Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.11-12 Pretoria nov./dic. 2020
http://dx.doi.org/10.17159/sajs.2020/7854
REVIEW ARTICLE
Progress in the management of Fusarium head blight of wheat: An overview
Sinegugu RN. Shude; Kwasi S. Yobo; Nokwazi C. Mbili
Discipline of Plant Pathology, School of Agricultural, Earth and Environmental Sciences, College of Agriculture, Engineering and Science, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
Fusarium head blight (FHB), also known as head scab, is a devastating fungal disease that affects small grain cereal crops such as wheat (Triticum aestivum L.). The predominant causal agent, Fusarium graminearum Schwabe (teleomorph: Gibberella zeae (Schwein.) Petch), is ranked the fourth most important fungal plant pathogen worldwide. Apart from yield and quality losses, mycotoxin production can occur from FHB infection, resulting in harmful effects on human and animal health. Some level of disease control may be achieved by using certain fungicides and agronomic practices plus host resistance. In South Africa, there are currently no registered fungicides or bio-fungicides, no resistant wheat cultivars and only limited control is achieved by cultural practices. Because effective disease reduction cannot be achieved by using a single strategy, the integration of multiple management strategies can enhance disease control. We review possible strategies for reducing the risk for FHB infections that are relevant to the context of South Africa and other wheat growing areas in Africa.
SIGNIFICANCE:
• The importance of the effect of FHB on wheat cannot be overemphasised. This review highlights and describes the various control options and their efficacies. It also describes the current state of research in an effort to control FHB and its associated mycotoxins.
• Wheat is one of the most produced crops worldwide and in South Africa, hence this review could promote and intensify research towards the development of more effective management strategies for FHB of wheat.
Keywords: Fusarium graminearum, Gibberella zeae, disease management, head scab, cereal grain
Introduction
Fusarium head blight (FHB), also known as head scab, is a devastating fungal disease that affects small grain cereal crops such as wheat (Triticum aestivum L.).1-3 It is regarded as a major limiting factor in wheat and barley (Hordeum vulgare L.) production across the world.4,5 The disease is caused by the FHB species complex which consists of more than 17 Fusarium species.68 However, in South Africa, FHB is predominantly caused by Fusarium graminearum Schwabe (teleomorph: Gibberella zeae (Schwein.) Petch).7 The FHB pathogen is capable of causing head blight or scab on wheat, barley, rice (Oryza sativa L.) and oats (Avena sativa L.), and Gibberella stalk and ear rot disease on maize (Zea mays L.). The pathogen may infect other host genera without causing disease symptoms. These genera include Agrostis, Bromus, Calamagrostis, Cortaderia, Cucumis, Echinochloa, Glycine, Lolium, Lycopersicon, Medicago, Phleum, Poa, Secale, Setaria, Sorghum, Spartina and Trifolium. Apart from F. graminearum, the Fusarium species that occur in South Africa are: F. acacia-mearnsii O'Donnell, T. Aoki, Kistler & Geiser, F. boothii O'Donnell, T. Aoki, Kistler & Geiser, F. brasilicum T. Aoki, Kistler, Geiser & O'Donnell, F. cortaderiae O'Donnell, T. Aoki, Kistler & Geiser and F. meridionale T. Aoki, Kistler, Geiser & O'Donnell.9
F. graminearum is distributed worldwide, and is especially prominent in temperate regions where its hosts are mostly cultivated.8 The pathogen infects spikelets at anthesis and thereafter colonises the entire head systemically, thus producing extensive blight symptoms.10,11 This happens when the presence of favourable environmental conditions coincide with high disease pressure and susceptible host tissue.8,11 Disease progress is accompanied by the production of trichothecene mycotoxins [primarily deoxynivalenol (DON), nivalenol (NIV)] and zearalenone (ZEA), which not only pose a threat to the health of humans and other animals, but also reduce grain quality.12,13
Challenges involved in the management of FHB are because the favourable conditions for disease development often coincide with the conditions that trigger anthesis. Moreover, the fast progress and epidemic development of FHB limits the effectiveness of certain control methods.14 Nevertheless, some management strategies have been reported to provide certain levels of FHB and DON reduction on infected hosts.15-21 There are no registered fungicides1 and no completely resistant wheat cultivars1,22 in South Africa or elsewhere, whilst only limited control is achieved by cultural control methods.1,9 Therefore, the development of more effective FHB management strategies is essential.
Wheat production
Wheat is a cereal grain that is native to the Levant region of the Near East and Ethiopian highlands.23 It is cultivated worldwide and is one of the three most produced cereal crops in the world.23,24 Wheat is a good source of carbohydrates (78.10%), proteins (14.70%), minerals (2.10%), fat (2.10%), B-group vitamins and dietary fibre.25 It can be consumed as an ingredient in foods such as bread, pasta, crackers, cakes, noodles and couscous.23,25
Epidemiology of F. graminearum
Wheat plants are mostly susceptible during anthesis, because during this stage the wheat anthers split to discharge pollen (a process known as anther extrusion), which serves as an opening and provides entry for the pathogen.26,27 The favourable conditions for infection are prolonged periods (48-72 hours) of high moisture or relative humidity (<90%), moderately warm temperature (15-30 °C), frequent rainfall and the occurrence of air currents.2,28,29 These conditions usually occur in spring. Trail et al.30 reported that an increase in relative humidity results in a build-up of turgor pressure within the ascus and consequently the forcible discharge of ascospores. Rainfall has been reported to cause the rupturing of the ascus wall which consequently encourages the dispersal of ascospores.14,30
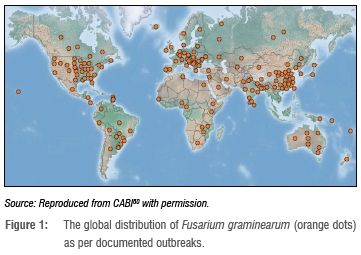
The occurrence of these conditions and the abundance of inoculum before, during and after anthesis of susceptible cultivars, therefore results in yield and quality losses as well as the development of severe epidemics.2,26,31 The host remains susceptible throughout the flowering stage; however, late infections have been associated with reduced disease severity and high DON accumulation.32 Due to differences in climatic requirements, and genetic and environmental adaptations within the FHB species complex, these species are capable of causing disease in a variety of conditions, resulting in the worldwide distribution of FHB (Figure 1).2,8 For example, F. culmorum (Fc) (W.G. Smith) and F. avenaceum (Fr.) Sacc are more predominant in cooler regions (such as Western Europe) whereas F. graminearum predominates in warmer and more humid regions of the world (such as North America and Australia).33
Disease cycle and symptom development
During overwintering or over-summering, the pathogen survives as a precursor to perithecia from which ascospores (primary inoculum) are forcibly discharged under favourable environmental conditions (warm, wet and moist) (Figure 2).11,30 The ascospores are dispersed by wind or rain splashes, land on susceptible plant tissue and colonise the plant surfaces (Figure 2).8,11,33 After entry, Fusarium runner hyphae grow intercellularly and asymptomatically in the inner tissue of the spikelets (palea, lemma and glumes).11,33
Thereafter, the hyphae grow intracellular^, which leads to plant cell death.11 This is accompanied by the production of mycotoxins such as DON which has virulent properties that lead to tissue necrosis.34 Mycotoxins are capable of disabling the plant defence mechanisms and defending the fungus from other microorganisms, thus promoting infection.35 A study by Boenisch and Schãfer36 revealed that F. graminearum forms lobate appressoria and infection cushions during FHB pathogen infection in wheat tissue and trichothecene biosynthesis occurs in these structures. The authors further reported that trichothecene biosynthesis is not necessary for the formation of these structures nor the initial infection of wheat tissue.36
Initial disease symptoms include water-soaked lesions on spikelets which later appear whitened or bleached.11,37 Thereafter, white or pinkish mycelia (Figure 3a) and pink or orange spore masses (Figure 3b) appear on the margin of the glumes of infected spikelets.37,38 Small purple-like or black spherical structures (perithecia) are produced (Figure 3c)37,38 which then sporulate and further infect healthy host tissue2. Infected kernels may appear shrivelled, shrunken and discoloured with a light-brown or pinkish-white appearance.2 These Fusarium-damaged kernels (FDKs) are often associated with high mycotoxin concentrations, reduced seedling emergence and reduced seedling vigour, making them unusable as food, feed or seed.38,39
Economic and social importance of FHB
According to Dean et al.40, FHB is currently ranked the fourth most scientifically and economically important plant fungal disease globally. Economic impacts (direct or indirect) caused by FHB are due to yield loss (production of FDKs), mycotoxin contamination, reduced animal productivity and human health costs.41,42 In the USA, yield and quality losses due to FHB disease on wheat and barley in the 1990s amounted to more than USD3 billion (which equates to ZAR10.5 billion based on the average annual exchange rate in the aforementioned years).43 Losses in Canada have ranged from USD50 million (ZAR183.5 million) to USD300 million (ZAR1.1 billion) annually since the early 1990s.44 According to Scott et al.45, a disease incidence of more than 70% was reported near Winterton in KwaZulu-Natal, South Africa.
FHB-infected grain can result in allergic reactions as well as breathing problems for handlers.46 In non-ruminants (e.g. pigs), feed refusal and reduced feed consumption have been reported as side effects of DON-contaminated feed ingestion.38,44,46 Ruminants (e.g. cattle) are reported to have a higher tolerance to DON concentrations than non-ruminants.35,38 Moreover, adult beef cattle have a higher tolerance to DON concentrations than calves and pregnant cows.44 DON has been reported to result in abortions, stillbirths and weak piglets, thus affecting pig markets.41
In addition to crop losses and mycotoxin production, the disease also leads to the selective loss of albumin and gluten proteins on contaminated grains.47 This results in yield and quality (economic) losses due to a reduction in the market grade of grains intended for feed, malting, baking, milling, trade (exports), biofuel and brewing industries.8,41,44,48
Chemical and physical methods for the detoxification of mycotoxin-contaminated grains have been previously studied.44 In a review by Jard et al.49, various techniques of mycotoxin decontamination are discussed which can be achieved by either adsorption or transformation. Current regulations, however, prohibit both the decontamination of grains with mycotoxin levels above the acceptable limits and the chemical treatment of products intended for human consumption.49 Moreover, obtaining an economical or commercially feasible method for the detoxification of contaminated grains has been unsuccessful thus far.44
Management strategies
Agronomic practices
Certain agronomic practices have been reported to contribute to the reduction of FHB incidence and severity.2,50,51 The most effective cultural control strategies that result in reduced pathogen inoculum and thus reduced FHB incidence and severity in succeeding seasons include: crop rotation with non-host crops; residue management; and tillage practices.9,51,52
Other practices that are of moderate or low efficacy in the control of FHB include: disease forecasting; early planting; the use of early maturing cultivars; the use of cultivars with agronomic traits that are unfavourable for FHB infection; weed control; irrigation management; and optimising crop nutrition.9,51,53 Post-harvest storage practices such as increasing the combine's fan speed, reducing moisture and temperature in the silos, and sorting and discarding broken and damaged kernels have also been reported to be effective in reducing FHB and DON contamination in grain batches.50,51 Because damaged kernels are lightweight and thus easily blown away, they can be separated by increasing the combine's fan speed.50,51
Environmental conditions can affect the efficacy of agronomic practices in the control of FHB. For example, the occurrence of rainy weather can encourage the dispersal of ascospores, thus resulting in FHB infections.8,30 Nevertheless, agronomic practices can lower the amount of inoculum present in the field and thus lower FHB infections on host plants.
Chemical control
In most parts of the world, several fungicides have been tested for their efficacy in reducing FHB on wheat.1,53 According to Haidukowski et al.54, the use of certain fungicides resulted in reductions of 77% and 89% in disease severity and mycotoxin contamination of infected grains, respectively. Fungicides in the demethylation inhibitor (DMI) class are widely used to control FHB and DON contamination on grain crops.53,55 In a study by Paul et al.56, the application of DMI fungicides on wheat anthers at Feekes 10.5.1 growth stage was the most effective treatment in reducing FHB index and DON.
According to Salgado et al.50 and Palazzini et al.16, past research has reported on the successful reduction of FHB severity and DON concentrations and consequently reduced yield and quality losses from the timely application of triazole-based fungicides. Cromey et al.17 observed a reduction up to 90% in FHB incidence and a 14% yield increase through the application of tebuconazole on FHB-infected wheat plants. Moreover, meta-analyses of fungicide trials conducted in the USA showed that metconazole, prothioconazole + tebuconazole, and prothioconazole were the best three fungicide treatments resulting in the highest increase in yield and test weight.57,58
Some fungicides used to control FHB have been reported to indirectly increase DON concentrations in grains.56,59 These include fungicides of the quinone inhibitor (QoI) class.56 In a study by Paul et al.56, the application of QoI fungicides on wheat anthers at either Feekes 9 or Feekes 10.5 growth stages increased mean DON concentrations compared to the non-treated checks. Previous research reports that the use of azoxystrobin resulted in the reduction of FHB caused by Microdochium nivale var. nivale (Fries) Samuels and Hallet, and M. majus (Wollenw.) Glynn & S.G. Edwards, no reduction of F. graminearum and F. culmorum and high DON concentrations on harvested grains.17,60 This could be attributable to the non-toxicity of M. nivale as mycotoxin production has been associated with increased virulence in Fusarium species.51,61 Moreover, azoxystrobin could have slowed down and not prevented the disease17 and also attacked other competitive microorganisms on wheat ears, thus encouraging the development of FHB51,35.
The timing of fungicide application is crucial as fungicides are most effective when applied within a week of early anthesis.1,62 Achieving this timing can be difficult due to the uneven flowering of tillers across cultivation fields as well as rainy weather.62 Moreover, the erratic nature of FHB epidemics can reduce fungicide efficacy.35 Regardless of the successful reduction of FHB and DON provided by certain fungicides, no fungicide has been reported to completely eradicate the disease on infected crops and even the best fungicides are not fully effective.1 Therefore, fungicides are best used in combination with other control strategies (such as cultural methods).1
Biological control
Several bacterial, fungal and yeast strains have been reported to provide effective reduction of FHB severity and/or DON concentrations in infected grains.15,63,64 These were reviewed by Legrand et al.65 and presented in appropriate tables. Biological control agents (BCAs) can be applied as residue, seed, spikelets and/or post-harvest treatments.65 According to Schmale and Bergstrom35, BCAs have been reported to be potentiated with the ability to provide extended protection of spikes even after flowering, when most control strategies (e.g. fungicides) cannot be applied.
Mycotoxin-binding and bio-transforming microorganisms can also be used to reduce mycotoxin contamination in grains by binding the mycotoxins or by converting them to less toxic metabolites, respectively.65 Unfortunately, the development of effective and safe detoxifying agents for use in grains intended for human consumption has been unsuccessful thus far.49,65
Bacteria as antagonists
Strains of Bacillus spp.18,42, Pseudomonas spp.64, Streptomyces spp.15 and Lactobacillus plantarum66have been tested against F. graminearum. These bacteria were isolated from various environments, applied on anthers and/or residues of host plants, and employed various mechanisms of biological control (such as antibiosis, competition and mycoparasitism) against F graminearum.65
In a study by Pan et al.67, Bacillus megaterium reduced FHB incidence and severity, and DON production under field conditions by 93%, 54% and 89.3%, respectively. Furthermore, a study by Palazzini et al.15 reported that Streptomyces sp. RC 87B reduced F. graminearum inoculum by 85% and 100% after 45 days and 90 days, respectively, when applied on wheat stubble. In a later study, Palazzini et al.68 reported that B. velezensis RC 218 and Streptomyces albidoflavus RC 87B effectively reduced FHB incidence (up to 30%), severity (up to 25%) and deoxynivalenol accumulation (up to 51%) on durum wheat under field conditions.
Fungi and yeasts as antagonists
In a study by Xue et al.63, significant reduction in mycelial growth (52.6%), spore germination (-100%), perithecial production (>99%), FHB index (58%), DON concentration (21%) and number of FDKs (65%) was obtained by using Clonostachys rosea strain ACM941 as a BCA against FHB under laboratory, greenhouse and field conditions. Resultantly, C. rosea strain ACM941 is believed to be a promising BCA of FHB. Other fungal species that have been tested against FHB include Trichoderma spp.69 and Microsphaeropsis spp.70 According to Gilbert and Haber14, there are only a few yeast strains that have been reported to be effective against FHB compared to bacteria and fungi. Field trials of three strains of Cryptococcus spp. showed a reduction in FHB severity by as much as 50-60%.71 Reduction in FHB severity by Cryptococcus spp. was also observed in other similar studies.18,19,21,72,73
Breeding for resistance
Many researchers believe that genetic resistance is the best, most cost-effective strategy that could provide meaningful, consistent and durable FHB control.51,74 According to Mesterházy et al.75, wheat resistance to FHB is not Fusarium species-specific, making it achievable by breeding for resistance to Fusarium species in general. Although there are variations in the susceptibility of different host plant species to FHB, there are no wheat or barley varieties that possess immunity against FHB.1,75
Recent wheat breeding programmes for FHB resistance focus on mapping quantitative trait loci (QTL) that confer a response on two or more types of FHB resistance1,76, such as the Fhb1 derived from the Chinese wheat cultivar Sumai 31,76. A list of wheat cultivars that have been evaluated for FHB resistance in China, the USA, Japan and Brazil are presented by Shah et al.77 These landraces provide moderate to high resistance to FHB and some of them have been used as parents in breeding programmes.77
Nonetheless, resistance breeding programmes have been slow, resulting in only a few partially resistant cultivars being produced thus far.1,53 This could be attributable to FHB resistance in small grains being complex and inherited quantitatively.1,53 Consequently, there are no resistant cultivars commercially available in most parts of the world, including South Africa.1,22 However, partially resistant cultivars can be used to reduce disease incidence and severity.75
Priming using resistance inducing chemicals
The use of resistance inducing chemicals such as jasmonic acid, ethylene and salicylic acid to enhance induced systemic resistance and systemic acquired resistance in wheat as means to control FHB has been previously studied.19,20 According to past research, salicylic acid signalling is believed to be responsible for basal resistance to FHB whereas jasmonic acid signalling reduces further infection by the pathogen.20,68 In a study by Palazzini et al.68, salicylic acid signalling was induced early (12 hours) after the inoculation off wheat spikes with F. graminearum whereas jasmonic acid signalling was induced later (after 48 hours). Nevertheless, further research (such as formulation development, optimum concentration and application timing) is required for resistance-inducing chemicals to be employed in FHB management programmes.19
Integrated control strategies
As much as some control strategies provide certain levels of reduction in FHB severity and mycotoxin concentration, no single control strategy will provide significant control of FHB, especially under environmental conditions favourable for disease development.1,19,21,53,73,78,79 Therefore, the use of integrated disease management strategies is considered the best way to control FHB on cereal crops due to the increased reduction of FHB severity and DON concentrations that could be achieved.9,53,78
In a study to test the efficacy of an integrated approach to FHB control, McMullen et al.79 observed that the use of crop rotation, crop rotation + tolerant cultivar, crop rotation + tolerant cultivar + fungicide application resulted in 50%, 80% and 92% reductions in FHB, respectively. In a similar study, the combination of ploughing, a moderately resistant variety and triazole fungicide application at heading resulted in a 97% DON reduction on FHB-contaminated wheat grains.78 BCAs can be combined with other control strategies (such as fungicides) or co-cultured with other BCAs in the integrated management of FHB.19,21,73 In wheat field trials conducted by Schisler et al.21, the co-culture of C. flavescens OH182.9 and C. aureus OH71.4 significantly reduced FHB severity compared to when each of the agents was applied alone. This shows that the integration of effective management strategies has the potential to enhance FHB reduction and should thus be further researched.
Way forward
Regardless of some reported efficacies, the inconsistency and lack of durability of BCAs65, and the residue and resistance development concerns associated with fungicides14,16 are major limitations in the development of FHB management strategies. Moreover, the use of agronomic practices in FHB management is not always feasible and/or economical in commercial farming systems. Some researchers believe that improving host genetic resistance could provide more meaningful, durable and consistent protection against FHB and its mainly produced mycotoxin, DON.51,74 Therefore, future research can be aimed at improving host resistance to FHB either by resistance breeding or by the use of resistance inducers. The isolation and testing of more effective natural antagonists of F. graminearum that can be integrated with other management strategies could help improve FHB control and reduce the risks associated with fungicide use.
Conclusion
FHB remains a major threat to wheat production worldwide. Although some strategies have provided some level of disease reduction, the current dependency on fungicides in FHB management practices poses concerns regarding fungicide resistance as well as environmental, human and animal health. Therefore, further research in the development of more effective and more reliable FHB management strategies is necessary.
Acknowledgements
This study was funded by the National Research Foundation (South Africa) and the University of KwaZulu-Natal Capacity Development Programme.
Competing interests
We declare that there are no competing interests.
Authors' contributions
S.PN.S.: Wrote the initial draft of the manuscript, implemented the comments after editing and revised the manuscript. K.S.Y: Student supervision, project leadership and management, funding acquisition and editing of manuscript. N.C.M.: Student co-supervision, funding acquisition and proofreading of the final draft.
References
1. Dweba CC, Figlan S, Shimelis HA, Motaung TE, Sydenham S, Mwadzingeni L, et al. Fusarium head blight of wheat: Pathogenesis and control strategies. Crop Prot. 2017;91:114-122. https://doi.org/10.1016/j.cropro.2016.10.002 [ Links ]
2. Lenc L. Fusarium head blight (FHB) and Fusarium populations in grain of winter wheat grown in different cultivation systems. J Plant Prot Res. 2015;55:94-109. https://doi.org/10.1515/jppr-2015-0013 [ Links ]
3. Yang F, Jacobsen S, J0rgensen HJL, Collinge DB, Svensson B, Finnie C. Fusarium graminearum and its interactions with cereal heads: Studies in the proteomics era. Front Plant Sci. 2013;4(37):1-8. https://doi.org/10.3389/fpls.2013.00037 [ Links ]
4. Dubin HJ, Glichrist L, Reeves J, McNab A. Fusarium head scab: Global status and prospects; 1997 October 13-17; El Batan, Mexico. Mexico: CIMMYT; 1997. p. 130. [ Links ]
5. Leonard KJ, Bushnell WR, editors. Fusarium head blight of wheat and barley. St. Paul, MN: APS Press; 2003. [ Links ]
6. O'Donnell K, Ward TJ, Geiser DM, Kistler HC, Aoki T. Genealogical concordance between the mating type locus and seven other nuclear genes supports formal recognition of nine phylogenetically distinct species within the Fusarium graminearum clade. Fungal Genet Biol. 2004;41:600-623. https://doi.org/10.1016/j.fgb.2004.03.003 [ Links ]
7. Boutigny AL, Beukes I, Viljoen A. Head blight of barley in South Africa is caused by Fusarium graminearum with a 15-adon chemotype. J Plant Pathol. 2011;93:321-329. http://dx.doi.org/10.4454/jpp.v93i2.1186 [ Links ]
8. Parry DW, Jenkinson P McLeod L. Fusarium ear blight (scab) in small grain cereals - A review. Plant Pathol. 1995;44(2):207-238. https://doi.org/10.1111/j.1365-3059.1995.tb02773.x [ Links ]
9. Schoeman A, Greyling-Joubert SM. Gibberella on maize, sorghum and wheat. Potchefstroom: Grain SA; 2017. Available from: https://www.grainsa.co.za/gibberella-on-maize,-sorghum-and-wheat [ Links ]
10. Sella L, Gazzetti K, Castiglioni C, Schafer W, Favaron F. Fusarium graminearum possesses virulence factors common to Fusarium head blight of wheat and seedling rot of soybean but differing in their impact on disease severity. Phytopathology. 2014;104:1201-1207. https://doi.org/10.1094/PHYTO-12-13-0355-R [ Links ]
11. Trail F. For blighted waves of grain: Fusarium graminearum in the postgenomics era. Plant Physiol. 2009;149:103-110. https://doi.org/10.1104/pp.108.129684 [ Links ]
12. Bottalico A, Perrone G. Toxigenic Fusarium species and mycotoxins associated with head blight in small grain cereals in Europe. Eur J Plant Pathol. 2002;108:611-624. https://doi.org/10.1023/A:1020635214971 [ Links ]
13. Desjardins AE. Fusarium mycotoxins: Chemistry, genetics, and biology. St. Paul, MN: APS Press; 2006. [ Links ]
14. Gilbert J, Haber S. Overview of some recent research developments in Fusarium head blight of wheat. Can J Plant Pathol. 2013;35:149-174. https://doi.org/10.1080/07060661.2013.772921 [ Links ]
15. Palazzini JM, Yerkovich N, Alberione E, Chiotta M, Chulze SN. An integrated dual strategy to control Fusarium graminearum sensu stricto by the biocontrol agent Streptomyces sp. RC 87B under field conditions. Plant Gene. 2017;9:13-18. https://doi.org/10.1016/j.plgene.2016.11.005 [ Links ]
16. Palazzini JM, Torres AM, Chulze ZN. Tolerance of triazole-based fungicides by biocontrol agents used to control Fusarium head blight in wheat in Argentina. Lett Appl Microbiol. 2017;66(5):434-438. https://doi.org/10.1111/lam.12869 [ Links ]
17. Cromey MG, Lauren DR, Parkes RA, Sinclair KI, Shorter SC, Wallace AR. Control of Fusarium head blight of wheat with fungicides. Australas Plant Pathol. 2001;30:301-308. https://doi.org/10.1071/ap01065 [ Links ]
18. Khan NI, Schisler DA, Boehm MJ, Slininger PJ, Bothast RJ. Selection and evaluation of microorganisms for biocontrol of Fusarium head blight of wheat incited by Gibberellazeae. Plant Dis. 2001;85:1253-1258. https://doi.org/10.1094/pdis.2001.85.12.1253 [ Links ]
19. Zhang S, Schisler DA, Boehm MJ, Slininger PJ. Utilization of chemical inducers of resistance and Cryptococcus flavescens OH 182.9 to reduce Fusarium head blight under greenhouse conditions. Biol Control. 2007;42:308-315. https://doi.org/10.1016/j.biocontrol.2007.05.020 [ Links ]
20. Makandar R, Nalam VJ, Lee H, Trick HN, Dong Y Shah J. Salicylic acid regulates basal resistance to Fusarium head blight in wheat. Mol Plant Microbe Interact. 2011;25:431-439. https://doi.org/10.1094/mpmi-09-11-0232 [ Links ]
21. Schisler DA, Slininger PJ, Boehm MJ, Paul PA. Co-culture of yeast antagonists of Fusarium head blight and their effect on disease development in wheat. Plant Pathol J. 2011;10:128-137. https://doi.org/10.2923-ppj.2011.128.137 [ Links ]
22. De Villiers IC. Glasshouse screening of CIMMYT wheat germplasm for Fusarium head blight response in South Africa. S Afr J Plant Soil. 2014;31:49- 51. https://doi.org/10.1080/02571862.2014.890752 [ Links ]
23. Muneera Al-kahtani DF. Isolation of fungi and their mycotoxin extract from stored wheat and other grains importer in Saudi Arabia. Am J Food Technol. 2014;9:370-376. https://doi.org/10.3923/ajft.2014.370.376 [ Links ]
24. Leplat J, Friberg H, Abid M, Steinberg C. Survival of Fusarium graminearum, the causal agent of Fusarium head blight. A review. Agron Sustain Dev. 2013;33:97-111. https://doi.org/10.1007/s13593-012-0098-5 [ Links ]
25. Kumar P Yadava RK, Gollen D, Kumar S, Verma RK, Yadav S. Nutritional contents and medicinal properties of wheat: A review. Life Sci Med Res. 2011; LMSR-22, 10 pages. [ Links ]
26. Brown NA, Bass C, Baldwin TK, Chen H, Massot F, Carion PWC, et al. Characterisation of the Fusarium graminearum-wheat floral interaction. JPathog. 2011; Art. #626345, 9 pages.https://doi.org/10.4061/2011/626345 [ Links ]
27. Rittenour WR, Harris SD. An in vitro method for the analysis of infection-related morphogenesis in Fusarium graminearum. Mol Plant Pathol. 2010;11(3):361-369. https://doi.org/10.1111/j.1364-3703.2010.00609.x [ Links ]
28. Muthomi JW, Ndung'u JK, Gathumbi JK, Mutitu EW, Wagacha JM. The occurrence of Fusarium species and mycotoxins in Kenyan wheat. Crop Prot. 2008;27:1215-1219. https://doi.org/10.1016/j.cropro.2008.03.001 [ Links ]
29. Lenc L, Czecholinski G, Wyczling D, Turów T, Kazmierczak A. Fusarium head blight (FHB) and Fusarium spp. on grain of spring wheat cultivars grown in Poland. J Plant Prot Res. 2015;55(3):266-277. https://doi.org/10.1515/jppr-2015-0038 [ Links ]
30. Trail F, Xu H, Loranger R, Gadoury D. Physiological and environmental aspects of ascospore discharge in Gibberella zeae (anamorph Fusarium graminearum). Mycologia. 2002;94:181-189. https://doi.org/10.1080/15572536.2003.11833223 [ Links ]
31. Shaner G. Epidemiology of Fusarium head blight of small grain cereals in North America. In: Leonard KJ, Bushnell WR, editors. Fusarium head blight of wheat and barley. St. Paul, MN: APS Press; 2003. p. 84-119. [ Links ]
32. Del Ponte EM, Fernandes JMC, Bergstrom GC. Influence of growth stage on Fusarium head blight and deoxynivalenol production in wheat. J Phytopathol. 2007;155:577-581. https://doi.org/10.1111/j.1439-0434.2007.01281.x [ Links ]
33. Bushnell WR, Hazen BE, Pritsch C. Histology and physiology of Fusarium head blight. In: Leonard KJ, Bushnell WR, editors. Fusarium head blight of wheat and barley. St. Paul, MN: APS Press; 2003. p. 44-83. [ Links ]
34. Jansen C, Von Wettstein D, Schãfer W, Kogel KH, Felk A, Maier FJ. Infection patterns in barley and wheat spikes inoculated with wild-type and trichothecene synthase gene disrupted Fusarium graminearum. Proc Natl Acad Sci USA. 2005;102:16892-16897. https://doi.org/10.1073/pnas.0508467102 [ Links ]
35. Schmale III DG, Bergstrom GC. Fusarium head blight in wheat. Plant Health Instr. 2003. https://doi.org/10.1094/PHI-I-2003-0612-01 [ Links ]
36. Boenisch MJ, Schãfer W. Fusarium graminearum forms mycotoxin producing infection structures on wheat. BMC Plant Biol. 2011;11:110. https://doi.org/10.1186/1471-2229-11-110 [ Links ]
37. Murray TD, Parry DW, Cattlin LD. Diseases of small grain cereal crops: A colour handbook. London: Manson Publishing Ltd; 2009. p. 2-4,132. https://doi.org/10.1201/b15911 [ Links ]
38. Mills K, Salgado JD, Paul PA. Fusarium head blight or head scab of wheat, barley and other small grain crops. Columbus, OH: CFAES Publishing, Ohio State University; 2016. Available from: https://ohioline.osu.edu/factsheet/plpath-cer-06 [ Links ]
39. Wegulo SN. Factors Influencing deoxynivalenol accumulation in small grain cereals. Toxins. 2012;4:1157-1180. https://doi.org/10.3390/toxins4111157 [ Links ]
40. Dean R, Van Kan JA, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, et al. The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol. 2012;13:414-430. https://doi.org/10.1111/j.1364-3703.2011.00783.x [ Links ]
41. Matny ON. Fusarium head blight and crown rot on wheat & barley: Losses and health risks. Adv Plants Agric Res. 2015;2:2-7. https://doi.org/10.15406/apar.2015.02.00039 [ Links ]
42. Bacon CW, Hinton DM. Potential for control of seedling blight of wheat caused by Fusarium graminearum and related species using the bacterial endophyte Bacillus mojavensis. Biol Sci Technol. 2007;17:81-94. https://doi.org/10.1080/09583150600937006 [ Links ]
43. Windels CE. Economic and social impacts of Fusarium head blight: Changing farms and rural communities in the northern great plains. Phytopathology. 2000;90:17-21. https://doi.org/10.1094/PHYTO.2000.90.L17 [ Links ]
44. Alberta Fusarium Action Committee. Alberta Fusarium graminearum management plan. Edmonton: Alberta Agriculture and Rural Development; 2012. Available from: https://www1.agric.gov.ab.ca/Sdepartment/deptdocs.nsf/all/agdex5210/$file/110_632-3.pdf?OpenElement [ Links ]
45. Scott DB, De Jager EJH, Van Wyk PS. Head blight of irrigated wheat in South Africa. Phytophylactica. 1988;20:317-319. [ Links ]
46. McMullen M, Zhong S, Neate S. Fusarium head blight (scab) of small grains. Fargo, ND: NDSU Extension Service; 2008. Available from: https://scabusa.org/pdfs/NDSU_PP-804_FHB-Small-Grains.pdf [ Links ]
47. Boyacioglu D, Hettiarachchy NS. Changes in some biochemical components of wheat grain that was infected with Fusarium graminearum. J Cereal Sci. 1995;21(1):57-62. https://doi.org/10.1016/S0733-5210(95)80008-5 [ Links ]
48. Nielsen LK, Cook DJ, Edwards SG, Ray RG. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int J Food Microbiol. 2014;179:38-49. https://doi.org/10.1016/].ijfoodmicro.2014.03.023 [ Links ]
49. Jard G, Liboz T, Mathieu F, Guyonvarc'h A, Lebrihi A. Review of mycotoxin reduction in food and feed: From prevention in the field to detoxification by adsorption or transformation. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:1590-1609. https://doi.org/10.1080/19440049.2011.595377 [ Links ]
50. Salgado JD, Wallhead M, Madden LV Paul PA. Grain harvesting strategies to minimize grain quality losses due to Fusarium head blight in wheat. Plant Dis. 2011;95:1448-1457. https://doi.org/10.1094/pdis-04-11-0309 [ Links ]
51. Jouany JP. Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Anim Feed Sci Tech. 2007;137:342-362. https://doi.org/10.1016/j.anifeedsci.2007.06.009 [ Links ]
52. Dill-Macky R, Jones RK. The effect of previous crop residues and tillage on Fusarium head blight of wheat. Plant Dis. 2000;84:71-76. https://doi.org/10.1094/pdis.2000.84.1.71 [ Links ]
53. Wegulo SN, Baenziger PS, Nopsa JH, Bockus WW, Hallen-Adams H. Management of Fusarium head blight of wheat and barley. Crop Prot. 2015;73:100-107. https://doi.org/10.1016/j.cropro.2015.02.025 [ Links ]
54. Haidukowski M, Pascale M, Perrone G, Pancaldi D, Campagna C, Visconti A. Effect of fungicides on the development of Fusarium head blight, yield and deoxynivalenol accumulation in wheat inoculated under field conditions with Fusarium graminearum and Fusarium culmorum. J Sci Food Agric. 2004;85:191-198. https://doi.org/10.1002/jsfa.1965 [ Links ]
55. McMullen M, Bergstrom G, De Wolf E, Dill-Macky R, Hershman D, Shaner G, et al. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012;96:1712-1728. https://doi.org/10.1094/PDIS-03-12-0291-FE [ Links ]
56. Paul PA, Bradley CA, Madden LV Dalla Lana F, Bergstrom GC, Dill-Macky R, et al. Meta-analysis of the effects of QoI and DMI fungicide combinations on Fusarium head blight and deoxynivalenol in wheat. Plant Dis. 2018;102:2602-2615. https://doi.org/10.1094/PDIS-02-18-0211-RE [ Links ]
57. Paul PA, McMullen MP Hershman DE, Madden LV. Meta-analysis of the effects of triazole-based fungicides on wheat yield and test weight as influenced by Fusarium head blight intensity. Phytopathology. 2010;100:160-171. https://doi.org/10.1094/PHYTO-100-2-0160 [ Links ]
58. Paul PA, Lipps PE, Hershman DE, McMullen MP Draper MA, Madden LV. Efficacy of triazole-based fungicides for Fusarium head blight and deoxynivalenol control in wheat: A multivariate meta-analysis. Phytopathology. 2008;98:999-1011. https://doi.org/10.1094/PHYTO-98-9-0999 [ Links ]
59. Pirgozliev SR, Edwards SG, Hare MC, Jenkinson P. Effect of dose rate of azoxystrobin and metconazole on the development of Fusarium head blight and the accumulation of deoxynivalenol (DON) in wheat grain. Eur J Plant Pathol. 2002;108:469-478. https://doi.org/10.1023/A:1016010812514 [ Links ]
60. loos R, Belhadj A, Menez M, Faure A. The effects of fungicides on Fusarium spp. and Microdochium nivale and their associated trichothecene mycotoxins in French naturally infected cereal grains. Crop Prot. 2005;24:894-902. https://doi.org/10.1016/j.cropro.2005.01.014 [ Links ]
61. Logrieco A, Vesonder RF, Petersen SW, Bottaico A. Re-examination of the taxonomic disposition of and deoxynivalenol production by Fusarium nivale NRRL 3290. Mycologia. 1991;83:367-370. [ Links ]
62. Freije AN, Wise KA. Impact of Fusarium graminearum inoculum availability and fungicide application timing on Fusarium head blight in wheat. Crop Prot. 2015;77:139-147. http://dx.doi.org/10.1016/j.cropro.2015.07.016 [ Links ]
63. Xue AG, Voldeng HD, Savard ME, Fedak G, Tian X, Hsiang T. Biological control of Fusarium head blight of wheat with Clonostachys rosea strain aCm941. Can J Plant Pathol. 2009;31:169-179. https://doi.org/10.1080/07060660909507590 [ Links ]
64. Wang LY, Xie YS, Cui YY, Xu J, He W, Chen HG. Conjunctively screening of biocontrol agents (BCAs) against Fusarium root rot and Fusarium head blight caused by Fusarium graminearum. Microbiol Res. 2015;177:34-42. http://dx.doi.org/10.1016/j.micres.2015.05.005 [ Links ]
65. Legrand F, Picot A, Cobo-Díaz JF, Chen W, Le Floch G. Challenges facing the biological control strategies for the management of Fusarium head blight of cereals caused by F graminearum. Biol Control. 2017;113:26-38. https://doi.org/10.1016/j.biocontrol.2017.06.011 [ Links ]
66. Baffoni L, Gaggia F, Dalanaj N, Prodi A, Nipoti P Pisi A, et al. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015;15:242. http://dx.doi.org/10.1186/s12866-015-0573-7 [ Links ]
67. Pan D, Mionetto A, Tiscornia S, Bettucci L. Endophytic bacteria from wheat grain as biocontrol agents of Fusarium graminearum and deoxynivalenol production in wheat. Mycotoxin Res. 2015;31:137-143. https://doi.org/10.1007/s12550-015-0224-8 [ Links ]
68. Palazzini J, Roncallo P Cantoro R, Chiotta M, Yerkovich N, Palaciois S, et al. Biocontrol of Fusarium graminearum sensu stricto, reduction of deoxynivalenol accumulation and phytohormone induction by two selected antagonists. Toxins. 2018;10(2):88. https://doi.org/10.3390/toxins10020088 [ Links ]
69. Matarese F, Sarrocco S, Gruber S, Seidl-Seiboth V, Vannacci G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology. 2012;158:98-106. https://doi.org/10.1099/mic.0.052639-0 [ Links ]
70. Bujold I, Paulitz TC, Carisse O. Effect of Microsphaeropsis sp. on the production of perithecia and ascospores of Gibberella zeae. Plant Dis. 2001;85:977-984. https://doi.org/10.1094/pdis.2001.85.9.977 [ Links ]
71. Khan NI, Schisler DA, Boehm MJ, Lipps PE, Slininger PJ. Field testing of antagonists of Fusarium head blight incited by Gibberella zeae. Biol Control. 2004;29:245-255. https://doi.org/10.1016/S1049-9644(03)00157-9 [ Links ]
72. Schisler DA, Khan NI, Boehm MJ, Slininger PJ. Greenhouse and field evaluation of biological control of Fusarium head blight on durum wheat. Plant Dis. 2002;86:1350-1356. https://doi.org/10.1094/pdis.2002.86.12.1350 [ Links ]
73. Schisler DA, Boehm MJ, Paul PA, Rooney AP Dunlap CA. Reduction of Fusarium head blight using prothioconazole and prothioconazole-tolerant variants of the Fusarium head blight antagonist Cryptococcus flavescens OH 182.9. Biol Control. 2015;86:36-45. https://doi.org/10.1016/j.biocontrol.2015.04.002 [ Links ]
74. Ruckenbauer P, Buerstmayr H, Lemmens M. Present strategies in resistance breeding against scab (Fusarium spp.). Euphytica. 2001;119(1):123-129. https://doi.org/10.1023/A:1017598523085 [ Links ]
75. Mesterházy Á, Bartók T, Kászonyi G, Varga M, Tóth B, Varga J. Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur J Plant Pathol. 2005;112:267-281. https://doi.org/10.1007/s10658-005-2853-9 [ Links ]
76. Jia H, Zhou J, Xue S, Li G, Yan H, Ran C, et al. A journey to understand wheat Fusarium head blight resistance in the Chinese wheat landrace Wangshuibai. Crop J. 2017;6:48-59. https://doi.org/10.1016/j.cj.2017.09.006 [ Links ]
77. Shah L, Ali A, Yahya M, Zhu Y, Wang S, Hi S, et al. Integrated control of Fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018;67:532-548. https://doi.org/10.1111/ppa.12785 [ Links ]
78. Blandino M, Haidukowski M, Pascale M, Palizzari L, Scudellari D, Reyneri A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crops Res. 2012;133:139-149. https://doi.org/10.1016/j.fcr.2012.04.004 [ Links ]
79. McMullen M, Halley S, Schatz B, Meyer S, Jordahl J, Ransom J. Integrated strategies for Fusarium head blight management in the United States. Cereal Res Commun. 2008;36:563-568. https://doi.org/10.1556/crc.36.2008.suppLb.45 [ Links ]
80. CABI. Gibberella zeae (head blight of maize). In: Invasive Species Compendium. Wallingford: CAB International; 2019. Available from: https://www.cabi.org/isc/datasheet/25167#toDistributionMaps [ Links ]
 Correspondence:
Correspondence:
Kwasi Yobo
Email: Yobok@ukzn.ac.za
Received: 23 Jan. 2020
Revised: 16 Apr. 2020
Accepted: 28 Apr. 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: National Research Foundation (South Africa); University of KwaZulu-Natal Capacity Development Programme














