Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.9-10 Pretoria sep./oct. 2020
http://dx.doi.org/10.17159/sajs.2020/7508
RESEARCH ARTICLE
Forest product harvesting in the Eastern Cape, South Africa: Impacts on habitat structure
Jessica Leaver; Michael I. Cherry
Department of Botany and Zoology, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
The Eastern Cape Province harbours 46% of South Africa's remaining indigenous forest cover, and is one of the country's poorest and least developed provinces. Forest resources thus represent a vital component of rural livelihoods in this region. Consequently, forest management policies aim to balance the needs of resource users with the ecological integrity of forest ecosystems. In a recent study, forest bird ranges were shown to have declined in the Eastern Cape over the past 20 years, despite increases in forest cover over the same time period, indicating that habitat degradation may be driving forest bird losses. Given that harvesting of forest products represents the primary human disturbance in forests in the Eastern Cape today, insight is needed regarding the link between resource use and habitat modification. We report on effects of harvesting of three key forest products - poles, timber and medicinal bark - on habitat structure at the ground, understorey and canopy layers in indigenous forests in the province. Harvest activities had considerable impacts on habitat structure, depending on the nature and extent of harvesting. Bark and timber harvesting resulted in canopy gaps, whereas pole harvesting reduced tree density, resulting in understorey gaps. Overall, harvest activities increased the frequency of canopy disturbance, and density of understorey layer foliage. Unsustainable bark harvesting practices increased the mortality rate of canopy trees, thereby increasing dead wood availability. By providing insight into human-mediated habitat modification in forests of the Eastern Cape, this study contributes to the development of ecologically informed sustainable resource management policies.
SIGNIFICANCE:
• Unregulated harvesting of forest products in state-managed indigenous forests of the Eastern Cape results in habitat modification.
• The nature and extent of habitat modification is dependent on the type and intensity of resource use, indicating that resource use may be sustainably managed.
• Timber and medicinal bark harvesting activities result in canopy disturbances, thereby altering natural canopy gap dynamics, with concomitant impacts on understorey habitat structure.
• Changes in forest habitat structure associated with high levels of resource use are likely to have ramifying effects on forest biodiversity.
Keywords: medical bark harvesting, timber harvesting, pole harvesting, habitat degradation, habitat modification
Introduction
Habitat loss and modification are currently the primary drivers of forest biodiversity loss globally.1 Unlike many parts of Africa, forest cover in the Eastern Cape, which harbours close to half (46%) of South Africa's remaining indigenous forest cover, has increased over the past 20 years2 - an increase which is attributed to the revegetation of previously cultivated fields in response to increasing trends of de-agrarianisation in rural areas3, together with carbon fertilisation4. Thus, while habitat loss appears not to be a major threat to forest biodiversity, degradation has been identified as a major concern.5-9 While much forest degradation in South Africa is attributed to extensive historical logging10, commercial-scale logging has not occurred in indigenous forests in the Eastern Cape for the past 80 years, after being outlawed in 1939 in all but one forest complex, where limited commercial harvesting was re-introduced in 19759. Consequently, informal harvesting of forest products now comprises the primary anthropogenic disturbance in forest habitats in the region5-9 and is largely related to poor socio-economic conditions in the province11. Thus, although forests comprise a mere 2.2% of provincial land cover12, their socioeconomic value is significant, with thousands of rural households dependent on forest resources for subsistence and commercial use13. While forest policies in South Africa aim to develop forests for sustainable use, several studies have reported unsustainable harvest rates occurring across the region7,8,12,14, largely attributed to a decline in the capacity of institutional and traditional structures to regulate resource use15,16. A de facto open-access system thus governs forest resource use in South Africa today, leading to increasing concern that unregulated resource use is degrading forest habitats and compromising the conservation of forest biodiversity.
Long-term harvesting of forest products has significant effects on temperate forest habitats, driving changes in habitat structure and tree species composition, even when occurring at relatively low levels.8,17 Moreover, the ecological impact of resource use depends on the plant part harvested and intensity of use.18 Thus, while grazing of livestock in forests may affect soil quality19 and increase exotic cover20, timber harvesting affects canopy closure, mean tree size and understorey density21,22. The extent to which a resource has been commercialised is also of consequence, as resources used to generate income, particularly in the context of de facto open-access systems, are often harvested more intensely, and frequently unsustainably, and thereby have more profound ecological impacts.12,14,23
Human activities that modify habitat structure, in turn, may influence faunal community assemblage in forests. For example, habitat features at the local scale relate to the occurrence of specific functional traits and community structure in avifaunal populations.24 Consequently, studies have shown forest faunal populations, including amphibians, bats, birds and reptiles, to be sensitive to human-mediated changes in habitat structure, with species specialised in their foraging or microhabitat requirement being particularly sensitive.25-30 Given the critical ecosystem functions provided by forest fauna - including seed dispersal, pest control and pollination31 - human activities that modify habitat structure may have ramifying effects on forest ecosystem functioning.
In a recent study, half of South Africa's forest-dependent bird species were shown to have experienced range declines in the past 20 years, with declines most notable in the Eastern Cape, despite forest cover increases in this region over the same time period.2 This finding suggests that habitat-scale disturbances rather than landscape-scale habitat loss may be driving bird declines in the region. We thus aimed to assess the effects of harvest activities on habitat structure (defined as the composition and arrangement of physical matter at a location32) at the forest scale. Specifically, we examined how different harvest activities modify habitat structure at the canopy, understorey and ground level in six forests, representative of five national forest types, across the Eastern Cape region. Resource use focused on extraction of live biomass from forests, namely understorey trees for poles, canopy trees for timber and crafts, and bark for medicinal purposes, as these represent key resource use types in the region.8
Methods
Study site
The study was conducted in the Eastern Cape Province of South Africa between April and July 2016. Forest cover in this region is discontinuous and highly fragmented (Figure 1).9 Within the study area, six forests were sampled, including five national forest types in the two main zones of forest, i.e. the lowland coastal and scarp forests of the subtropical coastal zone, and the warm-temperate mistbelt forests found on the south to southeastern aspect of inland mountain ranges (Figure 1).9 Specifically, the following forests were sampled: Mqaba (Pondoland Scarp Forest), Manubi (Transkei Coastal Forest) and Ntlaboya (Eastern Cape Dune Forest) of the lowland zone; and Gomo, Nqadu (Transkei Mistbelt Forest) and Pirie (Amathole Mistbelt Forest) of the montane zone (Figure 1). Within the Transkei mistbelt region, forests located within matrixes of timber plantations leased by the state to private companies are often deemed to be better protected than those which are not, so a forest in each category was sampled, with Nqadu associated with privately managed plantations while Gomo was associated with plantations managed by the South African Department of Environment, Forestry and Fisheries (DEFF). Study forests were selected based on their size, protected status, and the proximity of surrounding human settlements. Specifically, selected forests were greater than 150 ha, and unfenced; managed by DEFF; and had rural settlements within 4 km of the forest boundary. While most forest patches in the Eastern Cape are smaller than 150 ha, and prone to negative effects of fragmentation,5,9 study forests were selected to represent larger, more 'intact' forest patches within the region. This is because these forests have larger core areas (i.e. portion of forests unaffected by edge effects) and are thus of high biodiversity value, such that insight into anthropogenic pressures within these forests is of conservation priority.5 Furthermore, given that 70% of forests in the Eastern Cape region are managed by DEFF, and are often associated with communities in close proximity,5 study forests are representative of the current socio-political context within which larger, 'intact' forests in the region occur. Lastly, study forests have endured colonial logging,10 followed by subsistence harvesting in recent times, such that they are representative of the history of human impacts.
Study design
A total of 89 circular plots of 0.04 ha (radius 11.3 m) were sampled, with an average of 15 plots sampled per forest. Points for sampling plots in each forest were selected to represent varying levels of disturbance from resource use, based on detailed discussion and guided walks in each forest with DEFF staff (forest managers and/or forest guards), and local community members, in addition to visual assessment by J.L. of human use in each forest, conducted over two reconnaissance trips prior to sampling. Plot locations were selected to represent the continuum of harvesting disturbances present at each forest, from heavily harvested sites to those with little or no harvesting present. Heavily harvested plots were defined as those where >20% of available stems were harvested for poles, timber or bark. Where 10-20% of available stems were harvested, plots were described as intermediately harvested, while low levels of harvesting were defined as harvest levels of <5% of any resource at the plot level. This non-random sampling approach aimed to provide an objective overview of resource use within each forest, as well as samples from the full range of harvest activities and intensities, against which to investigate habitat changes and, in a linked study, avifaunal responses to resource use.24 Based on this categorisation, 6% (/7=8) of plots overall had no harvesting, while 27%, 30% and 34% had low, intermediate and high levels of harvesting, respectively. A minimum distance of 150 m was maintained between selected plots, and 50 m between plots and the forest edge (i.e. all survey sites were within the forest interior), while distance into the forest interior ranged from 50 m to 900 m.
Data collection
At each plot, microhabitat structure and foliage profile were recorded within three nested circular plots of 0.2 ha (radius of 25.2 m), 0.04 ha (radius of 11.3 m), and 0.01 ha (radius of 5.6 m), respectively. In the 0.2 ha plot, all standing dead trees (henceforth, snags) were recorded by diameter (cm) at 1.3 m above the ground, i.e. diameter at breast height (DBH), and cause of death, i.e. natural or due to bark harvesting. Natural snags include standing trees that have died due to factors other than harvesting, such as wind effects, senescence or disease. In the 0.04-ha plot, the following variables were recorded: DBH of all living stems (>5 cm DBH); percentage canopy cover; mean canopy height; percentage coverage of bare ground, leaf litter, grass cover and herbaceous cover; and foliage density at 0-0.5 m; 0.5-1 m; 1-2 m; 2-5 m; 5-10 m and 10-20 m. Foliage density at each height class was estimated using a 8-m-long telescoping pole and marked at each height interval. The pole was sequentially set up at eight evenly spaced points 11.3 m from the plot centre (i.e. along the 0.04-ha circular plot boundary) and visual estimates of foliage density (as a percentage) at each height class were made from the plot centre. Foliage density scores were further converted into a foliage height diversity index (FHDi) using the Shannon-Weiner Diversity Index formula, as follows:
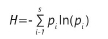
where p. is the proportion of the total foliage which lies in the ;th layer of the chosen horizontal layers. This index thus provided a measure of the vertical heterogeneity at each plot.
A rangefinder was used to assist with estimates of foliage density beyond the length of the telescoping pole, as well as to estimate mean canopy height at each plot. Abundance of coarse woody debris was measured based on the number of grounded dead logs (diameter >10 cm; length >1.5 m). Harvest activities were also measured in the 0.04-ha plot: stumps, i.e. trees harvested for poles or timber, were counted and diameter measured. Based on diameter, stumps were categorised as pole (5-19.9 cm diameter) or timber (>20 cm diameter) harvesting, after Obiri et al.8 Trees harvested for medicinal bark were recorded using DBH and extent of bark removal on individual trees up to 3 m on the tree stem (scored 1 - 6 based on percentage of bark removed, where 1 = 1-10%; 2 = 11-25%; 3 = 26-50%; 4 = 51-75%; 5 = ringbarked to any extent %; 6 = total ringbark, where ringbarked stems are those where bark has been removed from around the full circumference of the stem, after Cunningham33. In 0.01-ha plots, sapling (stem diameter 1-5 cm) abundance was recorded.
Data analyses
Pole and timber harvest intensities were calculated per plot for each size class based on the accumulated harvestable stems (stumps plus standing stems) as follows:
Tree harvest indexj = number stumpsj / (number stumpy + number stemsj),
where j represents the size class being assessed.
Bark harvest intensity was assessed based on a bark harvest index derived from summed bark removal scores assigned to individual bark-harvested trees, calculated at each plot, as follows:
Bark harvest index = summed bark removal score/no. individuals bark harvested
Harvest effects on forest structure were investigated using (1) linear mixed models for habitat variables measured on a continuous scale; (2) generalised linear mixed models for habitat variables measured as counts, and (3) beta regression for habitat variables measured as per cent cover. A mixed-modelling approach was used in all cases to account for the nested study design, with sample forests included as a random effect throughout the analysis to account for plots being nested within study forests. Separate models were used to assess the response of each habitat feature to harvesting, with pole, timber and bark harvest indices included as the explanatory variables in addition to, and in all possible combinations of two-way interactions with one another. The two-way interaction between timber and bark was not included, as bark and timber harvesting were seldom recorded within a single plot. Spearman's rank correlation test was used to test for significant correlations between harvest variables, to avoid issues related to multicollinearity. The test showed the harvesting variables to be uncorrelated (-0.4 < r > 0.4). Habitat variables measured as counts (tree, snag, sapling and grounded log abundance) were modelled using generalised linear mixed models, with a Poisson distribution and log-link. Response variables measured as per cent cover were converted to proportions and modelled using a beta regression. Model assumptions were verified by plotting residuals versus fitted values, and versus each covariate in the model. Where interaction terms did not improve model strength based on Akaike information criterion (AIC) values, they were removed from the final model. Data from Pirie were not included in these analyses as minimal harvesting was recorded in this forest, and these analyses aimed to assess effects in disturbed forests.
Results
Of the 18 measured structural variables, 12 were significantly impacted by harvesting activities, with responses dependent on the type and intensity of resource use (Figures 2-5; Table 1). Furthermore, the two-way interaction between pole and timber harvesting was shown to affect structural habitat heterogeneity (Figure 5; Table 1). Five habitat features were unaffected by harvest activities: canopy height; mean DBH; lower-understorey (0.5-1 m) foliage density; mid-storey (2-5 m) foliage density; and canopy layer (5-10 m) foliage density (Table 1).
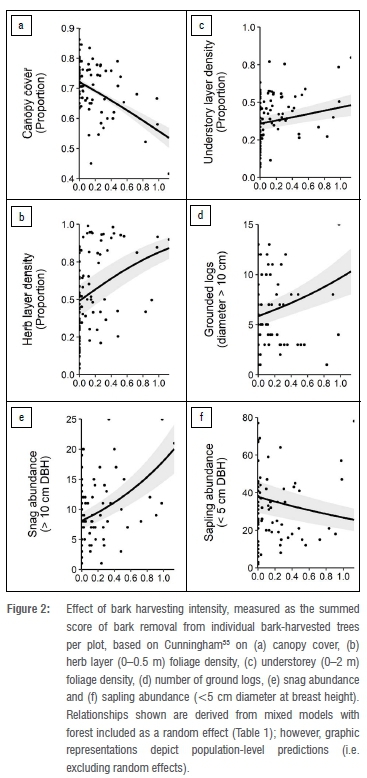


Bark harvesting
Increasing bark harvesting intensity negatively affected canopy cover and sapling abundance (<5 cm DBH), while herb layer (0-0.5 m) foliage density, overall understorey (0-2 m) foliage density, number of grounded logs and snag abundance (i.e. standing dead trees; >10 cm DBH) increased with bark harvesting intensity (Figure 2; Table 1).
Pole harvesting
Increasing pole harvesting intensity resulted in declines in tre abundance, sapling abundance, basal area per hectare and leaf litte cover. Conversely, herb layer (0-0.5 m) foliage density and herb covei increased with increasing pole harvesting intensity (Figure 3; Table 1).
Timber harvesting
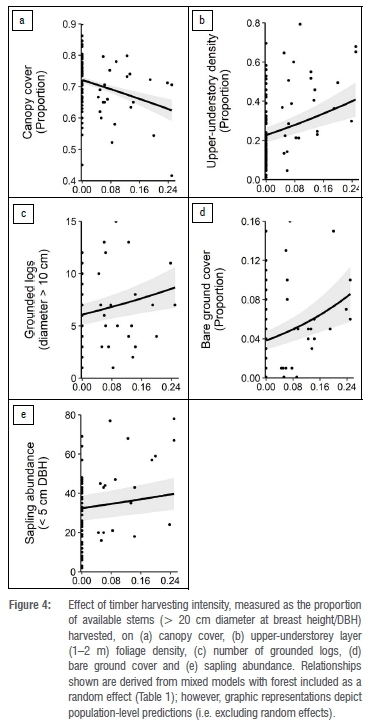
Increasing timber harvesting intensity resulted in a decline in canopy cover, while a positive relationship was found between the extent of timber harvesting and upper-understorey layer (1-2 m) foliage density, number of grounded logs, bare ground cover, and sapling abundance (Figure 4; Table 1).
Interacting harvest effects
Foliage height diversity index (FHDI) was negatively affected by the interaction between timber and pole harvesting. Specifically, FHDI increased in response to increasing timber harvest intensities where pole harvest levels were low (i.e. 5% of available stems), but declined in response to increasing timber harvest levels where pole harvest intensities were high (i.e. 20% of available stems; Figure 5; Table 1).
Discussion
The findings of this study show that unregulated harvesting of medicinal bark, poles and timber results in multiple structural modifications to forest habitats in state forests of the Eastern Cape. Specifically, bark and timber harvesting created canopy gaps, while pole harvesting created understorey gaps, with variable implications for ground and understorey layer microhabitat structure, respectively. Our findings are thus in agreement with those of previous studies which have shown significant impacts of resource use on forest habitat structure in South Africa.7,17,34 While the long-term ecological effects of harvest-mediated habitat modification are largely unknown34, they represent changes to the natural disturbance regime, and are thus likely to have ramifying effects on forest patterns and processes35, and faunal populations26. However, results of this study show that the extent of habitat modification is dependent on the nature and intensity of harvesting, and that different harvest activities, where occurring together at a fine spatial scale, may have interactive effects on habitat structure.
Bark harvesting
While several studies have examined the ecological implications of bark harvesting at individual and population levels36-39, concurrent impacts on habitat structure have been relatively under-studied. Increasing bark harvesting intensities resulted in a decline in canopy cover and sapling abundance, and an increase in herb layer (0-0.5 m) and understorey layer (0-2 m) foliage density, grounded logs, and snag density (i.e. standing dead trees). These habitat modifications are the result of excessive bark removal from tree stem circumferences, preventing the transport of photosynthetic products to tree roots, leading to root loss or death, thereby driving declines in canopy health and potential tree mortality.39,40 This creates gaps in the forest canopy, thereby increasing light availability to the forest floor such that ground and understorey layer foliage density increases. Over time, bark-harvested trees die and become snags which then decay, and dead branches drop to the ground, increasing the amount of grounded dead wood.
The substantial habitat-scale impacts of bark harvesting are perhaps best demonstrated by the close to 50% mean increase in snag abundance recorded across the four forests which experienced the highest levels of bark harvesting (Gomo, Manubi, Nqadu and Ntlaboya; Table 2), and associated increases in the number of grounded logs. While the ecological implications of the collection of dead wood for fuelwood from indigenous forests in South Africa have been cause for concern6,41, with negative effects on cavity-nesting mammals and birds42, few studies have highlighted the creation of dead wood in forests due to bark harvesting. The important ecological role of dead wood has long been recognised by ecologists.43 However, the value to forest taxa of harvest-mediated dead wood creation, at the cost of living canopy trees of a select few species and canopy cover, is currently unknown but likely to be multifaceted and taxon dependent.
Pole harvesting
Unlike bark and timber harvesting, pole harvesting did not affect the forest canopy, but resulted in a decline in basal area and stem density of trees and saplings. This finding reflects the nature of pole harvesting wherein multiple understorey trees are harvested at a fine spatial scale, thereby creating gaps in the understorey, as shown by Boudreau and Lawes17. Despite the lack of any major canopy disturbances, as pole harvesting intensity increased, multiple understorey layer features were affected: foliage density at the herb layer (0-0.5 m) and herb cover increased, while leaf litter cover declined. Thus, while declines in basal area, tree and sapling density were a direct effect of harvesting, altered understorey and ground layer conditions are likely to be an indirect response driven by increases in light availability and soil moisture content due to a reduction in tree density.17,44
Although beyond the scope of this study, increased herb cover in understorey gaps caused by pole harvesting may supress seedling establishment.44 Thus pole harvesting has the potential to alter not only structural habitat features, but also seedling recruitment, and therefore the maintenance of forest tree diversity. As indicated, changes in understorey conditions are dependent on the harvest intensity. Similarly, changes in seedling recruitment caused by pole harvesting are determined by understorey gap size,44 with larger gaps causing a potential successional shift in seedling recruitment. However, Boudreau and Lawes17 showed that under low harvesting intensity (11.6% of available pole-sized stems), pole harvesting did not negatively affect the long-term maintenance of tree diversity, suggesting that rates of pole harvesting measured in the current study (regional average of 7% of available pole-sized stems; Table 2) may not adversely affect tree species composition. However, modifications to understorey layer conditions may affect forest fauna. For example, leaf litter cover is a critical habitat for many forest invertebrates.45
Timber harvesting
At the habitat scale, timber harvesting resulted in canopy gaps through the selective extraction of canopy trees, driving an increase in upper-understorey (1-2 m) foliage density, sapling abundance, and bare ground cover. Furthermore, timber harvesting increased the number of grounded logs as a result of the large portions of harvested trees that are left in the forest after only the main stem of the harvested tree is removed. Furthermore, increases in dead wood may be associated with incidental tree damage associated with canopy-tree felling. Similar structural responses to selective timber harvesting have been shown by studies in tropical forests.21,22,46 The creation of canopy gaps in forest systems represents a vital component of natural forest disturbance regimes, given their important role in promoting regeneration, tree diversity and habitat heterogeneity.22,47-50 The gap phase represents a time of rapid plant growth49, attributed to increased resource availability and/or decreased resource competition51, demonstrated in the current study by the increased foliage density in the understorey. Furthermore, habitat conditions in canopy gaps compared to those in intact forest have been shown to differ significantly with respect to microclimate51,52, detritus27, productivity53, and plant species composition47,49. Consequently, multiple forest taxa, including birds, reptiles and invertebrates, have been shown to distinguish between canopy gap and intact habitats.22,46,50,54,55 This finding suggests that timber harvest activities, and concomitant habitat modifications, are likely to have ramifying effects on forest biodiversity.
The degree to which timber harvest activities affect forest biodiversity, beyond direct population-level impacts on target species, is likely to be dependent on the frequency of the disturbance, and the extent of incidental habitat damage. With regard to the former, selective harvesting practices in the Eastern Cape are likely to be less destructive than mechanised selective logging operations, which cause considerable damage through clearing for roads and log storage sites.46 Informal timber harvesting in the Eastern Cape is generally un-mechanised, with felled timber split in the forest, and carried out on foot along narrow footpaths (J.L. personal observation). The frequency of disturbance is thus likely to be more cause for concern, as the harvest-driven increase in the proportion of forest-under-gap conditions is likely to have implications for ecosystem functioning.35
Interacting harvest effects
The positive relationship between foliage height diversity and biodiversity has been well established, and is based on niche theory which predicts that a greater diversity of habitats supports a greater diversity of species.56 The decline in foliage height diversity in response to the interaction between pole and timber harvesting activities shown in this study indicates that, where these harvest types occur together at high rates, structural habitat complexity is reduced, and likely to negatively affect biodiversity at the habitat scale. This finding suggests that management strategies should limit the extent to which pole and timber harvesting activities occur together, and reduce the damage/lopping of smaller, non-target trees often associated with timber harvesting activities (J.L. personal observation), so as to maintain habitat heterogeneity in harvested areas.
Conclusion
The findings of this study indicate that resource use from state forests in the Eastern Cape has a significant impact on forest structure, although the nature and extent of the impact is dependent on the type and intensity of resource use. These results should be viewed within the context of forests that have a long history of human exploitation, from extensive colonial era logging to current subsistence and informal commercial harvesting of multiple forest products. However, the effects of long-term human exploitation are likely to have affected the current condition of all sampled forests, such that the findings of this study are indicative of habitat responses to more recent resource use disturbances. Similarly, while habitat structure is modified by random natural disturbances, such as windfalls, lightning or fire-spotting, which are vital components of natural disturbance-recovery regimes that maintain forest dynamics, resource use represents disturbances that occur in addition to these natural disturbances under which forest species have adapted, and thus may affect ecosystem persistence and resilience. Further research is needed to determine specific levels of resource use that can be sustained without negatively affecting forest biodiversity. Specifically, research regarding the impact of resource use on forest taxa at multiple trophic levels is needed to provide insight into ecosystem-wide implications of harvest-mediated habitat modification, and to contribute to the development of ecologically informed forest management policies.
Acknowledgements
We thank Welile Kedama of the South African Department of Environment, Forestry and Fisheries for granting permission to conduct research within state forests of the Eastern Cape, and to all Forestry personnel who assisted with fieldwork. C.J. Geldenhuys is thanked for meaningful discussion that helped guide this study. We are grateful to A. Wannenburgh for providing input on the first draft of this manuscript, and for the study site map. R. Duker is thanked for assistance in developing the data collection methodology. An anonymous reviewer is thanked for comments on the first draft of this manuscript. James Pryke is thanked for his comments on the thesis chapter from which this manuscript was derived, which have improved the manuscript.
Competing interests
We declare that there are no competing interests.
Data availability
The data sets generated and/or analysed during the current study are available from the corresponding author on request.
Authors' contributions
J.L. designed the study methodology, conducted data collection and data analysis, and wrote the initial draft of the manuscript. M.I.C. conceptualised the study, acquired funding, and contributed extensively to the writing of the manuscript revision.
References
1. Arroyo-Rodríguez V Melo FPL, Martínez-Ramos M, Bongers F, Chazdon RL, Meave JA, et al. Multiple successional pathways in human-modified tropical landscapes: New insights from forest succession, forest fragmentation and landscape ecology research: Multiple successional pathways. Biol Rev. 2017;92:326-340. https://doi.org/10.1111/brv.12231 [ Links ]
2. Cooper TJG, Wannenburgh AM, Cherry MI. Atlas data indicate forest dependent bird species declines in South Africa. Bird Conserv Int. 2017;27:337-354. https://doi.org/10.1017/S095927091600040X [ Links ]
3. Shackleton CM, Mograbi PJ, Drimie S, Fay D, Hebinck P Hoffman MT, et al. Deactivation of field cultivation in communal areas of South Africa: Patterns, drivers and socio-economic and ecological consequences. Land Use Policy. 2019;82:686-699. https://doi.org/10.1016/jJandusepoL2019.01.009 [ Links ]
4. Bond WJ, Midgley GF. A proposed CO2-controlled mechanism of woody plant invasion in grasslands and savannas. Glob Chang Biol. 2000;6:865-869. https://doi.org/10.1046/j.1365-2486.2000.00365.x [ Links ]
5. Berliner DD. Systematic conservation planning for South Africa's forest biome: An assessment of the conservation status of South Africa's forests and recommendations for their conservation [PhD thesis]. Cape Town: University of Cape Town; 2009. [ Links ]
6. Castley JG, Kerley GIH. The paradox of forest conservation in South Africa. For Ecol Manage. 1996;85:35-16. https://doi.org/10.1016/S0378-1127(96)03748-6 [ Links ]
7. Hoppe-Speer SCL, Adams JB, Bailey D. Present state of mangrove forests along the Eastern Cape coast, South Africa. Wetl Ecol Manag. 2105;23:371-383. https://doi.org/10.1007/s11273-014-9387-x [ Links ]
8. Obiri J, Lawes M, Mukolwe M. The dynamics and sustainable use of high-value tree species of the coastal Pondoland forests of the Eastern Cape Province, South Africa. For Ecol Manage. 2002;166:131-148. https://doi.org/10.1016/S0378-1127(01)00665-X [ Links ]
9. Von Maltitz G, Mucina L, Geldenhuys C, Lawes MJ, Eeley HAC, Adie H, et al. Classification system for South African indigenous forests: An objective classification for the Department of Water Affairs and Forestry. Pretoria: CSIR; 2003. [ Links ]
10. King NL. The exploitation of indigenous forests in South Africa. S Afr J Bot. 1941;48:455-480. https://doi.org/10.1080/03759873.1941.9631098 [ Links ]
11. Paumgarten F, Shackleton CM. The role of non-timber forest products in household coping strategies in South Africa: The influence of household wealth and gender. Popul Environ. 2011;33:108-131. https://www.jstor.org/stable/41487565 [ Links ]
12. Dold AP, Cocks ML. The trade in medicinal plants in the Eastern Cape Province, South Africa. S Afr J Sci. 2002;98:589-597. [ Links ]
13. Shackleton C, Shackleton S. The importance of non-timber forest products in rural livelihood security and as safety nets: A review of evidence from South Africa. S Afr J Sci. 2004;100:658-664. [ Links ]
14. Geldenhuys CJ. Bark harvesting for traditional medicine: from illegal resource degradation to participatory management. Scand J For Res. 2004;19:103-115. https://doi.org/10.1080/14004080410034182 [ Links ]
15. Obiri JAF, Lawes MJ. Attitudes of coastal-forest users in Eastern Cape Province to management options arising from new South African forest policies. Environ Conserv. 2002;29:519-529. https://doi.org/10.1017/S0376892902000371 [ Links ]
16. Von Maltitz GP Shackleton S. Use and management of forests and woodlands in South Africa: Stakeholders, institutions and processes from past to present. In: Lawes MJ, Eeley HAC, Shackleton CM, Geach BGS, editors. Indigenous forests and woodlands in South Africa: Policy, people and practice. Pietermaritzburg: University of KwaZulu-Natal Press; 2004. p. 109-112. [ Links ]
17. Boudreau S, Lawes MJ. Small understorey gaps created by subsistence harvesters do not adversely affect the maintenance of tree diversity in a subtropical forest. Biol Conserv. 2005;126:279-286. https://doi.org/10.1016/j.biocon.2005.06.004 [ Links ]
18. Ticktin T. The ecological implications of harvesting non-timber forest products: Ecological implications of non-timber harvesting. J Appl Ecol. 2004;41:11-21. https://doi.org/10.1111/j.1365-2664.2004.00859.x [ Links ]
19. Belsky AJ, Blumenthal DM. Effects of livestock grazing on stand dynamics and soils in upland forests of the interior west. Conserv Biol. 1997;11:315-327. https://doi.org/10.1046/j.1523-1739.1997.95405.x [ Links ]
20. Yates CJ, Norton DA, Hobbs RJ. Grazing effects on plant cover, soil and microclimate in fragmented woodlands in south-western Australia: Implications for restoration. Austral Ecol. 2000;25:36-47. https://doi.org/10.1111/j.1442-9993.2000.tb00005.x [ Links ]
21. Sekercioglu CH. Effects of forestry practices on vegetation structure and bird community of Kibale National Park, Uganda. Biol Conserv. 2002;107:229-240. https://doi.org/10.1016/s0006-3207(02)00097-6 [ Links ]
22. Wunderle JM, Henriques LMP Willig MR. Short-term responses of birds to forest gaps and understory: an assessment of reduced-impact logging in a lowland Amazon forest. Biotropica. 2006;38:235-255. https://doi.org/10.1111/j.1744-7429.2006.00138.x [ Links ]
23. Fashing PJ. Mortality trends in the African cherry (Prunus africana) and the implications for colobus monkeys (Colobus guereza) in Kakamega Forest, Kenya. Biol Conserv. 2004;120:449-459. https://doi.org/10.1016/j.biocon.2004.03.018 [ Links ]
24. Leaver J, Mulvaney J, Ehlers Smith DA, Ehlers Smith YC, Cherry MI. Response of bird functional diversity to forest product harvesting in the Eastern Cape, South Africa. For Ecol Manage. 2019;445:82-95. https://doi.org/10.1016/j.foreco.2019.04.054 [ Links ]
25. Asefa A, Davies AB, McKechnie AE, Kinahan AA, Van Rensburg BJ. Effects of anthropogenic disturbance on bird diversity in Ethiopian montane forests. Condor. 2017;119:416-430. https://doi.org/10.1650/CONDOR-16-8L1 [ Links ]
26. Bawa KS, Seidler R. Natural forest management and conservation of biodiversity in tropical forests. Conserv Biol. 1998;12:46-55. https://doi.org/10.1111/j.1523-1739.1998.96480.x [ Links ]
27. Cleary DFR, Boyle TJB, Setyawati T, Anggraeni CD, Loon EEV, Menken SBJ. Bird species and traits associated with logged and unlogged forest in Borneo. Ecol Appl. 2017;17:1184-1197. https://doi.org/10.1890/05-0878 [ Links ]
28. Gardner CJ, Jasper LD, Eonintsoa C, Duchene JJ, Davies ZG. The impact of natural resource use on bird and reptile communities within multiple-use protected areas: Evidence from sub-arid Southern Madagascar. Biodivers Conserv. 2016;25:1773-1793. https://doi.org/10.1007/s10531-016-1160-4 [ Links ]
29. Presley SJ, Willig MR, Wunderle JM, Saldanha LN. Effects of reduced-impact logging and forest physiognomy on bat populations of lowland Amazonian forest: Bat responses to logging in Amazonia. J Appl Ecol. 2007;45:14-25. https://doi.org/10.1111/j.1365-2664.2007.01373.x [ Links ]
30. Menon T, Sridhar H, Shahabuddin G. Effects of extractive use on forest birds in Western Himalayas: Role of local and landscape factors. For Ecol Manage. 2019;448:457-465. https://doi.org/10.1016/j.foreco.2019.06.033 [ Links ]
31. Sekercioglu CH. Increasing awareness of avian ecological function. Trends Ecol Evol. 2006;21:464-471. https://doi.org/10.1016/j.tree.2006.05.007 [ Links ]
32. Byrne LB. Habitat structure: A fundamental concept and framework for urban soil ecology. Urban Ecosyst. 2007;10:255-274. https://doi.org/10.1007/s11252-007-0027-6 [ Links ]
33. Cunningham AB. African medicinal plants: Setting priorities at the interface between conservation and primary health care. People and Plants Working Paper 1. Paris: UNESCO; 1993. [ Links ]
34. Lawes MJ, Griffiths ME, Boudreau S. Colonial logging and recent subsistence harvesting affect the composition and physiognomy of a podocarp dominated Afrotemperate forest. For Ecol Manage. 2007;247:48-60. https://doi.org/10.1016/j.foreco.2007.04.012 [ Links ]
35. Royo AA, Carson WP. On the formation of dense understory layers in forests worldwide: Consequences and implications for forest dynamics, biodiversity, and succession. Can J For Res. 2006;36:1345-1362. https://doi.org/10.1139/x06-025 [ Links ]
36. Chungu D, Muimba-Kankolongo A, Roux J, Malambo F. Bark removal for medicinal use predisposes indigenous forest trees to wood degradation in Zambia. Southern Forests. 2007;69:157-163. https://doi.org/10.2989/SHFJ.2007.69.3.4.354 [ Links ]
37. Gaoue OG, Ticktin T. Patterns of harvesting foliage and bark from the multipurpose tree Khaya senegalensis in Benin: Variation across ecological regions and its impacts on population structure. Biol Conserv. 2007;137:424-436. https://doi.org/10.1016/j.biocon.2007.02.020 [ Links ]
38. Guedje NM, Zuidema PA, During H, Foahom B, Lejoly J. Tree bark as a non-timber forest product: The effect of bark collection on population structure and dynamics of Garcinia lucida Vesque. For Ecol Manage. 2007;240:1-12. https://doi.org/10.1016/j.foreco.2006.09.029 [ Links ]
39. Vermeulen W, Geldenhuys C, Esler K. Response of Ocotea biilata, Curtisia dentata and Rapanea melanophloeos to medicinal bark stripping in the southern Cape, South Africa: Implications for sustainable use. Southern Forests. 2012;74:183-193. https://doi.org/10.2989/20702620.2012.717384 [ Links ]
40. Cunningham AB. Applied ethnobotany: People, wild plant use and conservation. London: Earthscan Publications; 2001. [ Links ]
41. Berry MG, Robertson BL, Campbell EE, Bredenkamp GJ. Impact of cutting and collecting of firewood associated with informal settlement in the southeastern Cape coastal zone. S Afr J Bot. 2005;71:179-190. https://doi.org/10.1016/S0254-6299(15)30131-9 [ Links ]
42. Du Plessis MA. The effects of fuelwood removal on the diversity of some cavity-using birds and mammals in South Africa. Biol Conserv. 1995;74:77-82. https://doi.org/10.1016/0006-3207(95)00016-w [ Links ]
43. Zhou L, Dai L, Gu H, Zhong L. Review on the decomposition and influence factors of coarse woody debris in forest ecosystem. J For Res. 2007;18:48-54. https://doi.org/10.1007/s11676-007-0009-9 [ Links ]
44. Louw SL. The effect of the spatial scale of tree harvesting on woody seedling establishment and tree dynamics at Ongoye Forest Reserve. Pietermaritzburg: University of KwaZulu-Natal; 2010. [ Links ]
45. Banks JE, Jackson C, Hannon LM, Thomas CM, Baya A, Njoroge L. The cascading effects of elephant presence/absence on arthropods and an Afrotropical thrush in Arabuko-Sokoke Forest, Kenya. Afr J Ecol. 2010;48:1030-1038. https://doi.org/10.1111/j.1365-2028.2010.01211.x [ Links ]
46. Thiollay JM. Influence of selective logging on bird species diversity in a Guianan rain forest. Conserv Biol. 1992;6:47-63. https://doi.org/10.1016/0006-3207(93)90360-d [ Links ]
47. Kneeshaw DD, Bergeron Y Canopy gap characteristics and tree replacement in the southeastern boreal forest. Ecology. 1998;79:783-794. https://doi.org/10.2307/176578 [ Links ]
48. Obiri JAF, Lawes MJ. Chance versus determinism in canopy gap regeneration in coastal scarp forest in South Africa. J Veg Sci. 2004;15:539-547. https://doi.org/10.1111/j.1654-1103.2004.tb02293.x [ Links ]
49. Brokaw NVL. Gap-phase regeneration in a tropical forest. Ecology. 1985;66:682-687. https://doi.org/10.2307/1940529 [ Links ]
50. Levey DJ. Tropical wet forest treefall gaps and distributions of understory birds and plants. Ecology. 1988;69:1076-1089. https://doi.org/10.2307/1941263 [ Links ]
51. Canham CD. An index for understory light levels in and around canopy gaps. Ecology. 1988;69:1634-1638. https://doi.org/10.2307/1941664 [ Links ]
52. Gray AN, Spies TA, Easter MJ. Microclimatic and soil moisture responses to gap formation in coastal Douglas-fir forests. Can J For Res. 2002;32:332-343. https://doi.org/10.1139/x01-200 [ Links ]
53. Prescott CE. The influence of the forest canopy on nutrient cycling. Tree Physiol. 2002;22:1193-1200. https://doi.org/10.1093/treephys/22.15-16.1193 [ Links ]
54. Richards LA, Windsor DM. Seasonal variation of arthropod abundance in gaps and the understorey of a lowland moist forest in Panama. J Trop Ecol. 2007;23:169-176. https://doi.org/10.1017/S0266467406003907 [ Links ]
55. Vitt LJ, Avila-Pires TCS, Caldwell JP Oliveira VRL. The impact of individual tree harvesting on thermal environments of lizards in Amazonian rain forest. Conserv Biol. 1998;12:654-664. https://doi.org/10.1111/j.1523-1739.1998.96407.x [ Links ]
56. MacArthur RH, MacArthur JW. On bird species diversity. Ecology. 1961;42:594-598. https://doi.org/10.2307/1932254 [ Links ]
 Correspondence:
Correspondence:
Jessica Leaver
Email: jes.leaver@gmail.com
Received: 22 Oct. 2019
Revised: 18 Mar. 2020
Accepted: 02 Apr. 2020
Published: 29 Sep. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: South Africa National Research Foundation through the Foundational Biodiversity Information Programme (FBIP 98871)














