Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.11-12 Pretoria nov./dic. 2020
http://dx.doi.org/10.17159/sajs.2020/8579
REVIEW ARTICLE
Potato virus Y and Potato leafroll virus management under climate change in sub-Saharan Africa
Kerstin KrijgerI, II; Jacquie E. van der WaalsII, III
IDepartment of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
IIForestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa
IIIDepartment of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
ABSTRACT
Potato has increased in importance as a staple food in sub-Saharan Africa, where its production is faced with a multitude of challenges, including plant disease development and spread under changing climatic conditions. The economically most important plant viruses affecting potatoes globally are Potato virus Y (PVY) and Potato leafroll virus (PLRV). Disease management relies mostly on the use of insecticides, cultural control and seed certification schemes. A major obstacle in many sub-Saharan Africa countries is the availability of disease-free quality seed potatoes. Establishment and implementation of quality control through specialised seed production systems and certification schemes is critical to improve seed potato quality and reduce PVY and PLRV sources. Seed could be further improved by breeding virus-resistant varieties adapted to different environmental conditions combined with management measures tailored for smallholder or commercial farmers to specific agricultural requirements. Innovative technologies -including more sensitive testing, remote sensing, machine learning and predictive models - provide new tools for the management of PVY and PLRV, but require support for adoption and implementation in sub-Saharan Africa.
SIGNIFICANCE:
• Potato virus Y (PVY) and Potato leafroll virus (PLRV) are the two major potato viruses threatening profitable seed potato production.
• High-quality seed shortage in many sub-Saharan Africa countries has been identified as a constraint to increasing yield.
• Specialised seed grower or seed certification programmes should be implemented to prevent virus transmission from seed to daughter tubers.
• Sustainable PVY and PLRV management in seed potatoes requires specific regional approaches to growth, farming and climatic conditions.
• Future research should include predictive models and new innovative technologies such as more sensitive testing, machine learning and remote sensing.
Keywords: PVY PLRV, seed certification, aphids, cultural control
Introduction
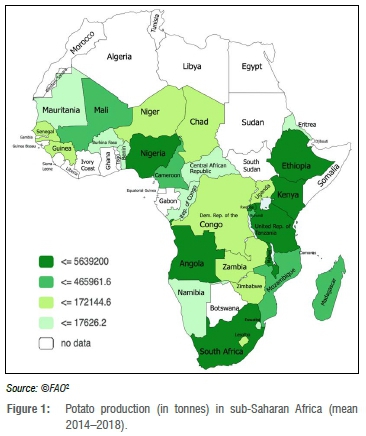
Potato (Solanum tuberosum L.; Solanaceae) is a high yielding cash crop in sub-Saharan Africa.1 Its production has experienced one of the largest increases in comparison with other staple food crops in the region.1 The potato production area more than doubled in sub-Saharan Africa between 1998 (655 447 ha) and 2018 (1.47 million ha), including in regions with high poverty rates, but yields vary greatly across the region (Figure 1).2 However, food security in sub-Saharan Africa remains a chronic problem.3 Although there is sufficient food to satisfy global average food consumption in lower-income countries, several countries in sub-Saharan Africa suffer from food insecurity due to low production with limited access to food produced in other countries.4 Food security is likely to worsen with the impact of climate change and a growing population.4 The effects of climate change are expected to be most severe in sub-Saharan Africa because of the high dependence on agriculture and its vulnerability to extreme weather events.3,5
Predicted climate change impact in sub-Saharan Africa is highly complex and region specific. Droughts and heat waves, unseasonal rainfall, and an increase in the frequency of extreme weather events are likely in future.6,7 The climate in sub-Saharan Africa has already changed with mean increases in temperature from a 1951-1980 baseline to 2019 of 1.5 °C, ranging from 1.0 °C in South Sudan and Eritrea to 2.3 °C in Namibia.2 Simultaneously, rainfall patterns have become even more variable with regional decreases in southern Africa and increases in eastern Africa.7 This variability is exacerbated by erratic severe drought episodes.8,9 Regional adaptations to maintain at least current yields in potato production in the increased area planted10 are therefore crucial as most potatoes are grown under dry-land conditions during specific rainy seasons11.
The impact of climate change on potatoes globally has been reviewed by Hijmans12, Haverkort and Verhagen13, Raymundo et al.14 and George et al.15 Little detailed information is available for sub-Saharan Africa. Simulation models for agro-ecosystems with continental and Mediterranean climates in South Africa suggest that increased CO2 levels will impact positively on water use by potato plants and thus yield, compensating for negative effects of increased temperature and reduced water availability, provided crops are grown at suitable times.16,17 However, potatoes grown under heat stress are likely to have a lower water use efficiency and reduced yields, even under increased CO2 levels.16,17 Changing climatic conditions could therefore reduce potato production in the lowlands in sub-Saharan Africa by up to 50% by 2050.14 In eastern Africa, where potatoes are grown mainly in the highlands, heat and water stress have been predicted to reduce yield, with the exception of Rwanda.18 Potato production regions in the Ethiopian Highlands, for example, are faced with a potential increase of 0.7 °C from 1975 to 2050.19
One of the major biotic limitations to potato production are plant diseases. Their impact may be even more severe in warmer regions where seed potato tubers are propagated over several generations, and year-round plantings are already under continuous pressure from insect-transmitted pathogens.20 Furthermore, an increase in temperature or milder winters may have a negative effect on seed potato systems that rely on cooler growing regions or cool winters for reducing plant virus inoculum and low insect vector pressure.20
More than 50 plant viruses that infect potatoes have been recorded20, of which two - Potato virus Y (PVY; genus Potyvirus, family Potyviridae) and Potato leafroll virus (PLRV; genus Polerovirus, family Luteoviridae) -affect profitable potato production globally20,21. PVY has overtaken PLRV as economically the most important of the potato viruses.20 PVY has been a challenge worldwide during the past 20 years due to the emergence of recombinant PVY variants.22,23 Both viruses result in dramatic yield and quality losses.21,24 Infection levels are increased by planting infected tubers, leading to increased infection levels over successive generations. Thus, PVY or PLRV infection can lead to downgrading or rejection of seed lots if tolerance levels set by seed potato certification schemes are exceeded.25-28 Tubers from informal seed systems, previous crops or tubers that are unmarketable are often used for planting29; this becomes a severe problem, especially for smallholder farmers in sub-Saharan Africa.
Both PVY and PLRV are transmitted to the new potato crop through aphid (Hemiptera: Aphididae) vectors (horizontal transmission; primary infection) or via infected seed tubers to daughter tubers (vertical transmission; secondary infection). The severity of infection depends on host plant tolerance, time of infection (with early infection leading to higher yield loss), environmental factors and virus strains involved.30,31 PVY and PLRV management relies largely on cultural preventative measures that limit virus inoculum or curative measures using insecticides to suppress aphid vector species.
Climate variability and climate change as well as agricultural changes (including introduction of new genotypes, germplasm movement and cultural intensification) add to the intricacy of the already complex virus-insect vector-plant-environment interactions.32,33 The impact of climate change on plant viruses has been recently reviewed by Islam et al.33, Jones34,35, Jones and Naidu36, and Trebicki37.
The impact of climate change on aphid vector species and abundance is complex, difficult to predict and region specific38-41 and depends on plant species, variety and age, and duration and severity of climate stressors and aphid species42,43
This review focuses on strategies to manage the spread of PVY and PLRV in potato crops in sub-Saharan Africa under changing climatic conditions. Research findings are reviewed and evaluated with specific reference to potato production in the sub-Saharan African context. Areas requiring further research to manage PVY and PLRV in sub-Saharan Africa are identified.
Potato virus Y
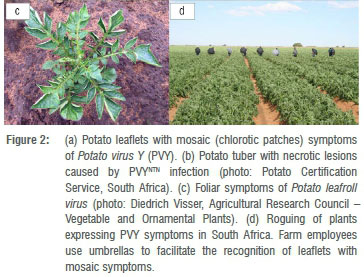
Potato virus Y (PVY) is a single-stranded RNA virus. Several strains have been identified - PVY0 (common strain), PVYN (tobacco veinal necrosis strain), and PVYC (stipple-streak strain, including potato virus C), and during the past two decades recombinant variants derived from PVY0 and PVYN have become prevalent worldwide, e.g. PVYN:0, PVYNTN (N-tuber necrotic), and PVYN-Wi (N-Wilga).22,23,44 PVY infection increases the number of undersized tubers. Foliar symptoms include mosaic (chlorotic patches; Figure 2a) that may be severe or mild and hardly detectable.45 Mild symptoms cause problems for virus management in potato seed production because symptomless plants remain unidentified and therefore are not eliminated and serve as virus inoculum.30 Some recombinant variants cause potato tuber necrotic ringspot disease (Figure 2b).45
PVY is primarily transmitted through aphid vectors, but can also be transmitted mechanically or by grafting but not through true seed.31 PVY is transmitted non-persistently. The virus does not replicate in the vector and there is no latent period (time between virus acquisition and when a vector becomes infective). Aphid vector species acquire and transmit PVY to an uninfected plant during short feeding probes of seconds to minutes of epidermal cells. Aphids tend to lose the ability to transmit PVY after probing one or two uninfected plants or virus non-host plants. They can become infected again when feeding on an infected plant. PVY can be transmitted by 65 aphid species or species groups that either breed on potato (colonising species; e.g. Myzus persicae, the most efficient vector) or are transient species that land and probe or feed but do not breed on potato (non-colonising species; e.g. Rhopalosiphum padi).30Transient species, although less efficient in PVY transmission30, are important because they may occur in high numbers. Therefore, both colonising and transient species are of importance when devising management strategies.30
PVY transmission and infection of plants is temperature dependent. High temperatures of 25-30 °C negatively influence virus transmission and virus titre at these temperatures decreases over time.46 Whereas virus establishment in Nicotiana benthamiana (Solanaceae) was highest between 20 °C and 30 °C, the virus titre decreased over time in plants at 25 °C and 30 °C.46
Potato leafroll virus
Potato leafroll virus (PLRV) is a single-stranded RNA virus. Primary infection symptoms include upward rolling of the leaflets (Figure 2c), while secondary infection symptoms include stunting of shoots and upward rolling of leaflets.21 Yield losses through stunting of plants and reduced tuber size and number are highest when planting infected tubers and through early infection of young virus-free plants by viruliferous aphids.31 Apart from reducing yield, PLRV causes internal net necrosis of tubers of some cultivars, rendering them unsuitable for seed, marketing and processing.20 Mature plants are less affected than young seedlings.31
PLRV is most commonly transmitted by aphids, and through grafting, but not mechanically nor through true seed.31 The virus is transmitted through aphid vector species in a persistent, circulative, non-propagative manner, i.e. individuals remain infected for life (the virus is passed on through moults), but PLRV does not replicate in the vector and the virus is not passed on to offspring.31 Vectors of PLRV must feed on the phloem sap of potato plants to acquire and transmit the virus. PLRV can be both acquired from infected plants and transmitted to healthy plants by aphid vectors with feeding times of 10-15 min but maximum virus acquisition occurs after feeding of approximately 12 h. Aphid vectors become infective after a latent period of 8-72 h.31 To date, 13 potato-colonising aphid species or species groups have been identified as vectors.47
The highest titre of PLRV was recorded between 20 °C and 30 °C in Physalis floridana (Solanaceae).46 The PLRV growth rate decreased from 25 °C and ceased at 35-40 °C, possibly due to gene silencing.46,48
PVY and PLRV management
Potato management, including disease management, requires an integrated approach and regional cooperation among growers.10,31 Potato virus control worldwide is currently largely achieved through seed quality and certification, vector control with insecticides and cultural control methods.28,31 Management of PVY poses an even greater challenge than that of PLRV, because it is transmitted non-persistently within seconds and insecticides may have a limited effect.49 Effective PVY management relies therefore on prevention.28 Depending on the prevalence of PVY or PLRV in a region and local conditions, a combination of various management strategies may be required for both viruses.
Climate change might affect commercial and smallholder farmers in different ways because of different production systems. In general, farmers may have to reassess current disease management practices. Apart from maximising soil and water conservation, integration of a variety of existing and innovative emerging strategies is likely to be required to cope with increasing uncertainty, and variable rainfall and temperatures.10,50 General reviews of plant virus management strategies can be found in Jones and Naidu36 and Kreuze et al.20 Radcliffe and Ragsdale31 and Dupuis et al.28 reviewed management strategies for PVY and Radcliffe and Ragsdale31 for PLRV. The following provides a brief overview of management options with emphasis on sub-Saharan Africa.
Seed quality and seed certification
The risk of PVY and PLRV spread can be minimised by planting disease-free seed potatoes.28 Even so, seed quality has been identified as a major limiting factor to successful potato production in a number of countries across sub-Saharan Africa1,10,29,51 where lack of specialised growers, informal seed systems and the use of unmarketable ware potatoes often result in poor-quality tubers, which produce low yields and tubers with low market values1,29.
Effective, usually government-regulated, seed potato certification programmes, together with virus testing regimes, have long been implemented in developed countries for the control of seed potato quality.2628 Certification thresholds or disease tolerances are set to limit secondary virus transmission from infected seed tubers to daughter tubers and to limit primary infection within a crop in the next season. However, the recent emergence of new strains, e.g. PVY™ and PVYN:O, together with potato cultivars that are tolerant, i.e. do not exhibit symptoms, pose new challenges to seed certification programmes.52 Furthermore, higher temperatures due to climate change may reduce levels of PVY and PLRV46 below the detection limit of enzyme-linked immunosorbent assays (ELISAs) used for detection in certification schemes. This is especially problematic for seed potatoes produced in low-lying seed potato regions in sub-Saharan Africa where temperature increases are expected to be more severe than in high-lying production areas. New technologies for reliable and sensitive virus detection methods, such as real-time reverse transcriptase polymerase chain reaction (qPCR) assays, should be implemented in schemes for seed certification to overcome the problem.
Some countries in sub-Saharan Africa, e.g. Kenya and South Africa, have introduced seed certification schemes.25,53 Both largely use ELISA for detection of PVY and PLRV. Due to underdetection, ELISA has been replaced with more sensitive molecular techniques in many European countries.28 The lack of capacity, aggravated by the cost of certification schemes, and a low demand because farmers are unwilling to pay the high price for quality seed1,29,54, has delayed the implementation of seed certification schemes in many countries in sub-Saharan Africa. In a survey in eastern Africa (Kenya, Uganda and Ethiopia), only 2% of seed potatoes were sourced from seed growers, whereas 65% originated from own fields and 31% from rural markets.55 This lack of certified seed could be overcome through government agencies that support smallholder farmers and provide advice through extension services and testing through national agencies. Furthermore, alternative strategies are being developed.1,29 These include the use of true seed of hybrid potato varieties, which is virus free and easier to transport, benefiting smallholder farmers in remote areas56, or prolonged seed health in informal systems in sub-Saharan Africa through an 'integrated seed health strategy' where disease resistance is combined with on-farm management29.
Disease-resistant potato varieties
Resistant varieties, in which the host limits pathogen multiplication, are thought to provide the most efficient way to manage viruses, but the majority of varieties grown currently in sub-Saharan Africa do not possess adequate resistance to PVY or PLRV.28,57 Furthermore, mature plant resistance has often been overcome by recombinant strains.58 Breeders have introduced genes for hypersensitive resistance and extreme resistance to PVY20,57 Hypersensitive resistance is mostly PVY-strain specific and the death of virus-infected cells leads to localised necrotic lesions. However, as hypersensitive resistance is temperature sensitive, the higher temperatures induced by climate change can be expected to neutralise its beneficial effect.24 Extreme resistance is effective against a broad range of PVY strains and does not cause visible lesions.20 Although various molecular techniques (e.g. RNA-mediated resistance, RNA interference and more recently cRlSPR/Cas technologies) are available for introducing resistance to viruses, only a few transgenic resistant potato varieties are available and none in developing countries.36,59 In many countries in sub-Saharan Africa, the introduction of transgenic crops remains a highly contested issue.
Breeding of potato varieties with glandular trichomes, that may reduce aphid settling and thus virus transmission, may be more successful for PLRV than PVY because of the difference in their mode of transmission (persistent versus non-persistent).57 However, it has been met with limited success because of negative effects on the growth period, tuber size and yield.57 For recent reviews on PVY-resistant varieties see Valkonen et al.60 and Kreuze et al.20, and for PLRV see Halterman et al.26.
Apart from planting virus-resistant varieties and increasing yield and nutrient content in varieties to maximise the use of the limited area available, more diversified disease-resistant varieties are needed that are at the same time more resource efficient, requiring less water, fertiliser and pesticides to provide greater resilience to extreme weather events.50,61-63 Some sub-Saharan African countries have initiated breeding programmes, frequently involving the National Agricultural Research System supported by the International Potato Center, to provide varieties suited for local growing conditions.3,64
Preventative cultural control
Spatial and temporal isolation
Tubers that exceed virus infection levels set by certification schemes or infected retained tubers in informal seed systems are unsuitable as seed potatoes but are frequently planted as commercial crops, which subsequently serve as virus reservoirs.47 Geographical isolation and temporal separation of seed from commercial potato plantings and other crops that are potential inoculum sources of PVY or PLRV have been listed amongst the most effective preventative management strategies28,31,47, which could also be applied by smallholder farmers in sub-Saharan Africa, although effective isolation distances are region specific31.
Roguing
Another strategy to reduce PVY and PLRV spread within a growing season is roguing - that is the visual inspection and removal of plants expressing symptoms within seed-potato crops (Figure 2d), especially when virus incidence is low.20,31 For roguing to be effective, seed potato fields require multiple inspections throughout the growing season and it is therefore labour intensive.31 Infected plants should be removed before aphid numbers increase.31 In order to recognise infected plants, cultivars expressing symptoms should be grown47, but this is in conflict with planting disease-resistant varieties that mask symptom expression. In this case, the only alternative to roguing for the elimination of infected plants is detection by laboratory testing, which therefore requires careful consideration.
Elimination of virus sources - volunteer potatoes and weed control
Volunteer potatoes, other susceptible crops and weed species that are hosts of PVY or PLRV are important virus reservoirs.28 Volunteer potatoes, which are plants that grow from tubers remaining in the soil after harvest, pose a significant threat as a source of virus inoculum and consequently viruliferous aphids in the next growing season, especially if warmer winters enhance their survival, and should be removed before the emergence of new plantings.28 Another aspect to reduce virus inoculum is weed management as the host ranges of PVY and PLRV span over several families.65,66
Crop mulching, intercropping, crop borders
Various options are available to reduce aphid landing in potato fields. The rationale is to create less contrast between the green plant canopy and the soil in order to lower aphid landing rates in seed potato fields.28 Straw mulches and intercropping with cereals, for example, may reduce PVY spread but may be expensive strategies that are effective only before the canopy closes and may require removal.28,47 Crop borders consisting of non-virus host plants rely on the same principle. Aphids tend to land in high numbers at field edges due to the contrast in light reflected from the soil and the plants.49 Aphid landing in the main crop is reduced by displacing the edge of the main field with a non-virus host plant. Crop borders for the management of non-persistent viruses have been reviewed by Schroder et al.49 Crop borders may be suitable for smallholder farmers in sub-Saharan Africa, whose fields tend to be smaller than those of commercial farmers, as the area needed for a border depends on field size. Crop borders consisting of non-virus crops that are already part of a farming system, e.g. maize or cereals, may provide additional support for smallholder farmers.
Aphid monitoring
Aphid vector species composition and abundance vary greatly with time of year and region.30,67 Thus, aphid monitoring is a key aspect of managing virus spread. Regular aphid monitoring data enable growers strategically to target location and timing of control measures, to optimise the timing of planting, haulm destruction and harvest. Aphid vector species composition together with their virus transmission efficiency and abundance are used to calculate vector pressure indices for specific regions.30
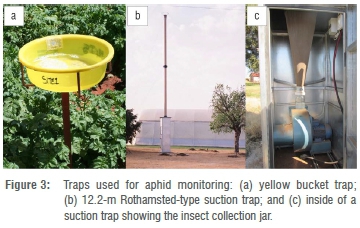
Aphid flight activity is usually monitored with suction traps, often in combination with yellow water traps to provide rapid information on vector pressure (Figure 3). The height of suction traps and the variation in topography determine the area over which aphids are sampled. Suction traps commonly used are 1.8, 8 or 12.2 m high.68 Traps above 10 m height provide random aerial samples of aphids. Suction traps with a height of 1.8 and 8 m usually monitor aphids at local level. However, depending on the landscape, trap catches of 8-m traps may be representative of aphid flight activity over a 30-km radius.69 South Africa has an extensive suction-trap network and seed potato farmers receive weekly information on aphid numbers and vector pressure to alert growers to the risk of PVY and PLRV spread.30 Establishment and maintenance of aphid monitoring programmes may be challenging, because of the cost involved and the difficulty in identifying aphid species, especially for smallholder farmers. However, government- or industry-supported aphid monitoring initiatives should be considered and are recommended as they can be hugely beneficial for controlling viral spread.
Planting and harvesting dates
The risk of virus transmission by an increase in aphid vector numbers can be reduced through adjustment of planting and harvesting dates to avoid peak vector activity47,70, which is region specific30,39,67. However, this strategy is dependent on aphid monitoring systems being in place.
Chemical control
The longer transmission time of PLRV compared to PVY makes it easier to manage virus spread with chemical control because insecticides may affect aphid vectors before they can transmit the virus.44 Synthetic insecticides may not be effective in preventing PVY transmission but may be effective in reducing aphid populations49 and are one of the main methods for the management of PVY and PLRV. Chemical control using insecticides and mineral oils has been reviewed by Dupuis et al.28, Lacomme et al.30 and Yang et al.71 Mineral oil may reduce PVY spread but its effectiveness depends on environmental conditions.28,71 In African countries, farmers have been reluctant to use mineral oils because of concerns of phytotoxicity at high temperatures and potential yield loss.72 Synthetic insecticides for smallholder farmers in sub-Saharan Africa are often not available or affordable.59
Predictive models
The complex interactions of different abiotic (e.g. climate change) and biotic stressors (e.g. plant pathogens and insect vectors) on plant growth make it challenging to predict the risk of plant disease development and spread. Climate modelling has progressed over the past years with models being able to guide farmers in planning climate change adaptation strategies.73 However, adoption of forecasting models for decision-making in sub-Saharan Africa by smallholder and commercial farmers alike has been limited due to lack of sufficient forecasting skills and perceived lack of relevance of forecasting models for specific farming decisions.74
New technologies
A variety of new and emerging technologies are being developed to improve virus disease management (e.g. through detection of diseased plants, insect infestations) or to alleviate climate change impact on virus outbreaks at various geographical levels.36 To improve virus detection, management and prediction, new detection technologies should be implemented, e.g. qPCR. Innovations to provide decision support and curb virus spread include remote sensing, machine learning, aerial surveillance, and precision agriculture.36,75-77 For example, Gómez et al.78 developed a model to predict potato yield using satellite remote sensing and machine learning. Griffel et al.79 proposed the use of support vector machine classification, based on machine learning and using different spectral profiles of PVY-infected and uninfected plants, for the detection of PVY infestations. Furthermore, technologies have been developed to identify areas of insect infestation, including aphids, based on plant stress.80
Conclusion
Current knowledge on the effect of climate change on plant disease management is limited. However, various measures may alleviate the predicted impact of climate change on the spread of PVY and PLRV and their aphid vectors. Future work in sub-Saharan Africa should concentrate on improving seed quality, currently a major constraint, especially for smallholder farmers, together with the development of region-specific management programmes that include cultural control methods. Another aspect is breeding for more disease-resistant varieties that (1) are more diversified to have a greater pool of varieties to choose from for different growth conditions, (2) provide greater resilience to extreme weather events, (3) have a higher nutrient content and yield to maximise the use of the area available, and (4) are more resource efficient. Further research should be directed at innovative technologies to improve disease detection, management and prediction. Considerable progress has been made in predictive modelling and its application in crop production, and research should be directed at improving the adoption and implementation thereof in sub-Saharan Africa. However, key to the implementation of any of these advances will be communication so that the benefits of these new technologies can be understood by stakeholders.
Competing interests
We declare that there are no competing interests.
Authors' contributions
K.K. and J.E.v.d.W. jointly conceived and wrote the article.
References
1. Demo P Lemaga B, Kakuhenzire R, Schulz S, Borus D, Barker I, et al. Strategies to improve seed potato quality and supply in sub-Saharan Africa: Experience from interventions in five countries. In: Low J, Nyongesa M, Quinn S, Parker M, editors. Potato and sweetpotato in Africa: Transforming the value chains for food and nutrition security. Wallingford: CABI; 2015. p. 155-167. [ Links ]
2. FAO. FAOSTAT Database [database on the Internet]. c2019 [cited 2020 Apr 23]. Available from: http://www.fao.org/faostat/en/#data/QC [ Links ]
3. Devaux A, Goffart J-P Petsakos A, Kromann P Gatto M, Okello J, et al. Global food security, contributions from sustainable potato agri-food systems. In: Campos H, Ortiz O, editors. The potato crop: Its agricultural, nutritional and social contribution to humankind. Cham: Springer International Publishing; 2020. p. 3-35. https://doi.org/10.1007/978-3-030-28683-5_1 [ Links ]
4. Alexandratos N, Bruinsma J. World agriculture towards 2030/2050: The 2012 revision. Rome: FAO; 2012. [ Links ]
5. Kotir JH. Climate change and variability in sub-Saharan Africa: A review of current and future trends and impacts on agriculture and food security. Environ Dev Sustain. 2011;13:587-605. https://doi.org/10.1007/s10668-010-9278-0 [ Links ]
6. Engelbrecht F, Adegoke J, Bopape M-J, Naidoo M, Garland R, Thatcher M, et al. Projections of rapidly rising surface temperatures over Africa under low mitigation. Environ Res Lett. 2015;10(8), Art. #085004. https://doi.org/10.1088/1748-9326/10/8/085004 [ Links ]
7. Davis-Reddy CL, Vincent K. Climate risk and vulnerability: A handbook for southern Africa. 2nd ed. Pretoria: CSIR; 2017. [ Links ]
8. Frischen J, Meza I, Rupp D, Wietler K, Hagenlocher M. Drought risk to agricultural systems in Zimbabwe: A spatial analysis of hazard, exposure, and vulnerability. Sustainability. 2020;12, Art. #752. https://doi.org/10.3390/su12030752 [ Links ]
9. Van der Waals JE, Steyn JM, Franke AC, Haverkort AJ. Grower perceptions of biotic and abiotic risks of potato production in South Africa. Crop Prot. 2016;84:44-55. https://doi.org/10.1016/j.cropro.2016.02.008 [ Links ]
10. Harahagazwe D, Condori B, Barreda C, Bararyenya A, Byarugaba AA, Kude DA, et al. How big is the potato (Solanum tuberosum L.) yield gap in sub-Saharan Africa and why? A participatory approach. Open Agric. 2018;3:180-189. https://doi.org/10.1515/opag-2018-0019 [ Links ]
11. Gildemacher PR, Kaguongo W, Ortiz O, Tesfaye A, Woldegiorgis G, Wagoire WW, et al. Improving potato production in Kenya, Uganda and Ethiopia: A system diagnosis. Potato Res. 2009;52:173-205. https://doi.org/10.1007/s11540-009-9127-4 [ Links ]
12. Hijmans RJ. The effect of climate change on global potato production. Am J Potato Res. 2003;80:271-280. [ Links ]
13. Haverkort AJ, Verhagen A. Climate change and its repercussions for the potato supply chain. Potato Res. 2008;51:223-237. https://doi.org/10.1007/s11540-008-9107-0 [ Links ]
14. Raymundo R, Asseng S, Robertson R, Petsakos A, Hoogenboom G, Quiroz R, et al. Climate change impact on global potato production. Eur J Agron. 2018;100:87-98. https://doi.org/10.1016/j.eja.2017.11.008 [ Links ]
15. George TS, Taylor MA, Dodd IC, White PJ. Climate change and consequences for potato production: A review of tolerance to emerging abiotic stress. Potato Res. 2017;60:239-268. https://doi.org/10.1007/s11540-018-9366-3 [ Links ]
16. Haverkort AJ, Franke AC, Engelbrecht FA, Steyn JM. Climate change and potato production in contrasting South African agro-ecosystems 1. Effects on land and water use efficiencies. Potato Res. 2013;56:31-50. https://doi.org/10.1007/s11540-013-9230-4 [ Links ]
17. Franke AC, Haverkort AJ, Steyn JM. Climate change and potato production in contrasting South African agro-ecosystems 2. Assessing risks and opportunities of adaptation strategies. Potato Res. 2013;56:51-66. https://doi.org/10.1007/s11540-013-9229-x [ Links ]
18. Adhikari U, Nejadhashemi AP, Woznicki SA. Climate change and eastern Africa: A review of impact on major crops. Food Energy Secur. 2015;4:110-132. https://doi.org/10.1002/fes3.61 [ Links ]
19. Sparks AH, Forbes GA, Hijmans RJ, Garrett KA. Climate change may have limited effect on global risk of potato late blight. Glob Change Biol. 2014;20:3621-3631. https://doi.org/10.1111/gcb.12587 [ Links ]
20. Kreuze JF, Souza-Dias JAC, Jeevalatha A, Figueira AR, Valkonen JPT, Jones RAC. Viral diseases in potato. In: Campos H, Ortiz O, editors. The potato crop: Its agricultural, nutritional and social contribution to humankind. Cham: Springer International Publishing; 2020. p. 389-130. https://doi.org/10.1007/978-3-030-28683-5_11 [ Links ]
21. Loebenstein G, Gaba V. Viruses of potato. In: Gad L, Hervé L, editors. Advances in virus research. San Diego, CA: Academic Press; 2012. p. 209246. https://doi.org/10.1016/b978-0-12-394314-9.00006-3 [ Links ]
22. Glais L, Bellstedt DU, Lacomme C. Diversity, characterisation and classification of PVY. In: Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV Jacquot E, editors. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. p. 43-76. https://doi.org/10.1007/978-3-319-58860-5_3 [ Links ]
23. Visser JC, Bellstedt DU, Pirie MD. The recent recombinant evolution of a major crop pathogen, Potato virus Y. PLoS ONE. 2012;7, e50631. https://doi.org/10.1371/journal.pone.0050631 [ Links ]
24. Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV, Jaquot E. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. https://doi.org/10.1007/978-3-319-58860-5 [ Links ]
25. Scheme TSASPC. Plant Improvement Act 1976 (Act no. 53 of 1976). South African Seed Certification Scheme. Pretoria: South African Department of Agriculture, Forestry and Fisheries; 2010. [ Links ]
26. Halterman D, Charkowski A, Verchot J. Potato, viruses, and seed certification in the USA to provide healthy propagated tubers. Pest Technol. 2012;6:1-14. [ Links ]
27. Hutton F, Spink JH, Griffin D, Kildea S, Bonner D, Doherty G, et al. Distribution and incidence of viruses in Irish seed potato crops. Ir J Agric Food Res. 2015;54:98-106. https://doi.org/10.1515/ijafr-2015-0011 [ Links ]
28. Dupuis B, Bragard C, Carnegie S, Kerr J, Glais L, Singh M, et al. Potato virus Y: Control, management and seed certification programmes. In: Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV Jacquot E, editors. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. p. 177-206. https://doi.org/10.1007/978-3-319-58860-5_7 [ Links ]
29. Thomas-Sharma S, Abdurahman A, Ali S, Andrade-Piedra JL, Bao S, Charkowski AO, et al. Seed degeneration in potato: The need for an integrated seed health strategy to mitigate the problem in developing countries. Plant Pathol. 2016;65:3-16. https://doi.org/10.1111/ppa.12439 [ Links ]
30. Lacomme C, Pickup J, Fox A, Glais L, Dupuis B, Steinger T, et al. Transmission and epidemiology of Potato virus Y. In: Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV Jacquot E, editors. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. p. 141-176. https://doi.org/10.1007/978-3-319-58860-5_6 [ Links ]
31. Radcliffe EB, Ragsdale DW. Aphid-transmitted potato viruses: The importance of understanding vector biology. Am J Potato Res. 2002;79:353-386. [ Links ]
32. Fargette D, Konaté G, Fauquet C, Muller E, Peterschmitt M, Thresh JM. Molecular ecology and emergence of tropical plant viruses. Annu Rev Phytopathol. 2006;44:235-260. https://doi.org/10.1146/annurev.phyto.44.120705.104644 [ Links ]
33. Islam W, Noman A, Naveed H, Alamri SA, Hashem M, Huang Z, et al. Plant-insect vector-virus interactions under environmental change. Sci Total Environ. 2020;701, Art. #135044. https://doi.org/10.1016/j.scitotenv.2019.135044 [ Links ]
34. Jones RAC. Plant virus emergence and evolution: Origins, new encounter scenarios, factors driving emergence, effects of changing world conditions, and prospects for control. Virus Res. 2009;141:113-130. https://doi.org/10.1016/j.virusres.2008.07.028 [ Links ]
35. Jones RAC. Future scenarios for plant virus pathogens as climate change progresses. In: Kielian M, Maramorosch K, Mettenleiter TC, editors. Advances in virus research. San Diego, CA: Academic Press; 2016. p. 87-147. https://doi.org/10.1016/bs.aivir.2016.02.004 [ Links ]
36. Jones RAC, Naidu RA. Global dimensions of plant virus diseases: Current status and future perspectives. Annu Rev Virol. 2019;6:387-409. https://doi.org/10.1146/annurev-virology-092818-015606 [ Links ]
37. Trebicki P. Climate change and plant virus epidemiology. Virus Res. 2020;286, Art. #198059. https://doi.org/10.1016/j.virusres.2020.198059 [ Links ]
38. Rouault G, Candau J-N, Lieutier F, Nageleisen L-M, Martin J-C, Warzée N. Effects of drought and heat on forest insect populations in relation to the 2003 drought in Western Europe. Ann For Sci. 2006;63:613-624. https://doi.org/10.1051/forest:2006044 [ Links ]
39. Van der Waals JE, Krüger K, Franke AC, Haverkort AJ, Steyn JM. Climate change and potato production in contrasting South African agro-ecosystems 3. Effects on relative development rates of selected pathogens and pests. Potato Res. 2013;56:67-84. https://doi.org/10.1007/s11540-013-9231-3 [ Links ]
40. Beetge L, Krüger K. Drought and heat waves associated with climate change affect performance of the potato aphid Macrosiphum euphorbiae. Sci Rep. 2019;9, Art. #3645. https://doi.org/10.1038/s41598-018-37493-8 [ Links ]
41. Macfadyen S, McDonald G, Hill MP. From species distributions to climate change adaptation: Knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agr Ecosyst Environ. 2018;253:208-219. https://doi.org/10.1016/j.agee.2016.08.029 [ Links ]
42. Huberty AF, Denno RF. Plant water stress and its consequences for herbivorous insects: A new synthesis. Ecology. 2004;85:1383-1398. [ Links ]
43. Pritchard J, Vickers LH. Aphids and stress. In: Van Emden HF, Harrington R, editors. Aphids as Crop Pests. 2nd ed. Wallingford: CABI; 2017. p. 132-147. [ Links ]
44. Jones RAC. Virus disease problems facing potato industries worldwide: Viruses found, climate change implications, rationalizing virus strain nomenclature, and addressing the Potato virus Y issue. In: Navarre R, Pavek MJ, editors. The potato: Botany, production and uses. Wallingford: CABI; 2014. p. 202-224. https://doi.org/10.1079/9781780642802.0202 [ Links ]
45. Lacomme C, Jacquot E. General characteristics of Potato virus Y (PVY) and its impact on potato production: An overview. In: Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV, Jacquot E, editors. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. p. 1-19. https://doi.org/10.1007/978-3-319-58860-5_1 [ Links ]
46. Chung BN, Canto T, Tenllado F, Choi KS, Joa JH, Ahn JJ, et al. The effects of high temperature on infection by Potato virus Y, Potato virus A, and Potato leafroll virus. Plant Pathol J. 2016;32:321-328. https://doi.org/10.5423/PPJ.OA.12.2015.0259 [ Links ]
47. Ragsdale DW, Radcliffe EB, DiFonzo CD. Epidemiology and field control of PVY and PLRV In: Loebenstein G, Berger PH, Brunt AA, Lawson RH, editors. Virus and virus-like diseases of potatoes and production of seed-potatoes. Dordrecht: Kluwer Academic Publishers; 2001. p. 237-273. [ Links ]
48. Kaiser WJ. Use of thermotherapy to free potato tubers of alfalfa mosaic, potato leaf roll, and tomato black ring viruses. Phytopathology. 1980;70:1119-1122. [ Links ]
49. Schroder ML, Glinwood R, Ignell R, Krüger K. The role of visual and olfactory plant cues in aphid behaviour and the development of non-persistent virus management strategies. Arthropod-Plant Interact. 2017;11:1-13. https://doi.org/10.1007/s11829-016-9463-7 [ Links ]
50. Muthoni J, Kabira JN. Potato production in the hot tropical areas of Africa: Progress made in breeding for heat tolerance. J Agric Sci. 2015;7:220-227. https://doi.org/10.5539/jas.v7n9p220 [ Links ]
51. Fuglie KO. Priorities for potato research in developing countries: Results of a survey. Am J Potato Res. 2007;84:353-365. https://doi.org/10.1007/BF02987182 [ Links ]
52. Davidson RD, Houser AJ, Sather K, Haslar R. Controlling PVY in seed: What works and what does not. Am J Potato Res. 2013;90:28-32. https://doi.org/10.1007/s12230-012-9290-z [ Links ]
53. Kimani E. Seed potato production and ceritifcation guidelines. Nairobi: Kenya Plant Health Inspectorate Service (KEPHIS); 2016. [ Links ]
54. Muthoni J, Mbiyu MW, Nyamongo DO. A review of potato seed systems and germplasm conservation in Kenya. J Agric Food Inform. 2010;11:157-167. https://doi.org/10.1080/10496501003680565 [ Links ]
55. Gildemacher PR, Demo P Barker I, Kaguongo W, Woldegiorgis G, Wagoire WW, et al. A description of seed potato systems in Kenya, Uganda and Ethiopia. Am J Potato Res. 2009;86:373-382. https://doi.org/10.1007/s12230-009-9092-0 [ Links ]
56. Danial D, de Vries M, Lindhout P. Exploring the potential of hybrid potato cultivars in East Africa [document on the Internet]. c2016 [cited 2020 Jul 01]. Available from: https://knowledge4food.net/wp-content/uploads/2017/02/161130-Report-Solynta-mission-to-East-Africa.pdf [ Links ]
57. Palukaitis P. Resistance to viruses of potato and their vectors. Plant Pathol J. 2012;28:248-258. https://doi.org/10.5423/ppj.Rw.06.2012.0075 [ Links ]
58. Torrance L, Cowan GH, McLean K, MacFarlane S, Al-Abedy AN, Armstrong M, et al. Natural resistance to Potato virus Y in Solanum tuberosum group Phureja. Theor Appl Genet. 2020;133:967-980. https://doi.org/10.1007/s00122-019-03521-y [ Links ]
59. Kreuze JF, Valkonen JPT. Utilization of engineered resistance to viruses in crops of the developing world, with emphasis on sub-Saharan Africa. Curr Opin Virol. 2017;26:90-97. https://doi.org/10.1016/j.coviro.2017.07.022 [ Links ]
60. Valkonen JP Gebhardt C, Zimnoch-Guzowska E, Watanabe KN. Resistance to Potato virus Y in potato. In: Lacomme C, Glais L, Bellstedt DU, Dupuis B, Karasev AV Jacquot E, editors. Potato virus Y: Biodiversity, pathogenicity, epidemiology and management. Cham: Springer; 2017. p. 207-241. https://doi.org/10.1007/978-3-319-58860-5_8 [ Links ]
61. Ghislain M, Douches DS. The genes and genomes of the potato. In: Campos H, Ortiz O, editors. The potato crop: Its agricultural, nutritional and social contribution to humankind. Cham: Springer; 2020. p. 139-162. https://doi.org/10.1007/978-3-030-28683-5_11 [ Links ]
62. Pradel W, Gatto M, Hareau G, Pandey SK, Bhardway V. Adoption of potato varieties and their role for climate change adaptation in India. Clim Risk Manage. 2019;23:114-123. https://doi.org/10.10167j.crm.2019.01.001 [ Links ]
63. Schulte-Geldermann E, Gildemacher PR, Struik PC. Improving seed health and seed performance by positive selection in three Kenyan potato varieties. Am J Potato Res. 2012;89:429-437. https://doi.org/10.1007/s12230-012-9264-1 [ Links ]
64. Kleynhans R, Myeza PN, Laurie SM, Visser A, Jansen Van Rensburg WS, Adebola PO. Collection, maintenance and utilization of plant genetic resources at Agricultural Research Council (ARC)-Roodeplaat Vopi, South Africa. 2nd All Africa Horticulture Congress. Acta Hortic. 2013;1007:993-998. https://doi.org/10.17660/ActaHortic.2013.1007.119 [ Links ]
65. Thomas PE, Hassan SA. First report of twenty-two new hosts of Potato leafroll virus. Plant Dis. 2002;86:561. https://doi.org/10.1094/PDIS.2002.86.5.561A [ Links ]
66. McDonald JG, Singh RP Host range, symptomology, and serology of isolates of Potato virus Y (PVY) that share properties with both the PVY and PVY0 strain groups. Am Potato J. 1996;73:309-315. https://doi.org/10.1007/BF02855210 [ Links ]
67. Hanafi A, Radcliffe EB, Ragsdale DW. Spread and control of potato leafroll virus in the Souss Valley of Morocco. Crop Prot. 1995;14:145-153. [ Links ]
68. Macaulay EDM, Tatchell GM, Taylor LR. The Rothamsted Insect Survey '12-metre' suction trap. Bull Entomol Res. 1988;78:121-129. [ Links ]
69. Harrington R, Hullé M, Plantegenest M. Monitoring and forecasting. In: Emden HFv, Harrington R, editors. Aphids as crop pests. Wallingford: CABI; 2007. p. 515-536. [ Links ]
70. Davis JA, Radcliffe EB, Ragsdale DW, MacRae I. Increasing in-row spacing enhances Potato virus Y and Potato leafroll virus spread in potato. Am J Potato Res. 2015;92:497-501. https://doi.org/10.1007/s12230-015-9462-8 [ Links ]
71. Yang Q, Arthurs S, Lu Z, Liang Z, Mao R. Use of horticultural mineral oils to control Potato virus Y (PVY) and other non-persistent aphid-vectored viruses. Crop Prot. 2019;118:97-103. https://doi.org/10.1016/j.cropro.2019.01.003 [ Links ]
72. Boukhris-Bouhachem S, Sellami MH, Chaieb I, Souissi R, El Fahem M. Can mineral oil protect seed potato against aphid transmission of Potato virus Y? In: Low J, Nyongesa M, Quinn S, Parker M, editors. Potato and sweetpotato in Africa: Transforming the value chains for food and nutrition security. Wallingford: CABI; 2015. p. 375-384. [ Links ]
73. Newbery F, Qi A, Fitt BDL. Modelling impacts of climate change on arable crop diseases: Progress, challenges and applications. Curr Opin Plant Biol. 2016;32:101-109. https://doi.org/10.1016/j.pbi.2016.07.002 [ Links ]
74. Chisadza B, Mushunje A, Nhundu K, Phiri EE. Opportunities and challenges for seasonal climate forecasts to more effectively assist smallholder farming decisions. S Afr J Sci. 2020;116(1/2), Art. #4649. https://doi.org/10.17159/sajs.2020/4649 [ Links ]
75. Zhang J, Huang Y Pu R, Gonzalez-Moreno R Yuan L, Wu K, et al. Monitoring plant diseases and pests through remote sensing technology: A review. Comput Electron Agric. 2019;165, Art. #104943. https://doi.org/10.1016/j.compag.2019.104943 [ Links ]
76. Mahlein A-K, Kuska MT, Behmann J, Polder G, Walter A. Hyperspectral sensors and imaging technologies in phytopathology: state of the art. Annu Rev Phytopathol. 2018;56:535-558. https://doi.org/10.1146/annurev-phyto-080417-050100 [ Links ]
77. Polder G, Blok PM, De Villiers HAC, Van der Wolf JM, Kamp J. Potato virus Y detection in seed potatoes using deep learning on hyperspectral images. Front Plant Sci. 2019;10, Art. #209. https://doi.org/10.3389/fpls.2019.00209 [ Links ]
78. Gómez D, Salvador R Sanz J, Casanova JL. Potato yield prediction using machine learning techniques and Sentinel 2 data. Remote Sens. 2019;11(15), Art. #1745. https://doi.org/10.3390/rs11151745 [ Links ]
79. Griffel LM, Delparte D, Edwards J. Using support vector machines classification to differentiate spectral signatures of potato plants infected with Potato virus Y. Comput Electron Agric. 2018;153:318-324. https://doi.org/10.1016/j.compag.2018.08.027 [ Links ]
80. Filho FHI, Heldens WB, Kong Z, De Lange ES. Drones: Innovative technology for use in precision pest management. J Econ Entomol. 2020;113:1-25. https://doi.org/10.1093/jee/toz268 [ Links ]
 Correspondence:
Correspondence:
Kerstin Kriger
Email: kkruger@zoology.up.ac.za
Received: 01 July 2020
Revised: 26 Oct. 2020
Accepted: 28 Oct. 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: None