Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.11-12 Pretoria nov./dic. 2020
http://dx.doi.org/10.17159/sajs.2020/8055
REVIEW ARTICLE
Emerging potato pathogens affecting food security in southern Africa: Recent research
Jacquie E. van der WaalsI, II; Kerstin KrügerII, III
IDepartment of Plant and Soil Sciences, University of Pretoria, Pretoria, South Africa
IIForestry and Agricultural Biotechnology Institute (FABI), University of Pretoria, Pretoria, South Africa
IIIDepartment of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
ABSTRACT
Potato is a staple crop that contributes to food security and poverty alleviation in developing nations. Despite this, yields in developing nations are often unsustainably low, due to various biotic and abiotic factors that negatively affect production. Some of the most important biotic constraints are pathogens, many of which are disseminated by seed tubers. The lack of functional or formal seed certification systems in many southern African countries results in a continual increase in pathogen pressure. Short rotation cycles, poor plant nutrition and inefficient control measures exacerbate the crop production challenges faced by resource poor growers. In this review, we discuss five of the most important diseases on potatoes in southern Africa, namely late blight, bacterial wilt, soft rot / blackleg, powdery scab and zebra chip. Management options for small-scale growers are provided.
SIGNIFICANCE:
• Potato production in southern Africa is threatened by tuber-borne pathogens.
• Establishment and implementation of seed certification systems in southern African countries will increase potato yields and subsequently contribute to food security.
• Late blight, bacterial wilt, soft rot / blackleg and powdery scab are important emerging diseases on potatoes in southern Africa.
• Improved understanding of the biology of pathogens and the epidemiology of diseases will contribute to the management thereof.
Keywords: potato, late blight, bacterial wilt, soft rot, powdery scab
Introduction
Potato is regarded as one of the most important crops in addressing the challenge of food security, particularly among smallholder farmers.1,2 Potato production has drastically increased since the 1960s in the developing world, in comparison to that in the developed world.3 However, the sustainability of potato production globally, and particularly in developing countries, is threatened by adverse abiotic conditions, pests and pathogens. Pest and pathogen control is difficult for subsistence potato growers in southern Africa - a region fraught with challenges, not least of which is the need to increase agricultural productivity in the face of climate change and a rapidly growing population.
Many of the important diseases affecting potato can be defined as emerging infectious diseases.4 Emerging infectious diseases are caused by pathogens that have '(i) increased in incidence, geographical or host range; (ii) have changed pathogenesis; (iii) have newly evolved or (iv) have been discovered or newly described'4. Similarly, Secor and Rivera-Varas5 classified important potato diseases as caused by emerging, changing or surviving pathogens. The primary reasons for the occurrence of emerging infectious diseases in potatoes and other crops are related to increased trade and travel, intensified and expanded land use, changes in agricultural practices, planting of new varieties, and extreme weather events linked to climate change.4-7
Using the classification proposed by Secor and Rivera-Varas5, important diseases on potatoes in the last decade (2010-2020) in southern Africa include late blight, bacterial wilt, soft rot and blackleg, and powdery scab. Zebra chip disease, although not recorded in southern Africa, has been included as a threat because of its severe impact on potato production. In this review, we investigate the drivers behind the increase in these diseases, mitigation measures and routes to prevent additional emerging infectious diseases from appearing in the southern African potato industry.
Late blight
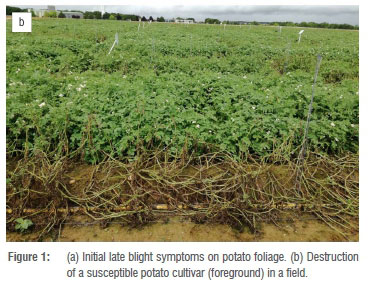
Late blight, caused by the oomycete Phytophthora infestans (Mont.) de Bary, is considered the most devastating potato disease globally, and causes severe yield losses when uncontrolled.4,7 The disease begins as small, light to dark green, circular to irregular-shaped water-soaked lesions on leaves. In cool, moist conditions the lesions expand to form dark brown to black lesions (Figure 1a), often surrounded by chlorotic halos. As the disease progresses, symptoms extend to petioles and haulms, eventually killing the plant (Figure 1b).8
In some countries in sub-Saharan Africa, including southern Africa, where the majority of potato crops are produced by small-scale or subsistence growers, losses due to late blight are estimated to be as high as 40-70% in susceptible varieties.9,10 In the South African commercial potato production industry, however, late blight is not considered the most important yield limiting disease. Climatic conditions during potato growing seasons in most of the 16 growing regions in South Africa are sub-optimal for the development of late blight.11 Given the climate change forecast for the next few decades, it is also unlikely that late blight will increase in severity - in fact, the contrary has been predicted.11 However, the SimCastMeta model simulation by Sparks et al.12 for five different potato growing regions globally, has indicated that blight units in some other southern African countries may increase slightly in the period 1975-2050. These possible increases in disease could, however, be mitigated by shifting planting dates to avoid conditions favourable for the development of late blight.12
A study by Pule et al.13 showed that the US-1 clonal lineage predominates in potato production areas of southern Africa. However, the recent first report of the EU_33_A2 clonal lineage of P. infestans causing late blight in Nigeria is concerning, as this lineage is suspected to be more widely spread than previously thought.14 The introduction of a new pathogen population into an area is often followed by an increase in disease severity and subsequent negative socio-economic impacts.15 This might be the case in southern Africa should EU_33_A2 spread south on the continent.
Late blight control in developed nations is achieved through seed certification, integrated management, intensive fungicide spray programmes, early planting dates, elimination of inoculum and planting resistant cultivars.10,15 However, in developing nations, where systemic fungicides are not easily accessible or are unaffordable, growers often use large amounts of low-cost dithiocarbamate-type contact fungicides, in particular mancozeb, which presents significant health hazards to farmworkers and their families.16 It has been suggested that late blight in developed countries can be managed by phosphonate applications, as a safer alternative to the hazardous dithiocarbamate derivatives currently used.16 Additional research is, however, needed to determine optimal timing, application rates, specific host reactions, environmental effects and interactions of phosphonates with other chemical compounds.16 The same level of control of the pathogen as that achieved by intensive spray programmes that incorporate both contact and systemic fungicides will not be attained by phosphonate application alone.
Host resistance may be the most effective way for resource poor growers to manage late blight.16,17 However, the adoption of resistant cultivars in developing countries is often slow, because of the scarcity of functional seed certification systems18 and because P. infestans rapidly overcomes cultivar resistance, and there is therefore a lack of truly resistant varieties19.
Bacterial wilt
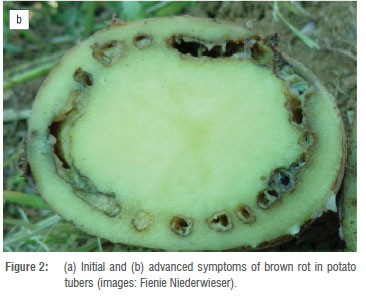
Bacterial wilt (or brown rot) is another serious disease of potatoes that can result in substantial yield losses. It is caused by members of the Ralstonia solanacearum species complex (RSSC). The first symptoms of the disease are wilting of the young leaves of plants, which is most commonly observed at the hottest time of day. As the disease progresses, plants become stunted, display general wilting and chlorosis, and will eventually die.20 The most obvious symptoms are noted in tubers, as an initial brown discolouration of the vascular tissue, followed by rotting thereof (Figure 2a and 2b). Members of the RSSC globally were previously assigned to four phylotypes, with phylotype classification based on phylogenetic analysis of the 16S-23S ItS region.21 However, Safni et al.22 elevated these phylotypes to species level. The phylotypes generally correlate with the geographical origin of the strains: phylotype II originating from the Americas was assigned to R. solanacearum, strains in phylotype IV from Indonesia were assigned to R. syzygii, and strains in phylotype I and III originating from Asia and Africa were assigned to R. pseudosolanacearum.23The most threatening potato pathogens, causing bacterial wilt and brown rot globally, fall into phylotype IIB sequevar 1 (IIB1), historically known as race 3, biovar 2, i.e. R. solanacearum.21,23Strains of both R. pseudosolanacearum and R. solanacearum can cause wilt in potato, but IIB1 strains are most persistent, can cause latent infections in tubers, are highly destructive and are cold tolerant.21
RSSC is considered to be one of the most important phytopathogenic bacteria globally, because of the destruction it can cause, its wide geographical distribution and host range, and its ability to survive for extended periods in soil, water, on plant debris and in asymptomatic hosts.24 In southern Africa, bacterial wilt has been reported in Angola, Malawi, Mozambique, South Africa, Swaziland, Zambia and Zimbabwe.25-27 A report by Nortje28 indicated that, historically, the most important phylotypes in Africa are I and II, including the virulent IIB1 strains. There is, however, a dearth of recent documentation on the species causing bacterial wilt of potatoes in southern African countries, but it is assumed that both R. pseudosolanacearum and R. solanacearum are present.
Management strategies for bacterial wilt include planting pathogen-free seed tubers, avoiding cutting or dipping seed tubers, good management of root-knot nematodes, planting of resistant varieties, soil fumigation, crop rotation with non-hosts, treatment of irrigation water, and disinfection of implements, machines and equipment.29 Messiha et al.30 demonstrated the potential of biological soil disinfestation to reduce soil populations of R. solanacearum through the incorporation of fresh organic matter into soil and then preventing the re-supply of oxygen into soils by covering with plastic. This practice results in shifts in the composition of bacterial communities, allowing biocontrol organisms to establish themselves in the soil.30 This practice may be a practical and affordable way for subsistence farmers in southern Africa to manage this devastating disease.
With the exception of South Africa, most southern African countries do not have formal or functional seed potato certification schemes. Growers thus rely on seed that is visually selected and saved from harvested tubers for the following seasons' planting.29 This practice has resulted in the spread of R. solanacearum, as growers usually select smaller tubers for seed, which are often latently contaminated with the pathogen.29 Numerous studies have shown that latently infected farm-saved seed is the primary source of inoculum and the pathway of dissemination of Ralstonia solanacearum IIB1 in many countries in sub-Saharan and southern Africa, thus contributing substantially to the severity of bacterial wilt in these countries.26,29,31 The importance of seed inoculum is emphasised by the success of the South African Potato Certification Service in drastically reducing the incidence of bacterial wilt in both registered seed potato and table potato plantings since the inception of the scheme.28 This decrease in the disease in the commercial potato production sector of South Africa can be ascribed to strict implementation of a formal seed certification scheme, and good disease management practices.
Despite the many methods by which bacterial wilt can be managed, it still remains a yield-limiting disease in many countries in southern Africa32,33, particularly for small-scale or subsistence growers. The wide host range and ability of the pathogen to survive for extended periods in soil and irrigation water renders many of the current potato practices useless in terms of reducing R. solanacearum soil inoculum. The wide genetic diversity of R. solanacearum hampers the success of cultivars bred for resistance against the pathogen, and makes detection and correct identification of the pathogen difficult, which in turn affects management decisions.28,33,34 An integrated pest management strategy - which combines numerous cultural, biological and chemical control measures - remains the most effective approach to reduce losses caused by R. solanacearum. It is vital that potato growers in southern Africa implement as many of these practices as possible to avoid serious economic losses.
Soft rot / blackleg disease complex
The soft rot / blackleg disease complex caused by Pectobacterium and Dickeya species is a potential threat to potato production worldwide.3538 The soft rotting Pectobacteriacea (SRp) cause systemic and vascular infections in potatoes, which result in the development of various symptoms on the stem and tubers.35-38 SRP, like R. solanacearum, are considered to be among the top 10 most important bacterial phytopathogens globally, and cause significant losses in crop yield and quality.24 SRP are primarily tuber-borne, but environmental sources and contamination through wind currents, insects and irrigation water are established secondary routes.39-43
Soft rot (Figure 3a) can occur in tubers at any stage (in the field, during harvest, post-harvest or in-store) and the disease will spread if a source of contamination is present and conditions are favourable for the development of the disease. The disease is most likely to occur in wet conditions with high temperatures.35 Blackleg (Figure 3b) develops when the bacteria in contaminated seed potatoes spread upwards in the stems.35,37 Aerial stem rot is a secondary soft rot of stems and leaves that develops after plants are wounded and subsequently infected by SRP.35,37
The disease complex is a major concern to the potato industry worldwide and a significant contributing factor to yield losses in southern Africa.44,45 This disease has increased in both severity and distribution, likely due to an increase in planting of susceptible varieties, and the effect of climate change on the composition of the pathogen population causing soft rot and blackleg in southern Africa.11 The majority of research on the SRP in southern Africa has been done in South Africa, and to a lesser extent in Zimbabwe.44-46 The most prevalent pathogen causing soft rot and blackleg in these countries is Pectobacterium brasiliense.44,45 Pectobacterium brasiliense has been shown to grow at temperatures between 20 °C and 38 °C47, which are the prevailing temperatures during the main potato growing seasons in southern Africa.
The primary means of management of SRP is planting disease-free seed potatoes37 and implementation of various cultural management practices, such as roguing of infected plants, increasing calcium fertilisation, avoidance of wounding at harvest and during grading, and storage in well-ventilated, low-temperature conditions. These practices have variable success rates due to latent infections of tubers, rapid reproduction of bacteria and subsequent disease development under favourable environmental conditions.37 Nevertheless, as with R. solanacearum, an effective seed certification system can help to reduce disease incidence.37,38
Powdery scab
Spongospora subterranea f. sp. subterranea (Sss), the causal agent of root galls (Figure 4a) and powdery scab (Figure 4b) on potatoes, has been described as an emerging pathogen on potatoes.5 For many years, powdery scab was considered a minor disease on potatoes; however, it is rapidly becoming an increasing threat to potato production globally.48-51 The global increase in powdery scab intensity can be attributed to increased potato production, planting of susceptible cultivars, more frequent use of irrigation, and discontinuation of mercury-based soil and seed treatments.48 Only a few published records on the distribution of Sss in southern Africa are available. According to Manditsvara52, powdery scab is a significant disease of potatoes in Zimbabwe. Likewise, research on powdery scab in South Africa has shown that this disease causes major economic losses to the potato industry in the country.53,54
Control of Sss is difficult, but a reduction in disease incidence and severity can be achieved through integration of a number of management measures. These measures include informed selection of pathogen-free fields, cultivar choice, seed and soil treatment, optimal plant nutrition, crop rotation with non-hosts or trapping crops, planting of disease- and pathogen-free tubers, and post-harvest hygiene.55 As with bacterial wilt and late blight, the implementation of many of these control methods by smallholder or subsistence growers in southern Africa is, however, often problematic or impractical. A compounding factor in the management of powdery scab is the correct identification of symptoms. The symptoms of common scab of potatoes, caused by Streptomyces spp., and powdery scab, are difficult to distinguish; although favourable conditions for disease development and control measures for the two diseases are different.56 Accurate identification of symptoms is thus critically important for the management and containment of the disease. Many African countries, however, do not have active and accurate pest and pathogen diagnostic services for low-value staple crops, in particular, due to lack of expertise and the costs involved in establishing such services.7,57
Most of the research on Sss in southern Africa has focused on management of powdery scab. Wright et al.53 demonstrated the importance of good hygiene and regular testing of growing media in the production of Sss-free mini-tubers in tunnels. Manditsvara52 and Simango and van der Waals54 established the potential of biofumigation and various soil treatments in suppressing root galling and powdery scab. These results may provide small-scale, subsistence and commercial growers with sustainable options for suppression of the pathogen in the soil.
The choice of crops in a rotation programme with potatoes is important. Growers should familiarise themselves with the host range of the pathogen58-60, and plant non-hosts or trapping crops in rotation with potatoes to prevent increase of the soil inoculum. No single management measure will control powdery scab; therefore, growers should use as many of the available options as possible to manage the disease.
Zebra chip disease
Zebra chip is an emerging potato disease that poses a serious threat to potato production worldwide, including in southern Africa. Symptoms in potato include leaf curling, yellowing or purpling of leaves or shoots, leaf chlorosis, shortened internodes with aerial tubers, and early necrosis.61 Potato tubers have necrotic flecking of the vascular tissue and streaks along the medullary rays.61 The name of the disease originates from potato slices that have brown blotches, stripes and streaks when fried. The disease is associated with the phloem-limited bacterium 'Candidatus Liberibacter solanacearum'. The pathogen is transmitted in the field by the potato psyllid Bactericera cockerelli (Hemiptera, Triozidae), to potato and other solanaceous crops. Other psyllid species have been identified as vectors of 'Ca. L. solanacearum' to plants within the Apiaceae. Another route of infection is planting of infected plant material.62 The insect vector is native to North and Central America.62 The disease has been introduced into Europe and New Zealand, with the cause of spread not yet clear.63 Pathways of introduction of the pathogen into southern Africa include importation of infected plant material or the infected insect vector.63 An integrated pest management approach is recommended, which includes the planting of certified disease-free material, conservation of natural enemies of the psyllid vector, application of insecticides and use of barriers for smaller areas to prevent the insect vector from reaching plants.63 The latter two strategies could be practically applied in southern Africa.
Discussion
Due to the vegetative propagation of potatoes by seed tubers, most of the important yield limiting pathogens are seed-borne and thus result in seed degeneration. Thomas-Sharma et al.64 define seed tuber degeneration as 'an increase in pest and/or pathogen incidence or severity, associated with reduction in yield or quality of seed tubers over successive cycles of vegetative propagation' and thus an indication of decreasing seed health. Most farmers in developing nations, such as the majority of those in southern Africa, use farm-saved or poor-quality seed, which is often infected with various pathogens, or is physiologically inferior.20,65-67 This, along with the use of low-yielding varieties with little or no resistance to important diseases, poor disease management, and nutritionally depleted soils, results in low yields in these countries. The Agricultural Research Council of South Africa has a dedicated potato breeding programme, the aim of which is to release varieties that are adapted to local growing conditions in Africa.68 If sufficiently supported, the varieties developed through this breeding programme could contribute positively to sustainable potato production in other southern African countries.
The introduction of specialised seed production systems, implementation of seed certification schemes69, reduction in the cost of good-quality seeds, and alleviation of the bottleneck in the seed supply in developing African countries will improve potato production and sustainability64-69. The production of seed tubers through a combination of tissue culture and aeroponics may also provide a viable alternative to conventional production of tubers for growers in developing countries.64,70-72 Aeroponics systems reduce the time and cost of production of seed, improve the growth and survival rate of plantlets, and are environmentally friendly. This approach will ensure the production of vast quantities of pathogen-free seed tubers for growers, and subsequently improve profitability of the farming operations.64,70-72
As the demand for potatoes in southern Africa increases, growers often repeatedly crop potatoes in succession on the same fields, which leads to increases in pathogen inoculum in the soil and subsequent yield losses.6,34,67 Numerous studies have shown the importance of crop diversification and rotation in the suppression of pathogens, the optimisation of nutrient use efficiency, crop productivity and, when legumes are included in the cycle, minimisation of the dependence on external nitrogen applications.73,74
Grower training plays a critical role in improving crop production.75 Demonstration plots and workshops run by regional extension officers should explain the importance of clean seed, crop rotation, hygiene, plant nutrition, and pest and pathogen management in potato production to growers. The success of such training efforts has been shown in Kenya and Swaziland inter alia.45,75
One of the challenges encountered in gathering the information for this review was the lack of published data on disease occurrence and epidemiology, type of pathogen strains present, prevalence of pathogens or effect of diseases on potato production in southern Africa. Smith et al.57 made similar observations on the reporting of plant pests and pathogens in Africa, with the number of reports of new pest introductions in Africa having dropped over the last century compared to Europe. These discrepancies could be attributed to either the actual rate of introductions or the lack of plant protection expertise and capacity to report introductions in many African countries.6,76 Potato yield losses due to major pathogens in (central) Africa may be more than 50%, compared with 24% in northern Europe, indicating clear differences in crop protection intensity between these two regions.77 These observations imply that Africa is ill-prepared for pest or pathogen introductions, which could threaten production of staple crops, such as potatoes.6 Improved border control, plant quarantine or monitoring of the movement of pathogens is imperative to prevent incursions of pathogens into new areas. Border porosity and lack of enforcement of plant quarantine of monitoring services in Africa results in unhindered movement of pests and pathogens across both regional and national borders on the continent27, and should receive urgent attention to ensure food security in southern Africa.
Competing interests
We declare that there are no competing interests.
Authors' contributions
J.E.v.d.W.: Conceptualisation; data curation; writing - initial draft. K.K.: Conceptualisation; writing - revision.
References
1. Birch PRJ, Bryan G, Fenton B, Gilroy EM, Hein I, Jones JT, et al. Crops that feed the world 8: Potato: Are the trends of increased global production sustainable? Food Secur. 2012;4:477-508. https://doi.org/10.1007/s12571-012-0220-1 [ Links ]
2. Devaux A, Kromann P Ortiz O. Potatoes for sustainable global food security. Potato Res. 2014;57:185-199. https://doi.org/10.1007/s11540-014-9265-1 [ Links ]
3. Gheysen G, Heremans B, Van Droogenbroek B, Custers R, Vossen JH, Visser RGF, et al. Durable cisgenic resistance to Phytophthora infestans in potato, and perspectives for applications in Africa. In: Low J, Nyongesa M, Quinn S, Parker M, editors. Potato and sweetpotato in Africa: Transforming the value chains for food and nutrition security. Oxfordshire: CAB International; 2015. p. 122-127. https://doi.org/10.1079/9781780644202.0122 [ Links ]
4. Anderson PK, Cunningham AA, Patel NG, Morales FJ, Epstein PR, Daszak P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol Evol. 2004;19:535-544. https://doi.org/10.1016/j.tree.2004.07.021 [ Links ]
5. Secor GA, Rivera-Varas VV. Emerging diseases of cultivated potato and their impact on Latin America. Rev Latinoamericana Papa (Suppl). 2004;1:1-8. [ Links ]
6. Waage JK, Woodhall JW, Bishop SJ, Smith JJ, Jones DR, Spence NJ. Patterns of plant pest introductions in Europe and Africa. Agr Syst. 2008;99:1-5. https://doi.org/10.1016/j.agsy.2008.08.001 [ Links ]
7. Fry WE, Birch PR, Judelson HS, Grunwald NJ, Danies G, Everts KL, et al. Five reasons to consider Phytophthora infestans a reemerging pathogen. Phytopathology. 2015;105:966-981. https://doi.org/10.1094/PHYTO-01-15-0005-FI [ Links ]
8. Nowicki M, Foolad MR, Nowakowska M, Kozik EU. Potato and tomato late blight caused by Phytophthora infestans: An overview of pathology and resistance breeding. Plant Dis. 2012;96:4-17. https://doi.org/10.1094/PDIS-05-11-0458 [ Links ]
9. Sengooba T, Hakiza JJ, editors. The current status of late blight caused by Phytophthora infestans in Africa, with emphasis on eastern and southern Africa. In: Proceedings of the Global Initiative on Late Blight Conference; 1999 March 16-19; Quito, Ecuador. [ Links ]
10. Ghislain M, Byarugaba AA, Magembe E, Njoroge A, Rivera C, Roman ML, et al. Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotechnol J. 2019;17:1119-1129. https://doi.org/10.1111/pbi.13042 [ Links ]
11. Van der Waals JE, Krüger K, Franke AC, Haverkort AJ, Steyn JM. Climate change and potato production in contrasting South African agro-ecosystems 3. Effects on relative development rates of selected pathogens and pests. Potato Res. 2013;56:67-84. https://doi.org/10.1007/s11540-013-9231-3 [ Links ]
12. Sparks AH, Forbes GA, Hijmans RJ, Garrett KA. Climate change may have limited effect on global risk of potato late blight. Glob Chang Biol. 2014;20:3621-3631. https://doi.org/10.1111/gcb.12587 [ Links ]
13. Pule BB, Meitz JC, Thompson AH, Linde CC, Fry WE, Langenhoven SD, et al. Phytophthora infestans populations in central, eastern and southern African countries consist of two major clonal lineages. Plant Pathol. 2013;62:154-165. https://doi.org/10.1111/j.1365-3059.2012.02608.x [ Links ]
14. Nnadi NE, Datiri AM, Pam DB, Ngene AC, Okonkwo FO, Sullivan L, et al. First report of the EU_33_A2 clonal lineage of Phytophthora infestans causing late blight disease of potato in Nigeria. New Dis Rep. 2019;40:20. https://doi.org/10.5197/j.2044-0588.2019.040.020 [ Links ]
15. Fry WE. Phytophthora infestans: New tools (and old ones) lead to new understanding and precision management. Annu Rev Phytopathol. 2016;54:529-547. https://doi.org/10.1146/annurev-phyto-080615-095951 [ Links ]
16. Kromann P Pérez WG, Taipe A, Schulte-Geldermann E, Sharma BP Andrade-Piedra JL, et al. Use of phosphonate to manage foliar potato late blight in developing countries. Plant Dis. 2012;96:1008-1015. https://doi.org/10.1094/PDIS-12-11-1029-RE [ Links ]
17. Blandón-Díaz JU. Insights into population structure and epidemiology of Phytophthora infestans from Nicaragua. Uppsala: Swedish University of Agricultural Sciences; 2011. [ Links ]
18. Thomas-Sharma S, Abdurahman A, Ali S, Andrade-Piedra JL, Bao S, Charkowski AO, et al. Seed degeneration in potato: The need for an integrated seed health strategy to mitigate the problem in developing countries. Plant Pathol. 2016;65:3-16. https://doi.org/10.1111/ppa.12439 [ Links ]
19. Forbes GA. Using host resistance to manage potato late blight with particular reference to developing countries. Potato Res. 2012;55:205-216. https://doi.org/10.1007/s11540-012-9222-9 [ Links ]
20. Genin S, Denny TP. Pathogenomics of the Ralstonia solanacearum species complex. Annu Rev Phytopathol. 2012;50:67-89. https://doi.org/10.1146/annurev-phyto-081211-173000 [ Links ]
21. Fegan M, Prior P. How complex is the "Ralstonia solanacearum species complex". In: Allen C, Hayward AC, Prior P editors. Bacterial wilt disease and the Ralstonia solanacearum species complex. St Paul, MN: American Phytopathological Society; 2005. p. 449-461. [ Links ]
22. Safni I, Cleenwerck I, De Vos P, Fegan M, Sly L, Kappler U. Polyphasic taxonomic revision of the Ralstonia solanacearum species complex: Proposal to emend the descriptions of Ralstonia solanacearum and Ralstonia syzygii and reclassify current R. syzygii strains as Ralstonia syzygii subsp. syzygii subsp. nov., R. solanacearum phylotype IV strains as Ralstonia syzygii subsp. indonesiensis subsp. nov., banana blood disease bacterium strains as Ralstonia syzygii subsp. celebesensis subsp. nov. and R. solanacearum phylotype I and III strains as Ralstonia pseudosolanacearum sp. nov. Int J Syst Evol Microbiol. 2014;64:3087-3103. https://doi.org/10.1099/ijs.0.066712-0 [ Links ]
23. Guidot A, Coupat B, Fall S, Prior P, Bertolla F. Horizontal gene transfer between Ralstonia solanacearum strains detected by comparative genomic hybridization on microarrays. ISME J. 2009;3:549-562. https://doi.org/10.1038/ismej.2009.14 [ Links ]
24. Mansfield J, Genin S, Magori S, Citovsky V Sriariyanum M, Ronald P et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol Plant Pathol. 2012;13:614-629. https://doi.org/10.1111/j.1364-3703.2012.00804.x [ Links ]
25. Muthoni J, Shimelis H, Melis R. Management of bacterial wilt [Ralstonia solanacearum] of potatoes: Opportunity for host resistance in Kenya. J Agric Sci. 2012;4(9):64. https://doi.org/10.5539/jas.v4n9p64 [ Links ]
26. Kagona JDZ. The incidence of bacterial wilt (Ralstonia solanacearum) in informal potato planting material used by farmers in Dedza and Ntcheu districts of Malawi [MSc thesis]. Ás: Norwegian University of Life Sciences; 2008. [ Links ]
27. Bentley JW, Robson M, Sibale BB, Nkhulungo E, Tembo Y Munthali F. Travelling companions: Emerging diseases of people, animals and plants along the Malawi-Mozambique border. Hum Ecol. 2012;40:557-569. https://doi.org/10.1007/s10745-012-9503-6 [ Links ]
28. Nortje P Bacterial wilt on potato: The South African experience [document on the Internet]. c2015 [cited 2020 Jun 29]. Available from: https://drive.google.com/file/d/16kzi6ntkiOY9cyh-HAKXy92s_uppqAHA/view [ Links ]
29. Okiro LA, Tancos MA, Nyanjom SG, Smart CD, Parker ML. Comparative evaluation of LAMP qPCR, conventional PCR, and ELISA to detect Ralstonia solanacearum in Kenyan potato fields. Plant Dis. 2019;103:959-965. https://doi.org/10.1094/PDIS-03-18-0489-RE [ Links ]
30. Messiha NAS, Van Diepeningen AD, Wenneker M, Van Beuningen AR, Janse JD, Coenen TGC, et al. Biological soil disinfestation (BSD), a new control method for potato brown rot, caused by Ralstonia solanacearum race 3 biovar 2. Eur J Plant Pathol. 2007;117:403-415. https://doi.org/10.1007/s10658-007-9109-9 [ Links ]
31. Gildemacher PR, Demo P, Barker I, Kaguongo W, Woldegiorgis G, Wagoire WW, et al. A description of seed potato systems in Kenya, Uganda and Ethiopia. Am J Potato Res. 2009;86:373-382. https://doi.org/10.1007/s12230-009-9092-0 [ Links ]
32. Sekani YK, Vernon K, Max WL, Patson CN. Production practices of potato (Solanum tuberosum L.) by farmers in Mzimba District, Northern Malawi. Afr J Agr Res. 2015;10:797-802. https://doi.org/10.5897/AJAR2014.9081 [ Links ]
33. Aloyce A, Ndakidemi PA, Mbega ER. Identification and management challenges associated with Ralstonia solanacearum (Smith), causal agent of bacterial wilt disease of tomato in Sub-Saharan Africa. Pak J Biol Sci. 2017;20:530-542. https://doi.org/10.3923/pjbs.2017.530.542 [ Links ]
34. Yuliar, Nion YA, Toyota K. Recent trends in control methods for bacterial wilt diseases caused by Ralstonia solanacearum. Microbes Environ. 2015;30:1-11. https://doi.org/10.1264/jsme2.ME14144 [ Links ]
35. Pérombelon MCM. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002;51:1-12. https://doi.org/10.1046/j.0032-0862.2001.Shorttitle.doc.x [ Links ]
36. Toth IK, Van der Wolf JM, Saddler G, Lojkowska E, Hélias V, Pirhonen M, et al. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011;60:385-399. https://doi.org/10.1111/j.1365-3059.2011.02427.x [ Links ]
37. Czajkowski R, Perombelon M, Van Veen JA, Van der Wolf J. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011;60:999-1013. https://doi.org/10.1111/j.1365-3059.2011.02470.x [ Links ]
38. Charkowski AO. The changing face of bacterial soft-rot diseases. Annu Rev Phytopathol. 2018;56:269-288. https://doi.org/10.1146/annurev-phyto-080417-045906 [ Links ]
39. Harrison MD, Franc GD, Maddox DA, Michaud JE, McCarter-Zorner NK. Presence of Erwinia carotovora in surface water in North America. J Appl Bacteriol. 1987;62:565-570. https://doi.org/10.1111/j.1365-2672.1987.tb02690.x [ Links ]
40. Rossmann S, Dees MW, Perminow J, Meadow R, Brurberg MB. Soft rot Enterobacteriaceae are carried by a large range of insect species in potato fields. Appl Environ Microbiol. 2018;84, e00281-18. https://doi.org/10.1128/AEM.00281-18 [ Links ]
41. Cappaert MR, Powelson ML, Franc GD, Harrison MD. Irrigation water as a source of inoculum of soft rot erwinias for aerial stem rot of potatoes. Phytopathology. 1988;78:1668-1672. https://doi.org/10.1094/Phyto-78-1668 [ Links ]
42. Laurila J, Ahola V, Lehtinen A, Joutsjoki T, Hannukkala A, Rahkonen A, et al. Characterization of Dickeya strains isolated from potato and river water samples in Finland. Eur J Plant Pathol. 2008;122:213-225. https://doi.org/10.1007/s10658-008-9274-5 [ Links ]
43. Van der Merwe JJ, Coutinho TA, Korsten L, Van der Waals JE. Pectobacterium carotovorum subsp. brasiliensis causing blackleg on potatoes in South Africa. Eur J Plant Pathol. 2009;126:175-185. https://doi.org/10.1007/s10658-009-9531-2 [ Links ]
44. Ngadze E, Brady CL, Coutinho TA, Van der Waals JE. Pectinolytic bacteria associated with potato soft rot and blackleg in South Africa and Zimbabwe. Eur J Plant Pathol. 2012;134:533-549. https://doi.org/10.1007/s10658-012-0036-z [ Links ]
45. Nxumalo KA, Dlamini BE, Masarirambi MT, Earnshaw DM. Management of postharvest diseases of vegetables in a tropical and sub-tropical environment of the Kingdom of Eswatini. Curr J Appl Sci Technol. 2020;38(6):1-13. https://doi.org/10.9734/cjast/2019/v38i630465 [ Links ]
46. Du Raan S, Coutinho TA, Van der Waals JE. Cardinal temperature differences, determined in vitro, between closely related species and subspecies of pectinolytic bacteria responsible for blackleg and soft rot on potatoes. Eur J Plant Pathol. 2015;144:361-369. https://doi.org/10.1007/s10658-015-0773-x [ Links ]
47. Toth IK, Sullivan L, Brierley JL, Avrova AO, Hyman LJ, Holeva M, et al. Relationship between potato seed tuber contamination by Erwinia carotovora ssp. atroseptica, blackleg disease development and progeny tuber contamination. Plant Pathol. 2003;52:119-126. https://doi.org/10.1046/j.1365-3059.2003.00821.x [ Links ]
48. Merz U, Falloon RE. Review: Powdery scab of potato-Increased knowledge of pathogen biology and disease epidemiology for effective disease management. Potato Res. 2008;52:17-37. https://doi.org/10.1007/s11540-008-9105-2 [ Links ]
49. Balendres MA, Tegg RS, Wilson CR. Key events in pathogenesis of Spongospora diseases in potato: A review. Aust Plant Pathol. 2016;45:229-240. https://doi.org/10.1007/s13313-016-0398-3 [ Links ]
50. Falloon RE, Merz U, Butler RC, Curtin D, Lister RA, Thomas SM. Root infection of potato by Spongospora subterranea: Knowledge review and evidence for decreased plant productivity. Plant Pathol. 2016;65:422-434. https://doi.org/10.1111/ppa.12419 [ Links ]
51. Merz U. Powdery scab of potato - Occurrence, life cycle and epidemiology. Am J Potato Res. 2008;85:241-246. https://doi.org/10.1007/s12230-008-9019-1 [ Links ]
52. Manditsvara H. Potential of Brassica napus and Trichoderma harzianum in control of powdery scab (Spongospora subterranea f. sp. subterranea) of potato (Solanum tuberosum L.) [MSc dissertation]. Gweru: Midlands State University; 2014. http://hdl.handle.net/11408/592 [ Links ]
53. Wright J, Lees AK, Van der Waals JE. Detection and eradication of Spongospora subterranea in mini-tuber production tunnels. S Afr J Sci. 2012;108(5/6), Art. #614, 4 pages. https://doi.org/10.4102/sajs.v108i5/6.614 [ Links ]
54. Simango K, Van der Waals JE. Effects of different soil treatments on the development of Spongospora subterranea f. sp. subterranea in potato roots and tubers in the greenhouse. Potato Res. 2017;60:47-60. https://doi.org/10.1007/s11540-017-9340-5 [ Links ]
55. Falloon RE. Control of powdery scab of potato: Towards integrated disease management. Am J Potato Res. 2008;85:253-260. https://doi.org/10.1007/s12230-008-9022-6 [ Links ]
56. Qu XS, Wanner LA, Christ BJ. Multiplex real-time PCR (TaqMan) assay for the simultaneous detection and discrimination of potato powdery and common scab diseases and pathogens. J Appl Microbiol. 2011;110:769-777. https://doi.org/10.1111/j.1365-2672.2010.04930.x [ Links ]
57. Smith JJ, Waage J, Woodhall JW, Bishop SJ, Spence NJ. The challenge of providing plant pest diagnostic services for Africa. Eur J Plant Pathol. 2008;121:365-375. https://doi.org/10.1007/s10658-008-9311-4 [ Links ]
58. Qu XS, Christ BJ. The host range of Spongospora subterranea f. sp. subterranea in the United States. Am J Potato Res. 2006;83:343-347. https://doi.org/10.1007/BF02871595 [ Links ]
59. Arcila IM, Gonzalez EP Zuluaga CM, Cotes JM. Alternate hosts of Spongospora subterranea f. sp. subterranea identification in Colombia by bioassay. Rev Fac Nal Agr Medellín. 2013;66:6987-6998. [ Links ]
60. Tsror L, Shapira R, Erlich O, Hazanovsky M, Lebiush S. Characterization of weeds and rotational crops as alternative hosts of Spongospora subterranea, the causal agent of powdery scab in Israel. Plant Pathol. 2019;69:294-301. https://doi.org/10.1111/ppa.13117 [ Links ]
61. Munyaneza JE. Zebra chip disease of potato: Biology, epidemiology, and management. Am J Potato Res. 2012;89:329-350. https://doi.org/10.1007/s12230-012-9262-3 [ Links ]
62. Vereijssen J, Smith GR, Weintraub PG. Bactericera cockerelli (Hemiptera: Triozidae) and Candidatus Liberibacter solanacearum in potatoes in New Zealand: Biology, transmission, and implications for management. J Integr Pest Manag. 2018;9. https://doi.org/10.1093/jipm/pmy007 [ Links ]
63. Teulon DAJ, Workman PJ, Thomas KL, Nielsen M-C. Bactericera cockerelli: Incursion, dispersal and current distribution on vegetable crops in New Zealand. NZ Plant Prot. 2009;62:136-144. https://doi.org/10.30843/nzpp.2009.62.4783 [ Links ]
64. Thomas-Sharma S, Andrade-Piedra J, Carvajal Yepes M, Hernandez Nopsa JF, Jeger MJ, Jones RAC, et al. A risk assessment framework for seed degeneration: Informing an integrated seed health strategy for vegetatively propagated crops. Phytopathology. 2017;107:1123-1135. https://doi.org/10.1094/PHYTO-09-16-0340-R [ Links ]
65. Gildemacher PR, Schulte-Geldermann E, Borus D, Demo P, Kinyae P, Mundia P, et al. Seed potato quality improvement through positive selection by smallholder farmers in Kenya. Potato Res. 2011;54:253-266. https://doi.org/10.1007/s11540-011-9190-5 [ Links ]
66. Schulte-Geldermann E. Potato research in Africa to improve farmers' livelihoods: Priorities in crop improvement, seed system, crop management, nutritional value, policies and marketing. Potato Res. 2018;60:287-289. https://doi.org/10.1007/s11540-018-9356-5 [ Links ]
67. Harahagazwe D, Condori B, Barreda C, Bararyenya A, Byarugaba AA, Kude DA, et al. How big is the potato (Solanum tuberosum L.) yield gap in sub-Saharan Africa and why? A participatory approach. Open Agriculture. 2018;3:180-189. https://doi.org/10.1515/opag-2018-0019 [ Links ]
68. Kleynhans R, Myeza PN, Laurie SM, Visser A, Jansen Van Rensburg WS, Adebola PO. Collection, maintenance and utilization of plant genetic resources at Agricultural Research Council (ARC)-Roodeplaat Vopi, South Africa. 2nd All Africa Horticulture Congress. Acta Hort. 2013;1007:993-998. https://doi.org/10.17660/ActaHortic.2013.1007.119 [ Links ]
69. Demo P Lemaga B, Kakuhenzire R, Schulz S, Borus D, Barker I, et al. Strategies to improve seed potato quality and supply in sub-Saharan Africa: Experience from interventions in five countries. In: Low J, Nyongesa M, Quinn S, Parker M, editors. Potato and sweetpotato in Africa: Transforming the value chains for food and nutrition security. Oxfordshire: CAB International; 2015. p. 155-167. https://doi.org/10.1079/9781780644202.0155 [ Links ]
70. Chiipanthenga M. Potential of aeroponics system in the production of quality potato (Solanum tuberosum L.) seed in developing countries. Afr J Biotechnol. 2012;11(17):3993-3999. https://doi.org/10.5897/AJB10.1138 [ Links ]
71. Njaika JA, Njoloma JP Zimba S, Mwase WF, Maliro MF, Bokosi JM, et al. The effectiveness of repeated shoot tip culture on pathogens load reduction in different local potato genotypes in Malawi. Int J Biotechnol. 2014;3:91-103. [ Links ]
72. Tsoka O, Demo P, Nyende AB, Ngamau K. Potato seed tuber production from in vitro and apical stem cutting under aeroponic system. Afr J Biotechnol. 2012;11(63):12612-12618. https://doi.org/10.5897/AJB10.1048 [ Links ]
73. Larkin RP, Griffin TS, Honeycutt CW. Rotation and cover crop effects on soilborne potato diseases, tuber yield, and soil microbial communities. Plant Dis. 2010;94:1491-1502. https://doi.org/10.1094/PDIS-03-10-0172 [ Links ]
74. Dias T, Dukes A, Antunes PM. Accounting for soil biotic effects on soil health and crop productivity in the design of crop rotations. J Sci Food Agric. 2015;95:447-454. https://doi.org/10.1002/jsfa.6565 [ Links ]
75. Nyamwaru RO, Ombati JM, Mwangi JG. Effectiveness of agricultural training centers' curriculum in promoting adoption of agricultural technologies: Evidence from small-scale potato farmers in Nyandarua County, Kenya. Int J Agr Manag Devel. 2014;4(2), Art. #246110. https://doi.org/10.22004/ag.econ.246110 [ Links ]
76. Olanya OM, Adipala E, Hakiza JJ, Kedera JC, Ojiambo P Mukalazi JM, et al. Epidemiology and population dynamics of Phytophthora infestans in sub-Saharan Africa: Progress and constraints. Afr Crop Sci J. 2001;9(1):185-194. https://doi.org/10.4314/acsj.v9i1.27638 [ Links ]
77. Oerke EC. Crop losses to pests. J Agr Sci. 2005;144:31-43. https://doi.org/10.1017/S0021859605005708 [ Links ]
 Correspondence:
Correspondence:
Jacquie van der Waals
Email:Jacquie.vdwaals@up.ac.za
Received: 13 Mar. 2020
Revised: 29 June 2020
Accepted: 29 June 2020
Published: 26 Nov. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: None