Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.9-10 Pretoria Sep./Oct. 2020
http://dx.doi.org/10.17159/sajs.2020/7846
RESEARCH ARTICLE
Nutrient uptake, yield and taste of oilseed rape (Brassica napus L.) and soil chemical properties following amendment with uncomposted and composted tobacco waste and cattle manure
Nothando DunjanaI; Rebecca ZengeniI; Charity PisaI, II; Menas WutaIII; Pardon MuchaonyerwaI
ISchool of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIDepartment of Natural Resources Management, Marondera University of Agricultural Sciences and Technology, Marondera, Zimbabwe
IIIDepartment of Soil Science and Agricultural Engineering, University of Zimbabwe, Harare, Zimbabwe
ABSTRACT
The inadequacy of the nutrient supply of most tropical and sub-tropical soils may be curbed through organic material recycling, thus reducing the need for mineral fertiliser use. To promote tobacco waste recycling in a smallholder food-cash crop production system, nutrient uptake, dry biomass yield and taste of oilseed rape (Brassica napus L.) and soil chemical properties were determined on a sandy loam soil under field conditions. The experiment was a randomised complete block design with three blocks and eight treatments, namely, control (no amendment), mineral fertiliser (121, 30.8, 24.6 kg/ha N, P and K, respectively), uncomposted tobacco leaf scrap (TSC) and compost of TSC and cattle manure (TSC-CM) at 5, 20 and 40 t/ha. N, P and K uptake and dry biomass yield of oilseed rape were higher (p<0.05) than control with 40 t/ha TSC-CM and mineral fertiliser application at 3 weeks after transplanting (WAT), while significant improvements with TSC were observed from 5 WAT. Mineral N, extractable P and exchangeable K were higher than control with TSC-CM at 20 t/ha and 40 t/ha at 3 WAT, and higher with TSC at 9 WAT. Soil organic carbon was more improved with TSC application than TSC-CM at 9 WAT. Organoleptic testing revealed an intensely bitter taste in oilseed rape with mineral fertiliser, 20 t/ha and 40 t/ha TSC at 3 WAT, although it diminished with time. A trade-off of nutrient uptake, yield, taste of oilseed rape and soil properties improvement is attainable with application of TSC-CM at 40 t/ha, while if composting is not feasible, TSC application at 20 t/ha is a viable alternative. Thus, judicious utilisation of tobacco waste offers a viable solution to the problem of low soil fertility on sandy soils and can reduce the need for mineral fertiliser use, while promising sustainable soil management.
SIGNIFICANCE:
• The study underlines the importance of recycling of organic materials as a viable, environmentally safe and low-cost soil fertility management practice.
• The study presents pragmatic practices that may be adopted so as to optimise the benefits of use of tobacco waste on food-cash crop smallholder farms, given the increasing popularity of such farming systems in Africa.
Keywords: compost, organic amendment, organoleptic test, soil organic carbon
Introduction
The cultivation of green leafy vegetables in smallholder farms across sub-Saharan Africa is widespread1,2 and essential for crop diversification. Crop diversification increases farm productivity, income and food variety while cushioning smallholder farmers against food price and climate shocks.3 The leaf biomass of Brassica provides fibre, vitamins, macronutrients, micronutrients and antioxidants to rural populations, which supplements nutrients obtained from the predominantly cereal-based staple diets.4 In the southern African context, like in Zimbabwe, oilseed rape (Brassica napus L.) is grown for its leaves and is one of the primary vegetable crops due to its high profitability, all-year round production and its short lifespan as compared to other leafy vegetables.5 However, oilseed rape has high nutrient requirements which can be met through mineral and/or organic fertiliser applications to attain optimum yield.6 Resultantly, continuous cropping without sufficient nutrient replenishment reduces productivity and poses a serious threat to the sustainable use of soil.
Soil infertility characterised by nutrient deficiencies, soil acidity and declining soil organic matter has been recognised as the fundamental biophysical constraint to crop productivity among smallholder farms,7 thus various efforts have been directed towards addressing this problem. Ganya et al.5 showed that nitrogen fertilisation at increasing rates significantly increases oilseed rape biomass yield, while leguminous trees' biomass yields have been tested in isolation and in combination with N-fertiliser with positive results.8,9 However, the prohibitive prices of mineral fertilisers10 and risk of soil acidification11 may discourage its recommendation. Additionally, Maereka et al.12 found a correlation between nitrate accumulation in Brassica juncea leaves and N-fertilisation, which resulted in a bitter taste of the vegetables, which raises the question of the suitability of intensive mineral fertiliser application on quality of vegetables. On the other hand, the adoptability of promising organic amendments including animal manures and agroforestry technologies is constrained by factors including unavailability in adequate quantities as well as variable quality.8,13,14 Accordingly, site-specific soil fertility management interventions are key in fostering relevance and adoptability.
Soil amelioration with tobacco waste (Nicotiana tabacum L.) has been shown to be beneficial in improving the productivity of various horticultural crops including lettuce (Lactuca sativa), spinach (Spinacia oleracea) and tomato (Lycopersicon esculentum).15-17Further, soil biological and physical properties have also been enhanced by tobacco waste application in other parts of the world.18,19 Tobacco waste nutrient levels in the range 1.97-2.38% N, 0.21-0.50% P 0.32-1.03% K and 38-41% C have been documented,19,20 pointing to its potential value as a nutrient resource. Meanwhile, tobacco production in smallholder farming systems has been on the rise in sub-Saharan Africa, particularly in leading tobacco-producing countries, such as Tanzania, Zimbabwe and Malawi, due to the policy reforms in the tobacco value chain and land resettlement initiatives.21,22 For example, in Zimbabwe, on average, production of tobacco occurs on 1.3-ha plots on the resettlement smallholder farms.23 Although tobacco production has been on the decrease in developed parts of the world, the economic benefits are prioritised over the health risks in developing countries.24 Subsequently, tobacco production creates a niche for organic waste generation in the form of tobacco leaf scrap (TSC). Tobacco leaf scrap is generated on-farm during harvesting, grading and packaging of the tobacco leaf. As such, TSC can be beneficially utilised to augment the limited organic resources in smallholder farming systems for the production of food crops, including oilseed rape.
Composting of tobacco waste with different organic materials such as cattle, pig and poultry manure has been widely employed as an environmentally friendly method of tobacco waste handling in tobacco processing industries, while generating a nutrient rich soil ameliorant.18,25 Composting ensures detoxification of nicotine before soil amelioration18,26,27 as a high concentration of nicotine is linked to depressed microbial activity27. However, labour constraints in smallholder food-tobacco producing farming areas28 as well as limited access to animal manures may deter adoption of composting technologies. On the other hand, other studies have attested to the positive benefits of direct application of tobacco waste such as favourable electrical conductivity (EC) of soil solution, increased microbial activity due to high carbon substrate, high macronutrient supply, insecticidal properties and improved soil aggregate stability due to phenolic properties.2931 Further, Nota et al.32 and Seckar et al.33 posit that the risk of nicotine toxicity with raw tobacco application is low due to rapid degradation in soil. As such, there is scope for evaluation of the potential of use of tobacco waste as a soil ameliorant so as to develop recommendations that are relevant across diverse smallholder farms.
Additionally, despite the extensive literature on tobacco waste use in vegetable production, no organoleptic (taste) studies relating tobacco waste application to vegetable taste have been done, yet the study by Maereka et al.12 attests to the key role that fertilisation plays on vegetable quality. Accordingly, given the limited access to fertiliser inputs and paucity of information on the use of tobacco waste under smallholder systems, we sought to investigate the comparative nutrient uptake, yield and taste of oilseed rape and selected chemical parameters of soil after amendment with uncomposted TSC and compost of TSC and cattle manure (TSC-CM) at increasing rates relative to recommended mineral fertiliser application rate. We hypothesised that TSC and TSC-CM would increase nutrient uptake and yield of oilseed rape and fertility status of the soil relative to the control and mineral fertiliser treatments. We further hypothesised that TSC would increase nitrogen and possibly nicotine uptake causing bitterness in oilseed rape relative to control, mineral fertiliser and TSC-CM treatments.
Materials and methods
Site description
The field experiment was conducted at the Marondera University of Agricultural Sciences and Technology (MUAST) farm, Mashonaland East Province in Zimbabwe. The MUAST farm lies at 18o21'56" S and 31o42'19" E in Svosve farming area. The area is typically characterised by hot, wet summers and cold, dry winters. An annual rainfall of 7001000 mm is received between the months October to April, marking the wet season, while winter season is experienced between May and August. The dominant soil type is the granite-derived sandy soils (Arenosol),34 which is typically highly leached and infertile.
Chemical composition of the organic amendments used in the experiment
Tobacco leaf scrap used for the experiment was collected from MUAST tobacco curing barns, grading and packaging sheds, while cattle manure (CM) was carefully swept off the top layers of the MUAST farm cattle pen. The TSC-CM was composted by mixing TSC and CM at a 50:50 w/w basis in 1-m3 perforated wooden bins. Aeration of compost was done weekly by turning of compost and moisture content was maintained at 60%. After 69 days, compost was deemed mature, with compost temperature matching ambient temperature. The chemical composition of TSC, CM and TSC-CM is given in Table 1.

Field experimentation
Nursery of oilseed rape (var. Hobson) was prepared in October 2017 by drilling seeds into a fine tilth seedbed, and lightly covering with soil, grass mulch and watering. Watering was done using a watering can fitted with a rose sprinkler every 2 or 3 days to ensure adequate moisture. Mulch was removed a week after seedling emergence. Five days prior to transplanting, seedlings were treated with Malathion 50% E.C and Carbaryl 50 WP to control aphids and cutworms. Seedlings were transplanted 28 days after emergence, prior to which they were hardened through water stressing for 7 days.
Seedlings were transplanted onto a sandy loam soil with pH (H2O) of 5.4, total N, extractable P and soil organic carbon (SOC) content of 0.7 g/kg, 18.7 mg/kg and 5 g/kg, respectively. The field was ploughed to a depth of 0.25 m using a tractor-drawn disc plough followed by levelling using a hand-held hoe. The experiment was laid out in a randomised complete block design with three blocks each consisting of eight plots measuring 3.5 x 3 m. Seedlings were transplanted at a plant density of 83 333 plants/ ha. The treatments consisted of a control with no fertility amelioration, mineral fertiliser, TSC and TSC-CM applied at 5, 20 and 40 t/ha. The mineral fertiliser treatment was 500 kg/ha compound D + 250 kg/ha ammonium nitrate supplying equivalents of 121 kg N/ha, 30.8 kg P/ha and 24.6 kg K/ha representing the high nutrition required for oilseed rape as recommended in the growers manual.6 The organic amendments were spread and incorporated into soil by digging with hand-held hoes to about 0.1 m, a day before transplanting, while basal mineral fertiliser was applied at transplanting. All plots were watered immediately after transplanting. Top dressing fertiliser was applied to the mineral fertiliser plots only, 3 weeks after transplanting (WAT). The crop was mainly rain-fed, with equal amounts of supplementary irrigation applied to all plots as required, using a watering can. The plots were kept weed free through hand hoeing. A repeat application of Malathion 50% E.C to control aphids was done at 5 WAT.
Biomass yield determination
Fresh oilseed rape leaves were harvested from a netplot, consisting of the four inner rows leaving two border rows on each side of the plot, at 3, 5, 7 and 9 WAT. Mature leaves were plucked from each plant, leaving three young leaves. Thereafter, the leaf samples were weighed to determine fresh biomass. Sub-samples of the biomass were oven dried at 70 °C for 72 h, weighed and processed for subsequent nutrient content determination. Dry biomass yield was calculated by multiplying the fresh biomass yield with a moisture correction factor. The remaining fresh leaf biomass samples were used for organoleptic testing.
Plant tissue nutrient and nicotine determination
Tissue nutrient concentration (total N, P, K, Fe, Zn, Cu and Mn) was obtained from digestion of oven dry plant tissue sample using sulfuric acid-selenium powder mixture on a block digester at 330 °C.35 This was followed by absorbance readings at 650 nm and 880 nm for total N and P respectively, after blue colour development using a UV-vis single beam spectrophotometer. Distilled water was used to dilute aliquots of the sample digest and then K and micronutrient concentrations were read off an atomic absorption spectrophotometer.35 Total uptake of N, P and K in kg/ha were separately calculated as:
Nutrient uptake (kg/ha) = concentration of the nutrient (%) *dry matter (kg/ha) / 100 Equation 1
Nicotine analysis was done at the Tobacco Research Board chemistry laboratory providing commercial analytical services. The procedure involved steam distillation of ground material under strongly basic conditions, followed by acidification of the distillate and ultraviolet absorbance reading at 236 mµ, 259 mµ and 282 mµ on a UV-vis spectrophotometer.36
Taste determination
Taste determination of fresh leaf biomass was done at each harvest, with the help of untrained panelists. The use of untrained panelists ensured a measure of general acceptance and not quality control as is the case with wine, tea and cheese tasting that require trained assessors.12 The taste panel of 15 people (aged 18-65) consisted of MUAST employees as well as surrounding community members who consented to participate in the study. About 25-30 leaves were composited from each replicate treatment, chopped and boiled for 5 min in 1 L of water in separate pots. The samples were cooled and served in small plastic boxes, labelled using numbers and arranged randomly. Tasting was done one person at a time to minimise bias. A glass of water was given to each panelist to rinse their palate after tasting each sample.12 Taste scoring was done on a scale of 1 to 4, with a score awarded for each tasted sample. The scores were recorded on a score sheet. A score of 1 represents 'not bitter', 2 - 'mildly bitter', 3 - 'bitter' and 4 - 'extremely bitter'.
Soil chemical analyses
Soil was randomly sub-sampled from the plots and composited at 3 and 9 WAT (first and last harvest) for soil pH, EC, total C, mineral N, extractable P and exchangeable K. Mineral N was measured from fresh soil samples while the rest of the assays were done on air-dry soil sub-samples. Soil pH and EC were read from a 1:2.5 w/v soil:deionised water supernatant suspension.37 Mineral N was extracted from soil using potassium sulfate followed by colourimetric determination of ammonium-N and nitrate-N which were summed together to obtain total mineral-N.35 Extractable P was determined according to Olsen's extraction method, while exchangeable K was determined using the ammonium acetate method at pH 5.8 followed by absorbance reading on an atomic absorption spectrophotometer.35 Total C was analysed following the Walkley-Black method with external heating.37 Soil nicotine content was determined as previously described for plant samples.
Statistical analysis
Dry biomass yield, macronutrient uptake, micronutrient and nicotine concentration, soil nutrient and nicotine content data, collected at each sampling interval, were subjected to a one-way analysis of variance (ANOVA) at p<0.05 using GenStat Discovery 14th edition.38 Tukey's honestly significant difference was used to separate between statistically different means. Taste data were recorded, cleaned and analysed using SPSS v.21.39 Taste data frequency rating for each treatment was derived by running descriptive statistical analysis in SPSS v.21 and frequency tables were generated. Thereafter, mean taste scores based on the rating of panelists for each treatment at each sampling interval were calculated and regressed against total N concentration in leaf biomass.40 Pearson correlation coefficients were used to assess the strength of association.
Results and discussion
Oilseed rape macronutrient uptake
Nitrogen uptake at 3 WAT was higher (p<0.05) with mineral fertiliser and 40 t/ha TSC-CM than in the control and TSC treatments (Figure 1), while P uptake was more improved with the mineral fertiliser treatment (Figure 2). K uptake followed a similar trend to N uptake, although 20 t/ha TSC was comparable to mineral fertiliser and the TSC-CM treatments at 3 WAT (Figure 3). This highlighted enhanced nutrient uptake during early growth due to mineral fertiliser and TSC-CM application. While mineral fertiliser supplied nutrients in a ready form, the difference between TSC-CM and TSC could be attributed to ready mineralisation in TSC-CM on account of the narrower C/N ratio, indicative of more N per degradable C as a consequence of composting.41 Additionally, depressed microbial activity associated with high nicotine content, as suggested by Adediran et al.16, could have been the cause. At 5 WAT, N, P and K uptake was approximately three times more (p<0.05) than in the control with TSC application at 20 t/ha and 40 t/ha and between 1.5-2.0 times more than in mineral fertiliser and TSC-CM. This finding was not surprising as TSC could potentially supply more nutrients than mineral fertiliser and TSC-CM. For instance, at 40 t/ha TSC could potentially supply 964 kg N/ha relative to 121 and 780 kg N/ha from mineral fertiliser and 40 t/ha TSC-CM, respectively. At 7 WAT, only 40 t/ha TSC resulted in significantly higher N, P and K uptake relative to mineral fertiliser and TSC-CM application at all rates (Figures 1-3) pointing to the longevity of nutrient supply at the higher application rate. Mostly, nutrient uptake in 5 t/ha TSC-CM and TSC did not significantly vary from the control, indicating limited effectiveness of such low application rates of organic amendments. Observations of higher nutrient uptake and yield with increasing application rates of tobacco waste have been reported in other studies.17,25
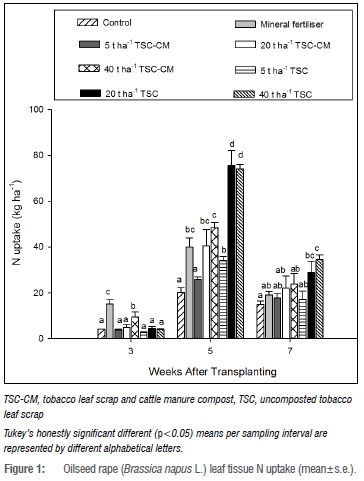
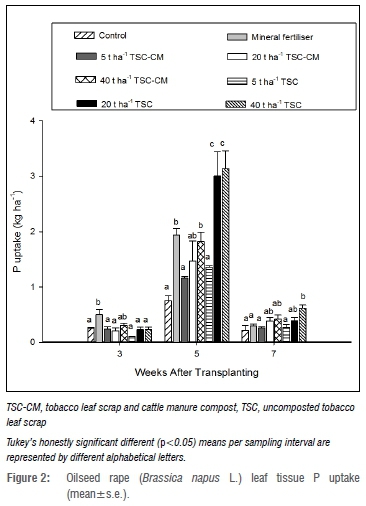
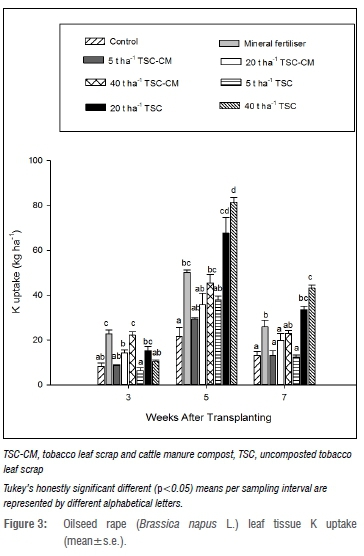
Oilseed rape micronutrient content
Application of TSC-CM at 20 t/ha and 40 t/ha significantly (p<0.05) improved Mn at 3 and 7 WAT, Zn at 5 WAT and Cu across all harvests, relative to control and mineral fertiliser (Table 2). Fe, Mn and Cu were improved by both 20 t/ha and 40 t/ha TSC relative to control and mineral fertiliser at selected sampling intervals (Table 2). Unlike macronutrients, micronutrient concentration, for example Cu and Fe, was also improved by 5 t/ha application rate at certain sampling intervals. Micronutrient concentration has increasingly been reported to be low in soils on most smallholder farms resulting in calls for supplementation through foliar applications or adoption of micronutrient-enriched mineral fertilisers in order to meet human dietary needs.42 Therefore, TSC-CM and TSC offer a cheaper source of micronutrients on smallholder farms as observed in this study and in other studies.27
Nicotine content and toxicity risk in oilseed rape
Nicotine content in all oilseed rape leaf sub-samples was below detectable levels (<0.01% of dry leaf matter) indicating assimilation in low levels. The nicotine concentration was thus below 500 mg/kg toxicity threshold according to European Union Regulations.43 This finding was expected, especially from TSC-CM which had undergone prior composting.16 With regard to TSC, rapid degradation of nicotine has been reported to occur between a few hours to a few days in soil in other studies,32,33 thus explaining its low assimilation by the plants. Subsequently, risk of nicotine toxicity from consumption of the oilseed rape fertilised with TSC was minimal.
Oilseed rape yield response to fertilisation
At 3 WAT, only mineral fertiliser and 40 t/ha TSC-CM treated plots had dry biomass yield that was higher (p<0.05) than that of the control, while those of the other treatments were not different (Figure 4). This finding was attributed to availability of nutrients in a ready form from mineral fertiliser, while only 40 t/ha application of TSC-CM ensured supply of nutrients in adequate amounts. On the other hand, although nutrient levels in TSC were comparatively higher (Table 1), there was wilting of seedlings in the 40 t/ha TSC treatment during the first week, resulting in gap filling being done in the second week. This was indicative of unfavourable conditions when high doses of TSC were applied. This observation is similar to that of Seckar et al.33 who reported inhibited early growth of lettuce head with high rates of tobacco waste amendment ascribed to high levels of nicotine in soil. In another study, Adediran et al.16 attributed poor seed germination of lettuce to high EC of tobacco waste and cabbage compost. Nonetheless, the inhibitory conditions were shown to subside within a week as successful gap filling was achieved in the subsequent week. This is consistent with the observations of Nota et al.32 and Seckar et al.33 who put forward that nicotine degradation in soils takes a few days, after which the risk of toxicity is extremely low. Consequently, this could have been the case in this study. Furthermore, the effects were only observable with the 40 t/ha application rate of TSC pointing to a dose-dependent effect.44 Although Delibacak and Ongun15 recommended a 1-month waiting period before planting or sowing activities when soil is amended with uncomposted tobacco waste, the present study indicated that a shorter waiting period of at least 1 week may be adequate. A shorter waiting period may reduce opportunities for nutrient loss, thus fostering optimum nutrient utilisation. At 5 WAT, 40 t/ ha TSC resulted in 116%, 85% and 30% more biomass yield than control, mineral fertiliser and 40 t/ha TSC-CM, respectively (Figure 4). Further, at 7 WAT, more biomass yield was realised from 20 and 40 t/ha TSC relative to control, mineral fertiliser and TSC-CM (Figure 4). At 9 WAT, TSC at 20 t/ha and 40 t/ha yielded approximately 0.25 t/ha and 0.34 t/ ha dry biomass, respectively (not shown on the figure), while the rest of the treatments did not produce any harvestable biomass, due to severe damage of plants by aphids (Aphidoidea spp.). Resultantly, cumulative biomass yield was significantly higher in 20 and 40 t/ha TSC treated plots relative to those of other treatments including mineral fertiliser and TSC-CM amended treatments (Table 3). This was attributed to supply of more nutrients from TSC relative to TSC-CM and mineral fertiliser as well as the additional biomass harvested at 9 WAT.


Oilseed rape organoleptic testing
The control was rated between 'not bitter' and 'mildly bitter' across the three sampling intervals, reflected by corresponding mean taste scores (Table 4). Extremely bitter taste of oilseed rape was observed with mineral fertiliser and TSC at 20 t/ha and 40 t/ha at 3 WAT and decreased with time (Table 4). On the other hand, TSC-CM at all rates and 5 t/ha TSC ranged between 'mildly bitter' and 'bitter' at 3 WAT, diminishing with time (Table 4). There was a positive but weak correlation between total N content and mean taste scores across all harvest periods (Figure 5), while no correlations were done with nicotine concentration owing to non-detectable levels. The intense bitterness of oilseed rape in TSC treatments at 20 t/ha and 40 t/ha confirmed the existing but undocumented concerns in the study area with TSC use, although it was not limited to TSC treatments - it also occurred with mineral fertilisation. While the bitterness with mineral fertilisation could be attributed to nitrogen and nitrate content in Brassica juncea leaves, as reported by Maereka et al.12, a weak correlation between taste and leaf N observed in this study indicated that nitrate could have had a more pronounced effect than total N. On the other hand, nitrate effects coupled with possible assimilation of by-products of nicotine breakdown could have been responsible for the bitter taste in TSC amended treatments,45 especially as bitterness was more intense during the early growth stages which coincided with periods of high nicotine degradation. Nevertheless, these effects subsided with time, thus pointing to an opportunity for utilisation.

Selected soil chemical properties during growth of oilseed rape
Soil pH ranged from 6.0 to 7.1 and did not significantly (p>0.05) vary across treatments at 3 WAT, although at 9 WAT it was significantly (p<0.05) higher with 20 and 40 t/ha TSC-CM (Table 5). The alkaline nature of TSC-CM (Table 1) most likely contributed to the increase in soil pH and this was desirable as the sandy soils in the study area are prone to acidity and increased risk of induced nutrient deficiencies.46 Consequently, TSC-CM application can be recommended to curb these problems. At 3 WAT, mineral fertiliser and TSC-CM produced higher EC than control and TSC applications, while at 9 WAT, both TSC-CM and TSC at 20 t/ha and 40 t/ha produced higher EC than both the control and mineral fertiliser did (Table 5). This indicated higher cationic and anionic concentrations, mostly likely mineralised from the organic amendments. Bertran et al.47 and Loper at al.48 attributed a similar increase of EC to cation release during organic material mineralisation. The increase in EC was variable and although 40 t/ha TSC resulted in 12 times higher EC than control, it was still below the critical limit of 4.0 dS/m and thus did not pose any detrimental salt effects.49 Soil organic carbon was significantly (p<0.05) higher with application of both TSC-CM (except at 5 t/ha) and TSC than the control and mineral fertiliser at 3 WAT (Table 5). Also at 9 WAT, both 20 t/ha and 40 t/ha application rates for TSC-CM and TSC resulted in significantly higher SOC over control and mineral fertiliser. This was by a margin of 85-169% with TSC-CM, while a wider margin of 137-229% was noted with TSC application. Higher SOC content with TSC at 9 WAT was in accordance with the higher C content in TSC due to availability of undegraded substrate. Additionally, carbon shielding associated with possible aggregate binding due to the release of phenolic substances during decomposition of TSC could have contributed to the higher SOC.31 On the other hand, the significantly (p<0.05) higher mineral N after addition of 20 t/ha and 40 t/ha TSC-CM and TSC relative to the control treatment (Table 5) was ascribed to the supply of nutrients from the organic amendments. Mineralisation of the organic materials also explained the observed high levels of extractable P and exchangeable K (Table 5). Generally, higher nutrient contents were observed in TSC-CM at 3 WAT than TSC owing to the narrower C/N ratio in the compost which favoured faster mineralisation.41 This was also confirmed by the nutrient uptake observations and pointed to more ready mineralisation of TSC-CM than TSC, while conversely, TSC presented an opportunity for prolonged supply of nutrients. Nicotine content in soil was below the 100 mg/kg detection level in all treatments across sampling periods (results not shown). As such, the risk of nicotine toxicity with TSC application was shown not to persist beyond 3 weeks. This observation attests to observations of swift degradation of nicotine in soil, as reported in other studies.32,33
Conclusion
The readiness of supply of nutrients by TSC-CM was highlighted at an application rate of 40 t/ha, and pointed to its importance in the promotion of early crop growth. On the other hand, higher nutrient supply and longevity of supply were realised with TSC application at 20 t/ha and 40 t/ha. In addition, while soil pH was improved with TSC-CM application, both TSC-CM and TSC improved soil nutrient levels including SOC, which is key towards organic matter build-up. Moreover, both TSC-CM and TSC were comparable in improving micronutrient concentration in oilseed rape relative to control and mineral fertiliser. Interestingly, there was low risk of nicotine toxicity in oilseed rape and soil with TSC application from 3 WAT. However, owing to the intensely bitter taste of oilseed rape in TSC-amended treatments, application of TSC-CM at 40 t/ha offers a trade-off of yield increase, nutrient uptake and oilseed rape taste and may, therefore, be more acceptable as an organic nutrient source relative to TSC. Notwithstanding, if composting is not feasible, TSC at 20 t/ha may be recommended, although the first harvest may have to be discarded. Thus, the viability of TSC-CM and TSC as practical solutions for nutrient supply and sustainable soil management were asserted. For further study, there is need for evaluation of performance of the organic materials based on the same N rate, evaluation of waiting periods after incorporation of TSC so as to enhance nutrient uptake during early growth stage as well as long-term application for sustainability.
Acknowledgements
We thank the Organisation for Women in Science for the Developing World for the PhD fellowship awarded to N.D. and the International Foundation for Science for a research grant (grant C/5764-1). We acknowledge the analytical services provided by the chemistry laboratory at the Tobacco Research Board.
Competing interests
We declare that there are no competing interests.
Authors' contributions
N.D.: Conceptualisation, methodology, data collection, sample analysis, validation, data curation, writing - the initial draft, writing - revisions, project leadership, project management, funding acquisition. R.Z.: Conceptualisation, methodology, student supervision, project management. C.P.: Methodology, data collection, sample analysis, writing - revisions. M.W.: Conceptualisation, methodology, student supervision, writing - revisions. PM.: Conceptualisation, methodology, student supervision, writing - revisions.
References
1. Mandiriza-Mukwirimba G, Kritzinger Q, Aveling T. A survey of brassica vegetable smallholder farmers in the Gauteng and Limpopo provinces of South Africa. J Agric Rural Dev Trop Subtrop. 2016;117:35-14. [ Links ]
2. Maseko I, Mabhaudhi T, Tesfay S, Araya HT, Fezzehazion M, Du Plooy CP. African leafy vegetables: A review of status, production and utilization in South Africa. Sustainability. 2018;10(1), Art. #16. https://doi.org/10.3390/su10010016 [ Links ]
3. Mango N, Makate C, Mapemba L, Sopo M. The role of crop diversification in improving household food security in central Malawi. Agric Food Secur. 2018;7(1), Art. #7. https://doi.org/10.1186/s40066-018-0160-x [ Links ]
4. Acikgoz FE. Mineral, vitamin C and crude protein contents in kale (Brassica oleraceae var. acephala) at different harvesting stages. Afr J Biotechnol. 2011;10:17170-17174. http://dx.doi.org/10.5897/AJB11.2830 [ Links ]
5. Ganya S, Svotwa E, Katsaruware RD. Performance of two rape (Brassica napus) cultivars under different fertilizer management levels in the smallholder sector of Zimbabwe. Int J Agron. 2018;2018, Art. #2351204, 7 pages. https://doi.org/10.1155/2018/2351204 [ Links ]
6. SNV (Netherlands Development Organisation). Smallholder horticultural production and business trainer's manual. Harare: SNV Zimbabwe; 2016. Available from: http://www.snv.org/public/cms/sites/default/files/explore/download/rarp_2016-horticulture-trainers-manual.pdf [ Links ]
7. Mungai LM, Snapp S, Messina JP Chikowo R, Smith A, Anders E, et al. Smallholder farms and the potential for sustainable intensification. Front Plant Sci. 2016;7:1-17. https://doi.org/10.3389/fpls.2016.01720 [ Links ]
8. Musara C, Chitamba J. Growth rate and yield of Brassica napus in response to Acacia angustissima leaf biomass application. J Anim Plant Sci. 2015;25:510-518. http://www.thejaps.org.pk/docs/v-25-02/26.pdf [ Links ]
9. Muchecheti BF, Madakadze IC. Yield and nitrogen recovery of rape (Brassica napus L.) in response to application of leguminous leaf litter and supplemental inorganic nitrogen. Exp Agric. 2016;52:518-536. https://doi.org/10.1017/S0014479715000228 [ Links ]
10. Cedrez BC, Chamberlin J, Guo Z, Hijmans RJ. Spatial variation in fertilizer prices in sub-Saharan Africa. PLoS ONE. 2020;15:1-20. http://dx.doi.org/10.1371/journal.pone.0227764 [ Links ]
11. Khan MN, Mobin M, Abbas ZK, Alamri SA. Fertilizers and their contaminants in soils, surface and groundwater. In: DellaSala DA, Goldstein MI, editors. The encyclopedia of the Anthropocene. Oxford: Elsevier Inc.; 2018. p. 225-240. https://doi.org/10.1016/B978-0-12-809665-9.09888-8 [ Links ]
12. Maereka EK, Madakadze RM, Mashingaidze AB, Kageler S, Nyakanda C. Effect of nitrogen fertilization and timing of harvesting on leaf nitrate content and taste in mustard rape (Brassica juncea L. Czern). J Food Agric Environ. 2007;5:288-293. [ Links ]
13. Mehdizadeh M, Darbandi EI, Naseri-rad H, Tobeh A. Growth and yield of tomato (Lycopersicon esculentum Mill.) as influenced by different organic fertilizers. Int J Agron Plant Prod. 2013;4:734-738. [ Links ]
14. Materechera SA. Utilization and management practices of animal manure for replenishing soil fertility among smallscale crop farmers in semi-arid farming districts of the North West Province, South Africa. Nutr Cycl Agroecosystems. 2010;87:415-128. https://doi.org/10.1007/s10705-010-9347-7 [ Links ]
15. Delibacak S, Ongun AR. Influence of composted tobacco waste and farmyard manure applications on the yield and nutrient composition of lettuce. Eurasian J Soil Sci. 2016;5:132-138. https://doi.org/10.18393/ejss.2016.2.132-138 [ Links ]
16. Adediran JA, Mnkeni PNS, Mafu NC, Muyima NYO. Changes in chemical properties and temperature during the composting of tobacco waste with other organic materials, and effects of resulting composts on lettuce (Lactuca sativa L.) and spinach (Spinacea oleracea L.). Biol Agriulture Hortic. 2004;22:101-119. https://doi.org/10.1080/01448765.2004.9754994 [ Links ]
17. Chaturvedi S, Upreti DK, Tandon DK, Sharma A, Dixit A. Bio-waste from tobacco industry as tailored organic fertilizer for improving yields and nutritional values of tomato crop. J Environ Biol. 2008;29:759-763. [ Links ]
18. Okur N, Hüsnü H, Lu K, Okur B, Delibacak S. Organic amendment based on tobacco waste compost and farmyard manure: Influence on soil biological properties and butter-head lettuce yield. Turksh J Agric For. 2008;32:91-99. [ Links ]
19. Gülser C, Candemir F. Changes in penetration resistance of a clay field with organic waste applications. Eurasian J Soil Sci. 2012;1:16-21. [ Links ]
20. Kayikcjog'lu HH, Okur N. Evolution of enzyme activities during composting of tobacco waste. Waste Manag Res. 2011;29:1124-1133. https://doi.org/10.1177/0734242x10392813 [ Links ]
21. Prowse M. A history of tobacco production and marketing in Malawi, 18902010. J East Afr Stud. 2013;7:691-712. https://doi.org/10.1080/17531055.2013.805077 [ Links ]
22. Scoones I, Mavedzenge B, Sukume C, Murimbarimba F. Tobacco, contract farming, and agrarian change in Zimbabwe. J Agrar Chang. 2017:1-21. https://doi.org/10.1111/joac.12210 [ Links ]
23. Masvongo J, Mutambara J, Zvinavashe A. Viability of tobacco production under smallholder farming sector in Mount Darwin District, Zimbabwe. J Dev Agric Econ. 2013;5:295-301. https://doi.org/10.5897/JDAE12.128 [ Links ]
24. Hu T, Lee AH. Tobacco control and tobacco farming in African countries. J Public Health Policy. 2015;36:1-9. https://doi.org/10.1057/jphp.2014.47 [ Links ]
25. Cercioglu M, Okur B, Delibacak S, Ongun AR. Effects of tobacco waste and farmyard manure on soil properties and yield of lettuce (Lactuca sativa L. var. Capitata). Commun Soil Sci Plant Anal. 2012;43:875-886. https://doi.org/10.1080/00103624.2012.653023 [ Links ]
26. Wang SN, Liu Z, Fang HZ, Meng J, Xu P Characterization of environmentally friendly nicotine degradation by Pseudomonas putida biotype A strain S16. Microbiology. 2007;153:1556-1565. https://doi.org/10.1099/mic.0.2006/005223-0 [ Links ]
27. Adediran JA, De Baets N, Mnkeni PNS, Kiekens L, Muyima NYO, Thys A. Organic waste materials for soil fertility improvement in the Border Region of the Eastern Cape, South Africa. Biol Agric Hortic. 2003;20:283-300. https://doi.org/10.1080/01448765.2003.9754974 [ Links ]
28. Dunjana N, Zengeni R, Muchaonyerwa P Wuta M. Typological characterisation of farms in a smallholder food-cash crop production system in Zimbabwe -opportunities for livelihood sustainability. J Agric Rural Dev Trop Subtrop. 2018;119:11-22. [ Links ]
29. Gulser C, Demir Z, Serkan I. Changes in some soil properties at different incubation periods after tobacco waste application. J Environ Biol. 2010;31:671-674. [ Links ]
30. Akhtar S, Shakeel S, Hamid A, Ahmad SR. Suitability of tobacco dust for agricultural purposes. J Biodivers Environ Sci. 2016;9:102-113. [ Links ]
31. Shakeel, S. Consideration of tobacco dust as organic amendment for soil: A soil & waste management strategy. Earth Sci. 2014,3:117-121. https://doi.org/10.11648/j.earth.20140305.11 [ Links ]
32. Nota G, Naviglio D, Ugliano M, Romano R. Determination of nicotine in the soil mixed with tobacco powder as fertilizer. Anal Lett. 2008;33:265-275. https://doi.org/10.1080/00032710008543051 [ Links ]
33. Seckar JA, Stavanja MS, Harp PR, Yi Y Garner CD, Doi J. Environmental fate and effects of nicotine released during cigarette production. Environ Toxicol Chem. 2008;27:1505-1514. https://doi.org/10.1897/07-284.1 [ Links ]
34. Food and Agriculture Organisation(FAO). World reference base for soil resources 2014: International soil classification system for naming soils and creating legends for soil maps. Rome: FAO; 2015. Available from: http://www.fao.org/soils-portal/soil-survey/soil-classification/world-reference-base/en/ [ Links ]
35. Okalebo JR, Gathua KW, Woomer PL. Laboratory methods of soil and plant analysis: A working manual. 2nd ed. Nairobi: TSBF-CIAT; SACRED Africa; 2002. [ Links ]
36. Willits C, Swain ML, Connelly JA, Brice BA. Spectrophotometric determination of nicotine. Anal Chem. 1950;22:430-433. https://doi.org/10.1021/ac60039a013 [ Links ]
37. Anderson JM, Ingram JSI. Tropical soil biology and fertility (TSBF). A hand book of methods. 2nd ed. Wallingford, UK: CAB International; 1993. [ Links ]
38. VSN International. GenStat for Windows. 14th ed. Hemel Hempstead, UK.: VSN International; 2013. [ Links ]
39. IBM SPSS. Statistics for Windows. Armonk, NY: IBM Corp.; 2012. [ Links ]
40. Tordoff MG, Sandell MA. Vegetable bitterness is related to calcium content. Appetite. 2009;52:498-504. https://doi.org/10.1016/j.appet.2009.01.002 [ Links ]
41. Bernal MP Alburquerque JA, Moral R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour Technol. 2009;100:5444-5453. http://dx.doi.org/10.1016/j.biortech.2008.11.027 [ Links ]
42. Manzeke GM, Mtambanengwe F, Nezomba H, Mapfumo P Zinc fertilization influence on maize productivity and grain nutritional quality under integrated soil fertility management in Zimbabwe. Field Crops Res. 2014;166:128-136. http://dx.doi.org/10.1016/j.fcr.2014.05.019 [ Links ]
43. Civilini M, Domenis C, Sebastianutto N, de Bertoldi M. Nicotine decontamination of tobacco agro-industrial waste and its degradation by micro-organisms. Waste Manag Res. 1997;15:349-358. https://doi.org/10.1177/0734242X9701500403 [ Links ]
44. Selmar D, Engelhardt UH, Hansel S, Thräne C, Selmar D, Engelhardt UH, et al. Nicotine uptake by peppermint plants as a possible source of nicotine in plant-derived products. Agron Sustain Dev. 2015;35:1185-1190. https://doi.org/10.1007/s13593-015-0298-x [ Links ]
45. Yu H, Tang H, Xu P Green strategy from waste to value-added-chemical production: Efficient biosynthesis of 6-hydroxy-3-succinoyl-pyridine by an engineered biocatalyst. Sci Rep. 2014;4:1-8. http://10.1038/srep05397 [ Links ]
46. Soropa G, Nyamangara J, Nyakatawa EZ. Nutrient status of sandy soils in smallholder areas of Zimbabwe and the need to develop site-specific fertiliser recommendations for sustainable crop intensification. S AfrJ Plant Soil. 2018;1-3. https://doi.org/10.1080/02571862.2018.1517901 [ Links ]
47. Bertran E, Sort X, Soliva M, Trillas I. Composting winery waste: Sludges and grape stalks. Bioresour Technol. 2004;95:203-208. https://doi.org/10.1016/j.biortech.2003.07.012 [ Links ]
48. Loper S, Shober AL, Wiese C, Denny GC, Stanley CD, Gilman EF, et al. Organic soil amendment and tillage affect soil quality and plant performance in simulated residential landscapes. HortScience. 2010;45:1522-1528. https://doi.org/10.21273/HORTSCI.45.10.1522 [ Links ]
49. Angelova VR, Akova VI, Artinova NS, Ivanov KI. The effect of organic amendments on soil chemical characteristics. Bulg J Org Agric Acad. 2013;19:958-971. [ Links ]
 Correspondence:
Correspondence:
Nothando Dunjana
Email: ntandodunjana@yahoo.com
Received: 22 Jan. 2020
Revised: 10 June 2020
Accepted: 22 June 2020
Published: 29 Sep. 2020
Editors: Teresa Coutinho, Salmina Mokgehle
Funding: Organisation for Women in Science for the Developing World; International Foundation for Science (grant C/5764-1)














