
15. Godonou I, Green KR, Oduro KA, Lomer CJ, Afreh-Nuamah K. Field evaluation of selected formulations of Beauveria bassiana for the management of the banana weevil (Cosmopolites sordidus) on plantain (Musa spp. AAB Group). Biocontrol Sci Technol. 2000;10:779-788. https://doi.org/10.1080/09583150020011726 [ Links ]
16. Kfir R. Seasonal abundance of the stem borer Chilo partellus Swinhoe (Lepidoptera: Pyralidae) and its parasites on summer grain crops. J Econ Entomol. 1992;85:518-529. https://doi.org/10.1093/jee/85.2.518 [ Links ]
17. Pathak MD, Khan ZR. Insect pests of rice. Los Banos, Philippines: International Rice Research Institute; 1994. [ Links ]
18. Parsa S, Ortiz V Vega FE. Establishing fungal entomopathogens as endophytes: towards endophytic biological control. J Ms Exp. 2013;74, e50360. https://doi.org/10.3791/50360 [ Links ]
19. Cherry AJ, Lomer CJ, Djegui D, Schulthess F. Pathogen incidence and their potential as microbial control agents in IPM of maize stem borers in West Africa. BioControl. 1999;44:301-327. https://doi.org/10.1023/A:1009991724251 [ Links ]
20. Kikuchi M, Haneishi Y Tokida K, Maruyama A, Godfrey A, Tsuboi T. The structure of rice retail markets in sub-Saharan Africa. Trop Agric Development. 2015;59:127-139. https://doi.org/10.11248/jsta.59.127 [ Links ]
21. Posada F, Aime MC, Peterson SW, Rehner SA, Vega FE. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol Res. 2007;111:748-757. https://doi.org/10.1016/j.mycres.2007.03.006 [ Links ]
22. Akello J, Dubois T, Gold CS, Coyne D, Nakavuma J, Paparu P Beauveria bassiana (Balsamo) Vuillemin as an endophyte in tissue culture banana (Musa spp). J Invertebr Pathol. 2007;96:34-12. https://doi.org/10.1016/j.jip.2007.02.004 [ Links ]
23. Pimentel D, Acquay H, Biltonen M, Rice P Silva M, Nelson J, et al. Environmental and economic costs of pesticide use. Bioscience. 1992;42:750-760. https://doi.org/10.2307/1311994 [ Links ]
24. Wagner BL, Lewis LC. Colonization of corn, Zea mays, by the entomopathogenic fungus, Beauveria bassiana. Appl Environ Microbiol. 2000;66:3468-3473. https://doi.org/10.1128/AEM.66.8.3468-3473.2000 [ Links ]
25. Ownley BH, Griffin MR, Klingeman WE, Gwinn KD, Moulton JK, Pereira RM. Beauveria bassiana: Endophytic colonization and plant disease control. J Invertebr Pathol. 2008;98:267-270. https://doi.org/10.1016/j.jip.2008.01.010 [ Links ]
26. Tefera T, Pringle K. Food consumption by Chilo partellus (Lepidoptera: Pyralidae) larvae infected with Beauveria bassiana and Metarhizium anisopliae and effects of feeding natural versus artificial diets on mortality and mycosis. J Invertebr Pathol. 2003;84:220-225. https://doi.org/10.1016/j.jip.2003.11.001 [ Links ]
27. Dobeski JW, Tribe HT. Isolation of entomogenous fungi from elm bark and soil with reference to ecology of Beauveria bassiana and Metarhizium anisopliae. Trans Br Mycol Soc. 1980;74:95-100. https://doi.org/10.1016/S0007-1536(80)80013-1 [ Links ]
28. Qazi SS, Khachatourians GG. Insect pests of Pakistan and their management practices: Prospects for the use of entomopathogenic fungi. Biopest Int. 2005;1:13-24. [ Links ]
29. Tefera T, Vidal S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009;54:663-669. https://doi.org/10.1007/s10526-009-9216-y [ Links ]
30. Vega FE, Posada F, Aime MC, Pava-Ripoll M, Infante F, Rehner SA. Entomopathogenic fungal endophytes. Biol Control. 2008; 46:72-82. https://doi.org/10.1016/j.biocontrol.2008.01.008 [ Links ]
31. Bing LA, Lewis LC. Suppression of Ostrinia nubilalis Hubner (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ Entomol. 1991; 20:1207-1211. https://doi.org/10.1093/ee/20.4.1207 [ Links ]
32. Bing LA, Lewis LC. Occurrence of the entomopathogen Beauveria bassiana (Balsamo) Vuillemin in different tillage regimes and in Zea mays L. and virulence towards Ostrinianubilalis (Hubner). Agric Ecosyst Environ. 1993;45:147-156. https://doi.org/10.1016/0167-8809(93)90065-W [ Links ]
33. Akello J, Dubois T, Coyne D, Hillnhutter C. Beauveria bassiana as an endophyte in tissue cultured banana plants: A novel way to combat the banana weevil, Cosmopolites sordidus. III International Symposium on banana: ISHS-ProMusa Symposium on recent advances in banana. Acta Hortic. 2009;828:129-138. https://doi.org/10.17660/ActaHortic.2009.828.12 [ Links ]
34. Maniania NK. Pathogenicity of entomogenous fungus (Hyphomycetes) to larvae of the stem borers, Chilo partellus Swinhoe and Busseola fusca Fuller. Int J Trop Insect Sci. 1992;13:691-696. https://doi.org/10.1017/S1742758400007918 [ Links ]
35. Akello J, Dubois T, Coyne D, Kyamanywa S. Endophytic Beauveria bassiana in banana (Musa spp) reduces banana weevil (Cosmopolites sordidus) fitness and damage. Crop Prot. 2008;27:1437-1441. https://doi.org/10.1016/j.cropro.2008.07.003 [ Links ]
36. Goulson D. Review: An overview of the environmental risks posed by neonicotinoid insecticides. J Appl Ecol. 2013;50:977-987. [ Links ]
37. Van Bael SA, Maynard Z, Rojas E, Mejia LC, Kyloo DA, Herre EA, et al. Emerging perspectives on the ecological roles of endophytic fungi in tropical plants. Mycol Series. 2005;23:181. [ Links ]
38. Gurulingappa P Sword GA, Murdoch G, McGee PA. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol Control. 2010;55:34-41. https://doi.org/10.1016/j.biocontrol.2010.06.011 [ Links ]
39. Cherry AJ, Banito A, Djegui D, Lomer C. Suppression of the stem borer Sesamia calamistis (Lepidoptera: Noctuidae) in maize following seed dressing, topical application and stem injection with African isolates of Beauveria bassiana. Int J Pest Manage. 2004;50:67-73. https://doi.org/10.1080/09670870310001637426 [ Links ]
40. Valda CAS, Reginaldo B, Edmilson JM, Jorge BT. Susceptibility of Plutella xylostella (L.) (Lepidoptera: Plutellidae) to the Fungi Beauveria bassiana (Bals.) Vuill. and Metarhizium anisopliae (Metsch.) Sorok Neotrop Entomol. 2003;32:653-658. https://doi.org/10.1590/S1519-566X2003000400016 [ Links ]
41. Godonou I, James B, Atcha-Ahowe C, Vodouhe S, Kooyman C, Ahanchede A, et al. Potential of Beauveria bassiana and Metarhizium anisopliae isolates from Benin to control Plutella xylostella L. (Lepidoptera: Plutellidae). Crop Prot. 2009;28:220-224. https://doi.org/10.1016/j.cropro.2008.10.009 [ Links ]
42. Tesfaye D, Seyoum E. Studies on the pathogenicity of native entomopathogenic fungal isolates on the cotton/melon aphid, Aphis gossypii Glover (Homoptera: Aphididae) under different temperature regimes. Afr Entomol. 2010;18:302-312. https://doi.org/10.4001/003.018.0215 [ Links ]
 Correspondence:
Correspondence:
Kwasi Yobo
Email: Yobok@ukzn.ac.za
Received: 31 Jan. 2020
Revised: 17 Apr. 2020
Accepted: 21 Apr. 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: Organization for Women in Science for the Developing World (OWSD), Plant Health Products (KwaZulu-Natal, South Africa)














