Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.11-12 Pretoria Nov./Dec. 2020
http://dx.doi.org/10.17159/sajs.2020/8508
RESEARCH ARTICLE
Effect of northern corn leaf blight severity on Fusarium ear rot incidence of maize
Maryke CravenI; Liesl MoreyII; Adrian AbrahamsI, III; Henry A. NjomI; Belinda Janse van RensburgI
IGrain Crops, Agricultural Research Council, Potchefstroom, South Africa
IIBiometry Unit, Agricultural Research Council, Pretoria, South Africa
IIIDepartment of Biotechnology and Food Technology, University of Johannesburg, Johannesburg, South Africa
ABSTRACT
Northern corn leaf blight (NCLB) caused by Exserohilum turcicum and Fusarium ear rot caused by Fusarium verticillioides, are economically important maize diseases in South Africa. The effect of induced plant stress by NCLB on F. verticillioides ear rot and fumonisin production is unknown. Four field trials were conducted during 2016/2017 and 2017/2018 (November and December planting dates) at the Agricultural Research Council - Grain Crops in Potchefstroom (South Africa). Three maize cultivars with varying resistance levels to NCLB were selected (IMP50-10B - susceptible, BG3292 - moderately susceptible, DKC 61-94BR - resistant). NCLB severities were created through eight treatments: TMT1 - maximum control (three fungicide applications); TMT2 - standard control (two fungicide applications) and TMT3 - natural control (not inoculated or sprayed). The remaining treatments were inoculated with a cocktail of five NCLB races (Race 3, 3N, 23, 23N and 13N): TMT4 (five weeks after planting / WAP); TMT5 (five and six WAP); TMT6 (five, six and seven WAP); TMT7 (six and seven WAP); and TMT8 (seven WAP). Maize ears were naturally infected with F. verticillioides. Fifteen random plants were labelled at dent stage and NCLB severity (%), area under the disease progress curve, ear rot diseased area, ear rot severity (%), ear rot incidence (%) and total fumonisins (FB1 + FB2+FB3; ug/kg) were established. Low levels of cob rot severity and fumonisins were obtained in all four trials. NCLB severity did not affect ear rot related parameters measured. Mean fumonisin levels were below the South African tolerance levels. Fumonisin concentrations differed significantly between cultivars but was not affected by NCLB severity or the cultivar x treatment interaction.
SIGNIFICANCE:
• This is the first study to investigate the effect of NCLB severity as a predisposing factor of ear rot incidence and severity of maize.
• The study confirmed that ear rot incidence and severity are not impacted by secondary stressors induced by NCLB, and that the cultivation of NCLB-resistant varieties would not bring about lower ear rot incidences.
Keywords: AUDPC, Exserohilum turcicum, fumonisin, Fusarium verticillioides, HPLC
Introduction
Northern corn leaf blight (NCLB), caused by Exserohilum turcicum (Pass.) K.J. Leonard and E.G. Suggs, is one of the most prominent leaf diseases of maize (Zea mayze) in South Africa. This disease occurs predominantly in the KwaZulu-Natal production areas and is particularly severe under irrigation systems.1 Typical yield losses attributed to the disease generally range between 15% and 30%, but yield losses of up to 50% have been documented.2,3 A potential yield reduction of 2-8% exists for every 10% increase in disease severity.4,5
Internationally, reference has been made to the development of secondary complications in maize due to severe leaf desiccation owing to infection by foliar pathogens. Latterell and Rossi6 reported severe lodging and up to 100% yield loss due to stalk deterioration of maize brought about by grey leaf spot (Cercospora zeae-maydis Tehon & E.Y Daniels). Stalk deterioration was attributed to the covering of the photosynthetic surfaces of the plant by lesions, which led to extreme water loss, but no report was given on whether stalk rot pathogens were conversely responsible for the stalk deterioration. NCLB has similarly been shown to potentially predispose maize plants to attack by both stalk7,8 and root rot pathogens9 when severe enough, by inducing sufficient stress in plants to weaken their natural defence mechanisms.
Despite the presence of Fusarium ear rot over the whole maize production area, the disease only gained importance when the mycotoxin-producing capabilities of its causal organism became evident.10 Fusarium ear rot caused by Fusarium verticillioides (Sacc.) Nirenberg (syn. Fusarium moniliforme J. Sheldon, Fusarium section Liseola)11, negatively affects crop yield and quality. The species can produce secondary metabolites (fumonisins) associated with a wide range of noxious effects on humans and livestock upon ingestion.12 Locally, high natural infection rates of F. verticillioides and resulting fumonisin concentrations were reported in warmer production areas including the Northern Cape, North-West and Free State Provinces of South Africa.13 South African regulations stipulate a tolerance of 4000 ug/kg for fumonisins in maize grain intended for further processing, while processed products that are ready for human consumption may not contain more than 2000 ug/kg of fumonisins.14
High temperatures, drought, poor fertilisation and stiff competition for nutrients are some of the conditions known to weaken the plant's natural defence, which predisposes the plant to increased ear rot infections.15,16 These conditions can promote colonisation by mycotoxigenic Fusarium spp. in maize grain during the growing season. Although it is commonly accepted that severe leaf diseases can potentially result in an increase in stalk rot incidence, it is not yet established whether a similar association could be drawn for ear rot infections (such as F. verticillioides) and subsequent fumonisin production in maize grain.
In the course of 2016, the Agricultural Research Council - Grain Crops, initiated a project in which field trials were conducted over a 2-year period to ascertain to what extent NCLB severity would impact on the manifestation of secondary diseases in maize cultivars with differing NCLB resistance statuses. Key to these trials was that NCLB would be the only disease introduced artificially, whilst the response of the cultivars pertaining to the development of secondary diseases through natural infection would be monitored. Of interest in the current study was whether NCLB-resistant varieties would assist in minimising the risk associated with ear rot infections and subsequent severity, and whether such a cultivar trait could be utilised in an integrated pest management strategy to not only reduce inoculum pressure, but also to minimise input costs. Weighing the cost associated with fungicide applications against the benefit of both natural resistance of NCLB-resistant varieties and the additional benefits of reduced ear rot infections potentially provided by NCLB-resistant varieties, will be useful to producers, allowing for informed decisions to be made regarding which cultivars to plant.
The current study reports on the observed influence of northern corn leaf blight severity on F. verticillioides ear rot infection and fumonisin production in the grain of three South African maize hybrids with varying NCLB disease resistance in the field.
Materials and methods
Inoculum preparation, field trials and treatment application
The five E. turcicum races (Race 3, 3N, 23, 23N and 13N) used in this study were ascertained through replicated growth chamber studies by means of differential sets of varying backgrounds.17 NCLB races were inoculated into maize seedlings and re-isolated from lesions. Mycelial plugs of each race were grown on potato dextrose agar for 2 weeks before mycelial plugs were transferred to autoclaved maize kernels in fruit flasks prepared according to Flett and McLaren18. Flasks were incubated at room temperature and shaken daily. After 2 weeks, the contents of the flasks were dried for 3 days after which the maize kernels were ground in a standard maize mill.19 The races were kept separate at all times and the mill was thoroughly cleaned after each isolate batch. After milling, equal amounts of each of the 10 isolates were added and thoroughly mixed to obtain an inoculation mixture.
Four field trials were conducted during 2016/2017 and 2017/2018. Two trials were planted during November and December, during each growing season, on the grounds of the Agricultural Research Council - Grain Crops (ARC-GC), Potchefstroom (North West Province; 26.743594.27 S, 27.069491 E). Three maize cultivars with varying resistance levels to NCLB were selected based on their performance in the national cultivar evaluation trials of ARC-GC under natural NCLB infection and included IMP50-10B (susceptible), BG3292 (moderately susceptible) and DKC 61-94BR (resistant). Various levels of NCLB were created through the application of eight treatments, including three control treatments: tMT1 - maximum control (three fungicide applications); TMT2 - standard control (two fungicide applications); TMT3 - natural control (not inoculated or sprayed). The remaining treatments were inoculated at various dates with the cocktail consisting of five NCLB races that included Race 3, 3N, 23, 23N and 13N: TMT4 - inoculated five weeks after planting (WAP); TMT5 - inoculated five and six WAP; TMT6 - inoculated five, six and seven WAP; TMT7 - inoculated six and seven WAP and TMT8 - inoculated 7 WAP. Each plant was inoculated with approximately 6 g inoculum placed in the whorl. TMT1 and TMT2 received two foliar fungicide formulations used in rotation every season i.e. Abacus® (pyraclostrobin/epoxiconazole - 1L/ha, BASF SA, Johannesburg, South Africa) and Sparta SC (flusilazole/carbendazim -500 mL/ha, Villa Crop Protection, Johannesburg, South Africa) together with an adjuvant Picanta (150 mL/ha, Villa Crop Protection). Fungicides were applied at 3-week intervals, with TMT1 receiving its first fungicide application at V8 leaf stage and TMT2 at flowering. Fungicides were applied using a CO2 gas operated knapsack sprayer and a four-nozzle (flat fan; 0.9 m spaced) boom. The knapsack sprayer was calibrated to a spray volume of 78 L/ha.
Each trial was planted in a split-plot design with treatment as the main plot and cultivar as the sub-plot, replicated three times. Each sub-plot consisted of two border rows flanking four rows per cultivar with 0.9-m inter-row spacing, 15 m in length. Intra-row spacing was 30 cm, with two kernels planted per hill. Four weeks after planting the plants were thinned out to one plant per hill. Fertiliser was applied according to soil analysis (150 kg/ha 3:2:1, 200 kg/ha LAN top dressing - 6 weeks after planting). Callisto (mesotrione - 480 g/L Syngenta SA, Centurion, South Africa) and Dual (s-metolachlor - 915 g/L, Syngenta SA) were applied pre-emergence and Basagran® (bendioxide - 480 g/L, BASF SA) was applied post-emergence to prevent weed encroachment. Directly after inoculation, approximately 15 mm water was applied through overhead irrigation over a 4-h period. Thereafter irrigation was supplied supplementary to rainfall as needed throughout the season to ensure that the trials received water weekly. Maize ear rot was initiated from natural infection by F. verticillioides. Weather data were captured by the ARC weather station situated on the Potchefstroom research farm.
Screening and sampling
Fifteen randomly selected plants were labelled in the first of the four middle rows of each plot and screened for NCLB development at V12, flower, milk, soft dough and dent stage.20 Disease was quantified as the percentage infected leaf material per plant per plot using a modified scale of 0.0, 0.5, 1.0, 5.0 10.0, 25.0, 50, 70 and >85%.19,21Area under the disease progress curve (AUDPC) was determined for each plot.
At physiological maturity, ears from the 15 marked plants were harvested separately from the remaining plants in the allocated row and screened for ear rot severity. Ear rot incidence and area affected (cm2) were established. Area affected (cm2) was established by using a 1 cm x 1 cm transparent plastic grid placed over the ear and the number of squares in which diseased areas could be observed, were counted. The ears were threshed and the kernel weight determined. A representative milled sample from each plot was stored at -20 °C until determination of total fumonisin concentrations. Fumonisins were analysed using the HPLC-VICAM method.22 Fumonisin standards were obtained from the Cape Peninsula University of Technology. A standard curve was generated by evaporating standards and reconstitution with a calibration standard solution ranging from 0.31 to 5 ug/kg. Fluorescence was performed at excitation and emission wavelengths of 335 nm and 440 nm, respectively, using a Waters 2475 multi A fluorescence detector equipped with a Symmetry C18 (5 um 3.9 x 150 mm) analytical column (Waters, Milford, USA). The LOD of the method used was 16 ug/kg and R2values were >99%. Total fumonisins were determined as the sum of FB1+FB2+FB3.
The remainder of the plants in the allocated row were harvested and yield established per plot by combining the kernel weight of the 15 marked plants and the remainder of the plants in the designated row. Yield was calculated at 12.5% moisture (l/ha).
Statistical analysis
Each trial was designed as a randomised block design with three replicates. The treatment design was a split-plot with the eight treatments and four cultivars randomised within each whole plot. Data of the various parameters measured from each trial were subjected to a split-plot analysis of variance to test for significant differences between treatments, cultivars and the interaction. Means of significant source effects were separated using Fisher's protected t-least significant difference (LSD) at a 5% significance level. In cases in which the interaction effect was non-significant, but either of the main effects indicated significant differences, treatment x cultivar interaction means were separated using Fisher's unprotected t-LSD.23 All the analyses were conducted using GenStat for Windows 18th edition. Regression analyses were performed to ascertain whether a relationship (linear or non-linear) existed between NCLB disease and ear rot parameters measured. Regressions were performed per cultivar, per trial.
Results
As environmental conditions during the flowering period determine the potential for ear rot development, reigning conditions during this period were of interest in the current study. Temperature and rainfall data during January (2017 and 2018) coincided with the general flowering period of the November planting dates (2016 and 2017), whilst February (2017 and 2018) coincided with that of the December planting dates (2017 and 2018) (Table 1). The 2016/2017 season experienced higher rainfall (658 mm) than that of 2017/2018 (414.27 mm), with the majority recorded during the month of February (2017). Average maximum temperatures were slightly higher during both January and February of 2018 than the same period during 2017. Temperatures for the remainder of the months for both seasons were very similar with the exception of December 2016, which was in general warmer than December 2017.
Ears of the 15 marked plants were inspected for all types of ear rot. Fusarium verticillioides ear rot was, however, the only type of ear rot present in all four trials. No Gibberella ear rot (Gibberella zeae) or Diplodia ear rot (Stenocarpella maydis) was observed.
Northern corn leaf blight severity and AUDPC
Aside from the November planting of 2017/2018, the various treatments allowed for a range of NCLB severity levels to be produced within each trial (Tables 2-5) that allowed a comprehensive view of the possible impacts that different severity levels have on ear rot development. During 2017/2018, untimely or continuous rainfall was experienced, which resulted either in fungicide applications not being applied at the optimum time or fungicide that was applied being washed off after application. This resulted in little to no control in TMT1 and TMT2, especially in the November 2017/2018 planting (Table 4). Although DKC61-94BR was included as the resistant cultivar, the use of a mixture of NCLB races lead to similar NCLB severities in this hybrid compared to that of the more susceptible hybrids (BG3292 and IMP50-10B). Average NCLB disease severities realised within the eight treatments accordingly were in the ranges of 0.7-70.7% (Table 2), 6.7-60.1% (Table 3), 38.5-61.3% (Table 4) and 16.7-55.5 % (Table 5) in the various trials. Both cultivar and treatment differed significantly in all four trials, with the cultivar x treatment interaction differing significantly in the December (2016/2017) and November (2017/2018) trials. Of the three cultivars included, DKC61-94BR consistently gave the lowest NCLB severity, whist TMT5 yielded the greatest NCLB severities in three of the trials. The general trend for AUDPC data generated mirrored that of NCLB severities achieved at dent stage. With the exception of the November 2016/2017 trial (Table 2), cultivar differences were observed in the AUDPC data. In all three trials, DKC61-94BR produced significantly lower AUDPC values (Tables 3-5). Similarly to the NCLB severity, TMT5 yielded the highest AUDPC in three of the trials (Tables 2, 4 and 5). Average AUDPCs achieved within the eight treatments in the various trials were in the ranges 24-1465 (Table 2), 227-1005 (Table 3), 703-1198 (Table 4) and 103-771 (Table 5). Sufficient ranges of AUDPCs were generated to effectively evaluate the potential impact of NCLB on ear rot severity.
Ear rot affected area
In general, low levels of area affected were observed in all four trials. Cultivar differences were observed in three of the four trials (Tables 2-1). BG3292 attained significantly greater ear rot affected areas in all three trials, which varied between 3.7 cm2 (November, 2016/2017 planting; Table 2) and 10.7 cm2 (November, 2017/2018 planting; Table 4). The remaining two cultivars had similar ear rot affected areas in all three trials. Only in one trial (November, 2016/2017 planting; Table 2) did the treatments result in significant differences, with TMT2 yielding a significantly greater average ear rot affected area (2.9 cm2) over the three cultivars included. A significant cultivar x treatment interaction was observed in the December 2017/2018 season, with TMT8, TMT1, TMT5, TMT2 and TMT6 of BG3292 achieving the highest area affected (Table 4).
Ear rot severity
Ear rot severity, similar to ear rot affected area, was very low in all four trials with trial means of 1.1%, 0.6%, 3.6% and 2.62%, respectively (Tables 2-5). Cultivar differences were observed in both the 2016/2017 trials as well as the November 2017/2018 planting trial, with BG3292 yielding significantly greater ear rot severity in all three trials (2.4%, 1.4% and 6.3% respectively; Tables 2-4). Neither the treatment effect nor the cultivar x treatment interaction was significant.
Ear rot incidence
Cultivar differences were observed in both the 2016/2017 trials as well as the November 2017/2018 trial. In all cases, BG3292 gave significantly greater ear rot incidence, which varied from 31.7% of the ears having some degree of ear rot (November 2017/2018 planting; Table 4) to 51% of the ears in the November 2016/2017 planting (Table 1).
Fumonisin
The average fumonisin concentration detected per trial in the sampled material ranged between 2 ug/kg (December 2016/2017 planting; Table 3) and 235 ug/kg (November 2017/2018 planting; Table 4). Cultivar differences occurred in the two 2016/2017 trials (Tables 2 and 3) as well as the November 2017/2018 planting (Table 4). BG3292 achieved the highest average fumonisin concentration in the grain in all three trials (3.8, 2.9 and 381 ug/kg, respectively). Significant differences between treatments in terms of fumonisin concentrations in the grain were only observed for the 2016/2017 November planting (Table 2), with TMT1 (5.3 ug/kg) followed by TMT8 (4 ug/kg). No significant cultivar x treatment interaction was observed. Fumonisin concentrations measured did not exceed 1407 ug/kg (Table 5) in any of the trials.
Regression analyses
Regression analyses were initially conducted against NCBL severity (at dent stage) and AUDPC for each of the ear rot related parameters. This was done per cultivar per season. As none of the regression analyses (either linear or non-linear) was significant (data not shown), the possibility was considered that external factors (other than NCLB severity) had contributed to the random effects observed over seasons. Data were accordingly pooled across the trials for each treatment, as pooling of data aids in minimising any effect that external factors, not linked to NCLB severity, might have had on the ear rot parameters measured. Linear, exponential and polynomial regression analyses were again conducted. Ear rot incidence was the only parameter that demonstrated a potential relationship with NCLB severity (R2= 0.67; Figure 1a) and AUDPC (R2 = 0.65; Figure 2a) for IMP50-10B; however, the relationship was not significant in either circumstance.
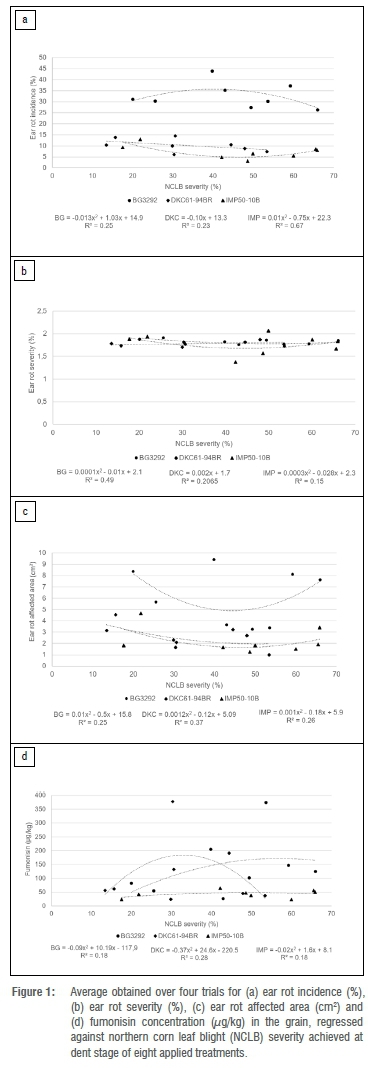
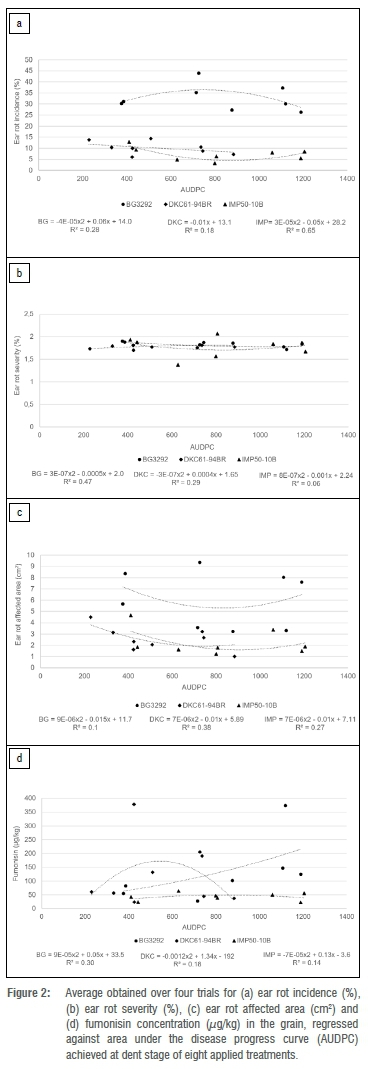
Discussion
The objective of this study was to establish whether the ear rot severity observed in three maize cultivars with varying degrees of NCLB resistance, would be impacted by NCLB severity suffered during the growing season. Multiple season trials were conducted together with an intensive E. turcicum inoculation approach to ensure that different degrees of NCLB were created to assess whether NCLB would predispose the maize plant to greater ear rot infections and subsequent fumonisin production in maize grain. Despite the fact that high levels of NCLB were achieved in all four trials, very low levels of ear rot (less than 11% obtained in the November 2017/2018 planting) were nonetheless observed. Fumonisin levels detected in the grain were also well below the accepted 2000 ug/kg concentration for grain. The averages in the trials varied between 2 ug/kg and 235 ug/kg.
Internationally, it is accepted that F. verticillioides gains access to the ear by one or more of three main access pathways: (1) fungal spores germinating on the silks and then fungal mycelia growing down the silks to infect the kernels and the ear (rachis); (2) systemic infection of the ear through infected stalks that generate infected seeds and (3) through wounds on the ear generated by insects, birds or hail damage.11,24 It is also common knowledge that ear rot incidence and severity as well as associations with mycotoxins vary with environmental conditions, genotype, and location.11,25 In general, higher temperatures and drier weather during flowering (26 °C and higher), higher temperatures during kernel maturation, more rainfall before harvest, drought stress as well as insect damage stress are factors known to increase ear rot severity and fumonisin content at harvest.11,26,27 Weather conditions during flowering are, however, considered critical for primary infection as well as for toxin synthesis in grain.28-30 For the current study, it was imperative that moist conditions were maintained throughout the duration of trials to ensure effective NCLB infection and subsequent high NCLB disease severity. Although leaf blight data indicate high and variable levels of disease, the extremely low ear rot levels raised the question of whether these low levels were due to the absence of epidemiologically competent inoculum, the absence of predisposition or possibly the end result of inherent cultivar resistance.
As all four trial sites of the current study were situated in the same area where ear rot related field experiments have been regularly conducted over numerous seasons, and entailed artificial inoculation with multiple F. verticillioides isolates22,31, it was assumed that present-day isolates at the trial site area would be more than capable of infecting maize ears, provided environmental conditions were conducive for ear rot infection and development. Although maximum temperatures during all four trials were in the required range for Fusarium ear rot development, drier conditions (which would have enhanced ear rot development)26,27 did not occur during flowering due to the irrigation applied to ensure NCLB development. The question to be addressed was whether NCLB severity would place the plant under sufficient stress to induce a water stress associated situation6 in the plant, which would unlock a similar response in the plant as would drought stress. One way in which this could happen is if NCLB infection results in stalk rot develoment7, which would hamper the plant's ability to access water and nutrients. NCLB severity at flowering stage was low with average NCLB severities of between 3% and 14% over the four trials (data not shown). Desiccation due to NCLB was accordingly most likely not severe enough at this critical stage to induce a form of water loss6 that would aid colonisation by the F. verticillioides pathogen and result in fumonisin production26.
Even though heritable resistance has been identified in maize32,33, Small et al.34 were the first to report potentially resistant maize inbred lines locally adapted to southern African production conditions. Very little is, however, known regarding the adoption rate of such lines by local breeding companies, especially as Fusarium ear rot resistance has been established to be a quantitative trait determined by polygenes.35,36 The respective seed companies could not confirm the Fusarium ear rot resistance of the three cultivars included. Based on what is known internationally, it would nevertheless be highly unlikely that these cultivars would pose such high levels of resistance that could be linked to limited ear rot infection observed over multiple seasons for all three cultivars, as no highly resistant genotypes suited to the production regions in southern Africa exist.37 A form of indirect resistance through the presence of the Bt gene, which would reduce damage by insects and subsequent infection by the pathogen, might have contributed to lower ear rots being observed. Of the three cultivars included, only DKC 61-94BR contains MON89034. BG3292, which accordingly does not contain Bt genes, consistently had the highest degree of ear rot, but never exceeded levels greater than 10.6% severity in any of the trials (Table 4). Irrespective of how the fungus infected, one would expect that - should stress induced by NCLB create favourable conditions for ear rot infection and growth - greater ear rot infections should have been observed in a cultivar such as BG3292, which consistently had high average NCLB severity over four trials.
Regression analyses conducted over multiple seasons and cultivars point to no significant association between NCLB and natural F. verticillioides infection. The possible exception is the fact that BG3292, which consistently had high NCLB severity over four trials, was identified as the cultivar with the highest degree of ear rot and fumonisin concentration observed in the ears (albeit at very low levels). The latter observation nevertheless speaks more to the hybrid's ability to cope with both the diseases individually, than to the link between the two diseases. In essence, the higher levels of NCLB in BG3292 did not result in an increase in ear rot or related parameters in any of the trials conducted.
It has lastly already been established that F. verticillioides can also infect through wounds on the ear11,31; hence artificial inoculations which make use of techniques which inject the pathogen into the ear are commonly used22,31. Although it has been established with the current study that NCLB severity was not able to induce greater ear rot incidence or severity under natural infection of F. verticillioides, follow-up research which includes artificial inoculation of F. verticillioides would shed additional light on the ability of NCLB to predispose the plant to greater ear rot infection in situations in which ears are damaged by insects, hail or birds.
Conclusion
In the current study, natural ear rot development was monitored in an area in which numerous field studies have been conducted in the past with epidemiological competent F. verticillioides ear rot isolates. Very low levels of ear rot severity were nonetheless obtained in all four trials. Without artificial interference, the local F. verticillioides isolates were not able to naturally infect the ears, most likely because conditions were too wet during flowering, which was a necessity to ensure sufficient NCLB development. Environmental conditions during flowering are determinant for ear rot development. Although high and variable degrees of NCLB severity were achieved in the current study, blight severity at flowering was not severe or sufficient enough to induce a stress response in the plants, which would simulate water stress conditions that would allow for greater ear rot development. Additional studies which include artificial inoculation of the ears, would aid in clarifying the potential effect of NCLB severity in scenarios in which ear rot development is brought about by insect, bird or hail damage. Based on fitted regression models, NCLB severity did not, however, affect natural ear rot development in three maize cultivars with varying NCLB resistance levels.
Acknowledgements
This work is based on research supported in part by the National Research Foundation of South Africa (grant number: 105981) and the Maize Trust.
Competing interests
We declare that there are no competing interests.
Authors' contributions
M.C.: Conceptualisation, methodology, data collection, writing - initial draft, funding acquisition. L.M.: Data analyses, validation, data curation. A.A.: Data collection, sample analyses, writing - revision, project leadership. H.N.: Data collection, sample analyses, writing - revision, project leadership. B.J.v.R.: Data collection, sample analyses, writing -revision.
References
1. Craven M, Morey L. Characterisation of South African ultra-short season maize hybrids: Evaluation of hybrid reaction to Exserohilum turcicum inoculation. S Afr J Plant Soil. 2011;28(3):163-171. https://doi.org/10.1080/02571862.2011.10640015 [ Links ]
2. Perkins JM, Pedersen WL. Disease development and yield losses associated with northern corn leaf blight on corn. Plant Dis.1987;71:940-943. https://doi.org/10.1094/pd-71-0940 [ Links ]
3. Pataky JK. Relationships between yield of sweet corn and northern leaf blight caused by Exserohilum turcicum. Phytopathology.1992;82:370-375. https://doi.org/10.1094/phyto-82-370 [ Links ]
4. Fisher DE, Hooker AL, Lim SM, Smith DR. Leaf infection and yield loss caused by four Helminthosporium leaf diseases of corn. Phytopathology. 1976;66:942-944. https://doi.org/10.1094/phyto-66-942 [ Links ]
5. Bowen KL, Pedersen WL. Effects of northern leaf blight and detasseling on yield and yield components on corn inbreds. Plant Dis.1988;72:952-956. https://doi.org/10.1094/pd-72-0952 [ Links ]
6. Latterell FM, Rossi AE. Gray leaf spot of corn: A disease on the move. Plant Dis. 1983;67:842-847. https://doi.org/10.1094/pd-67-842 [ Links ]
7. Dodd JL. The role of plant stresses in development of corn stalk rots. Plant Dis.1980;64:533-537. https://doi.org/10.1094/pd-64-533 [ Links ]
8. Raymundo AD, Hooker AL. Measuring the relationship between northern leaf-blight and yield losses. Plant Dis. 1981;65:325-327. https://doi.org/10.1094/pd-65-325 [ Links ]
9. Fajemisin JM, Hooker AL. Top weight, root weight, and root rot of corn seedlings as influenced by three Helminthosporium leaf blights. Plant Dis Rep.1974;58:313-317. https://doi.org/10.1094/phyto-64-1496 [ Links ]
10. White DG, editor. Compendium of corn diseases. 3rd ed. St. Paul, MN: APS Press; 1999. [ Links ]
11. Munkvold GP. Epidemiology of Fusarium disease and their mycotoxins in maize. Eur J Plant Pathol. 2003;109:705-713. https://doi.org/10.1023/A:1026078324268 [ Links ]
12. Rose LJ, Okoth S, Flett BC, Janse van Rensburg B, Viljoen A. Preharvest management strategies and their impact on mycotoxigenic fungi and associated mycotoxins. In: Njobeh PB, Stepman F, editors. Mycotoxins - Impact and management strategies. IntechOpen; 2018. https://doi.org/10.5772/intechopen.76808 [ Links ]
13. Janse van Rensburg B, McLaren NW, Flett BC, Schoeman A. Fumonisin producing Fusarium spp. and fumonisin contamination in commercial South African maize. Eur J Plant Pathol. 2015;141:491-504. https://doi.org/10.1007/s10658-014-0558-7 [ Links ]
14. Cosmetics & Disinfectants Act, 1972 (Act no. 54 of 1972), South Africa. Regulations governing tolerances for fungus-produced toxins in foodstuffs: Amendment. Government Gazette no. 40250; 2016. Available from: www.gov.za/sites/default/files/gcis_document/201609/40250gon987.pdf [ Links ]
15. Trento SM, Irgang H, Reis EM. Effect of crop rotation, monoculture and the density of plants in the incidence of grain burned in corn. Braz J Plant Physiol. 2002;27:609-613. https://doi.org/10.1590/S0100-41582002000600009 [ Links ]
16. Bruns HA. Controlling aflatoxin and fumonisin in maize by crop management. Toxin Rev. 2003;22:153-173. https://doi.org/10.1081/txr-120024090 [ Links ]
17. Craven M. Final maize report: Characterisation of Exserohilum turcicum isolates within South African maize production areas. Potchefstroom: Agricultural Research Council - Grain Crops; 2016. [ Links ]
18. Flett BC, McLaren NW. Optimum disease potential for evaluating resistance to Stenocarpella maydis ear rot in maize hybrids. Plant Dis. 1994;78:587-589. https://doi.org/10.1094/PD-78-0587 [ Links ]
19. Adipala E, Lipps PE, Madden LW. Reaction of maize cultivars from Uganda to Exserohilum turcicum. Phytopathology.1993;83:217-223. https://doi.org/10.1094/phyto-83-217 [ Links ]
20. Hanway JJ. How a corn plant develops. Special Report 38. Ames, IA: Iowa State University Cooperative Extension Service; 1966. http://lib.dr.iastate.edu/specialreports/38 [ Links ]
21. Elliott C, Jenkins MT. Helminthosporium turcicum leaf blight of corn. Phytopathology.1946;36:660-666. [ Links ]
22. Janse van Rensburg B, McLaren NW, Schoeman A, Flett BC. The effects of cultivar and prophylactic fungicide spray for leaf diseases on colonisation of maize ears by fumonisin producing Fusarium spp. and fumonisin synthesis in South Africa. Crop Protection. 2016;79:56-63. http://dx.doi.org/10.1016/j.cropro.2015.10.009 [ Links ]
23. Wei J, Carrol RJ, Harden KK, Wu G. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids. 2012;42(5):2031-2035. https://doi.org/10.1007/s00726-011-0924-0 [ Links ]
24. Munkvold GP McGee DC, Carlton WM. Importance of different pathways for maize kernel infection by Fusarium moniliforme. Phytopathology. 1997;87:209-217. https://doi.org/10.1094/phyto.1997.87.2.209 [ Links ]
25. Yamashita A, Yoshizawa T, Aiura Y Sanchez PC, Dizon EI, Arim RH, et al. Fusarium mycotoxins (fumonisins, nivalenol, and zearalenone) and aflatoxins in corn from Southeast Asia. Biosci Biotech Biochem. 1995;59:1804-1807. https://doi.org/10.1271/bbb.59.1804 [ Links ]
26. Shelby RA, White DG, Burke EM. Differential fumonisin production in maize hybrids. Plant Dis. 1994;78:582-584. https://doi.org/10.1094/pd-78-0582 [ Links ]
27. De la Campa R, Hooker DC, Miller JD, Schaafsma AW, Hammond BG. Modeling effects of environment, insect damage, and Bt genotypes on fumonisin accumulation in maize in Argentina and the Philippines. Mycopathologia. 2005;159:539-552. https://doi.org/10.1007/s11046-005-2150-3 [ Links ]
28. Maiorano A, Reyneri A, Magni A, Ramponi C. A decision tool for evaluating the agronomic risk of exposure to fumonisins of different maize crop management systems in Italy. Agric Syst. 2009;102:17-23. https://doi.org/10.1016/j.agsy.2009.06.003 [ Links ]
29. Maiorano A, Reyneri A, Sacco D, Magni A, Ramponi C. A dynamic risk assessment model (FUMAgrain) of fumonisin synthesis by Fusarium verticillioides in maize grain in Italy. Crop Prot. 2009;28:243-256. https://doi.org/10.1016/j.cropro.2008.10.012 [ Links ]
30. Cao A, Santiago R, Ramos AJ, Souto XC, Aguín O, Malvar RA, et al. Critical environmental and genotypic factors for Fusarium verticillioides infection, fungal growth and fumonisin contamination in maize grown in northwestern Spain. Int J Food Microbiol. 2014;177:63-71. https://doi.org/10.1016/j.ijfoodmicro.2014.02.004 [ Links ]
31. Schoeman A, Flett BC, Janse van Rensburg J. Evaluating three commonly used growth media for assessing fumonisin analogues FB1, FB2 and FB3 production by nine Fusarium verticillioides isolates. Food Add Contam. 2017;34(2):291-298. http://doi.org/10.1080/19440049.2016.1266397 [ Links ]
32. Clements MJ, Maragos CA, Pataky JK, White DG. Sources of resistance to fumonisin accumulation in grain and Fusarium ear and kernel rot of corn. Phytopathology. 2004;94:251-260. https://doi.org/10.1094/phyto.2004.94.3.251 [ Links ]
33. Afolabi CG, Ojiambo PS, Ekpo EJA, Menkir A, Bandyopadhyay R. Evaluation of maize inbred lines for resistance to Fusarium ear rot and fumonisin accumulation in grain in tropical Africa. Plant Dis. 2007;91:279-286. https://doi.org/10.1094/pdis-91-3-0279 [ Links ]
34. Small IM, Flett BC, Marasas WFO, McLeod A, Stander MA,Viljoen A. Resistance in maize inbred lines to Fusarium verticillioides and fumonisin accumulation in South Africa. Plant Dis. 2012;96:881-888. https://doi.org/10.1094/pdis-08-11-0695 [ Links ]
35. Robertson-Hoyt LA, Jines MP Balint-Kurti PJ, Kleinschmidt CE, White DG, Payne GA, et al. QTL mapping for Fusarium ear rot and fumonisin contamination resistance in two maize populations. Crop Sci. 2006;46:1734-1743. https://doi.org/10.2135/cropsci2005.12-0450 [ Links ]
36. Eller M, Robertson-Hoyt LA, Payne GA, Holland JB. Grain yield and Fusarium ear rot of maize hybrids developed from lines with varying levels of resistance. Maydica. 2008;53:231-237. [ Links ]
37. Aquino P Carrión F, Calvo R, Flores D. Selected maize statistics. In: Pingali L, editor. CIMMYT 1999-2000 world maize facts and trends: Meeting world maize needs: Technological opportunities and priorities for the public sector. Mexico: CIMMYT; 2001. p. 45-60. [ Links ]
 Correspondence:
Correspondence:
Maryke Craven
Email: CravenM@arc.agric.za
Received: 15 June 2020
Revised: 27 July 2020
Accepted: 03 Aug. 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: South African National Research Foundation (grant no. 105981); Maize Trust














