Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.7-8 Pretoria Jul./Aug. 2020
http://dx.doi.org/10.17159/sajs.2020/5963
RESEARCH ARTICLES
Revised estimates of Taung's brain size growth
Robert C. McCarthyI; Emily ZimelII
IDepartment of Biological Sciences, Benedictine University, Lisle, Illinois, USA
IIDepartment of Physical Sciences, Benedictine University, Lisle, Illinois, USA
ABSTRACT
Cranial capacity, a proxy for the volume of the brain and associated cranial contents, is an important yardstick used to compare early hominin species because increasing brain size is a key characteristic of our lineage. In 1925, Raymond Dart claimed that a natural endocast found at the Buxton Limeworks near Taung, South Africa (which he named Australopithecus africanus), showed signs of neural reorganisation, but its juvenile status complicated comparison to other hominoid species. In an attempt to put its brain size and reorganisation into a comparative context, subsequent researchers have tried to estimate Taung's adult cranial capacity by comparison to coarse-grained hominoid growth data. In this study, we simulated brain growth in A. africanus using asymptotic growth models in known-age mountain gorillas, chimpanzees and modern humans, and show that, at just under 4 years old, Taung's brain had already finished or nearly finished growing according to hominoid developmental schedules. Percentage-growth remaining estimates are lower here than in previous studies using cross-sectional ontogenetic samples of unknown chronological age. Our new adult estimates (between 404 cm3 and 430 cm3 overall and 405-406 cm3 for chimpanzee models) are smaller than previous estimates with a 'starting' cranial capacity of 404 cm3, supporting the hypothesis that Taung's adult brain size would have fallen toward the lower end of the A. africanus range of variation and strengthening the case that Taung was female.
Significance: •This is one of several recent studies to show that brain growth is completed in African apes and humans earlier than previously appreciated. •New adult cranial capacity estimates for Taung are lower than previous estimates, supporting the hypothesis that Taung was female. •Cessation of brain growth in hominoids at earlier ages than previously reported suggests that adult cranial capacities for hominin juvenile specimens have been overestimated
Keywords: brain growth, cranial capacity, brain ontogeny, growth curves, Australopithecus africanus
Introduction
The type specimen of Australopithecus africanus, Taung, is a juvenile skull consisting of a partial face with fragmentary pieces of the basicranium attached, a mandible, and a natural hemi-endocast.1 Taung has been the subject of intensive research focus because of its potential to resolve questions about hominin brain size and reorganisation2-7 and A. africanus craniofacial growth8-11, dental maturation12-18 and brain ontogeny19-21.
Raymond Dart originally estimated Taung's cranial capacity to be 520 cm3 based on a reconstruction of the hemi-endocast.2 Subsequent estimates have ranged between 382 cm3 and 530 cm3 (Tables 1-2). The most frequently cited estimate for Taung's cranial capacity - 404 cm3 - was derived from an independent reconstruction of the hemi-endocast22 and was recently corroborated by digital reconstruction of the endocast and endocranial cavity23. At 404 cm3, Taung's cranial capacity is already at the lower end of the range of A. africanus variation (Table 2), even though the Taung juvenile died after gingival eruption of the first molars but before the they had moved into functional occlusion, and so still had several years remaining to reach adulthood.12-19 Taung's importance to studies of hominin brain evolution and the scarcity of relatively complete crania and endocasts of adult A. africanus specimens have tempted researchers to estimate Taung's adult cranial capacity. Adult estimates range between 404 cm3 and 785 cm3 for 'starting' values ≥404 cm3 (Table 1). Researchers working with different age estimates and differing ideas about A. africanus growth trajectories have sometimes modelled Taung with a large percentage-growth remaining, producing adult cranial capacity estimates >600 cm3,2,3,19,24-26 which are larger than any known adult A. africanus specimens. Uncertainty about brain growth parameters in fossil species makes it difficult to estimate how much growth Taung had already attained (and how much remained),23,27-29 making it difficult to estimate adult cranial capacity. Size of the adult brain, which is approximated to some extent by cranial capacity, is an important parameter for understanding adaptive shifts in brain size and neural reorganisation early in human evolutionary history.
Previous adult predictions are based on overestimates of the amount of brain growth remaining in hominoids. At the time Taung was discovered in 1924, there were several misconceptions in the scientific literature about great ape growth and development. For example, it was thought that brain growth continued throughout the entire juvenile growth period until eruption of the third molars, or even beyond19(and references therein),25,34,37,38, with humans reaching 81-88%24,25,38 and great apes 85-92%19,24 of adult brain size in the period just prior to eruption of the first molars; and that chimpanzees followed a tooth eruption schedule similar to that of modern humans, with the first permanent molar erupting at the end of the sixth year19 or early in the fifth year25. Based in part on these misconceptions, Keith24,25 and Dart2,3 increased Taung's juvenile cranial capacity by 15-20% to produce adult cranial capacity estimates of 520-625 cm3. Zuckerman19 increased Taung's juvenile cranial capacity by 3% to as much as 57% based on the supposed amount of growth expected in chimpanzees and gorillas between eruption of the first molars and adulthood (Table 1). More recent estimates, while lower, have mostly relied on data from Ashton and Spence33 who found that 92.5% of adult cranial capacity is attained on average prior to eruption of the first molar in cross-sectional samples of hominoids (including orangutans, gorillas, chimpanzees and modern humans).
Here we reconsider evidence for Taung's adult cranial capacity, taking into account the most up-to-date estimates of chronological age, cranial capacity and brain growth trajectories in African apes and modern humans; and use these new estimates to reassess brain size variability in A. africanus.
Materials and methods
We used mountain gorilla (Gorilla beringei), chimpanzee (Pan troglodytes) and modern human (Homo sapiens) cranial capacity growth curves to produce developmental simulations10,11,23 of Taung's brain growth trajectory and to calculate the per cent changes that would have occurred between a given set of 'starting' ages and brain growth completion (Figure 1). We used starting age estimates of 3.73, 3.83 and 3.93 years based on a comparison of Taung's root length and crown development to A. africanus specimen Stw 40217, but also considered 3.3-year8,9,12 and 3.5-year13,15 estimates (based on patterns of root and crown development) and 4-year24,39, 4.5-5.5-year40 and 5-7-year41 estimates (based on varying interpretations of dental development and tooth eruption timing) in order to assess the impact of different starting ages on adult cranial capacity estimates.
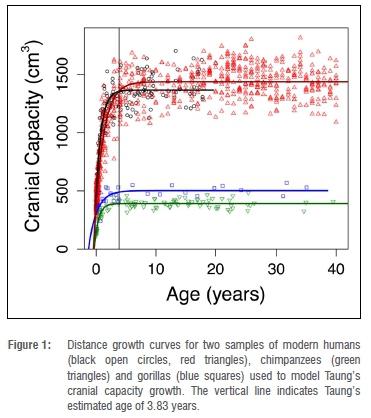
We collated cranial capacities and/or brain masses for mountain gorilla, chimpanzee and modern human comparative samples from the literature42-47 and set up each data set - including deleting outliers - following previously determined criteria42,44,48,49. Because raw data were not available in one instance47, we digitised axes and data points from the original paper using WebPlotDigitizer50. We transformed brain mass data from previous studies42-46 into cranial capacities following equations in Cofran and DeSilva51. As we are modelling the amount of growth that occurs between a given 'starting' age and asymptotic growth cessation, results would differ minimally (if at all) based on different methods for adjusting between brain mass (in grams) and cranial capacity (in cubic centimetres or millilitres). In this case, we prioritised maintaining all the data in the same units as the fossil data (cm3) over leaving each data set in its original units. After visually examining growth curves for modern humans, chimpanzees and mountain gorillas, we decided to cap three data sets at 40 years of age in order to limit the known impact that brain shrinkage at older ages can have on curve-fitting.43,46,48,52 The fourth (modern human) data set included only individuals up to 18 years.47
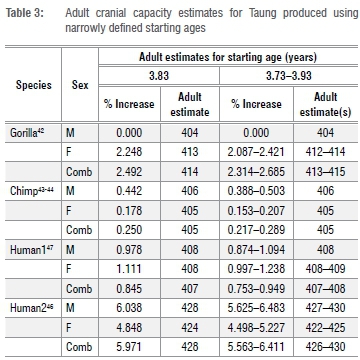
We fit the data in R (R Core Development) using a non-linear asymptotic regression model with a vertical offset (SSasymp), which is a 'self-starting' function that iteratively fits a model using initial estimates of the horizontal asymptote ('Asym'), the natural logarithm of the rate constant ('lrc'), and a numeric parameter corresponding to the response value when the input is zero ('R0'). We estimated cranial capacities from the regression fit at given starting ages for Taung and calculated percentage-change values for cranial capacities between each starting age and the maximum age for each data set (in each case the growth curve had reached asymptotic stability). We then estimated adult values for Taung by increasing Taung's juvenile cranial capacity by these percentage-change values. The R code for each of these operations is presented in the supplementary material. As noted above, we produced estimates for the most realistic 3.73-3.93-year-old starting values (Table 3), but also tested the effects of different juvenile starting cranial capacity estimates (Supplementary table 1) and different starting ages (Supplementary table 2). In this study, we prioritised testing the effects of different starting juvenile cranial capacity and age estimates over fitting confidence intervals, which adds a layer of complexity that will be addressed in a follow-up study.
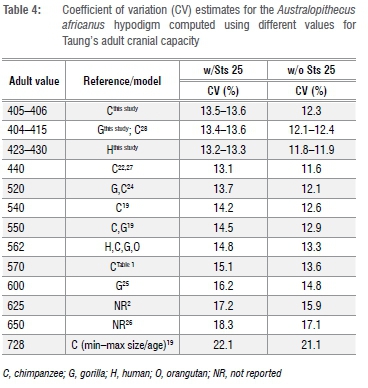
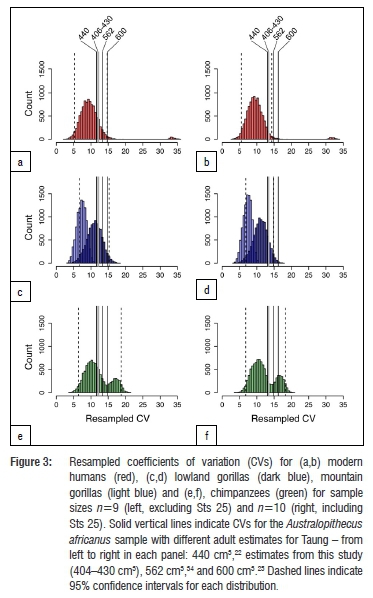
To assess variability in the A. africanus sample, we calculated coefficients of variation (CV)53 for the A. africanus hypodigm with different adult estimates for Taung (Table 4), and compared these values to bootstrapped samples of adult Gorilla gorilla + G. berengei, P. troglodytes, and H. sapiens matching the sample size of the A. africanus sample (n=10 including Sts 25, n=9 excluding Sts 25; see explanation below), as well as to historical CV values for A. africanus (Supplementary table 3). We did not include cranial capacity data for the recently published specimen StW 573 from the Silberberg Grotto at Sterkfontein because its taxonomic status is still under debate, and its endocast has not yet been virtually reconstructed to correct for distortion during the fossilisation process.54
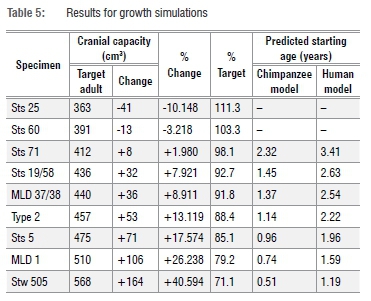
We also simulated A. africanus cranial capacity growth by looking at per cent changes necessary to grow Taung to different A. africanus 'target' adults, thereby simulating different models for growth increases in Taung's cranial capacity10,11 (Table 5). We compared these percentages to values for comparative samples to assess the likelihood that Taung's remaining brain growth would be sufficient to produce target adult cranial capacity values.
Results
Adult cranial capacity estimates for Taung fall within a relatively narrow range regardless of narrowly defined starting age and choice of species- or sex-specific models (Table 3). By 3.83 years of age, cranial capacity has reached 97.5% of adult size in gorillas (100% in males, 97.8% in females), 99.8% in chimpanzees (99.5% in males, 99.8% in females), and 94.0-99.3% in modern humans (94.4-99.0% in males, 95.2-99.0% in females). Because there is so little growth remaining between 3.73-3.93 years of age and adulthood (Figures 1-2), juvenile estimates increase by only 0-26 cm3 (Table 3) - a maximum increase of ~6.5%. There is a 3-26 cm3 (0.75-6.48%) increase according to modern human growth curves, but chimpanzees and gorillas have, respectively, <1% and <3% growth remaining (Table 3, Figure 2, Supplementary table 1). Adult cranial capacity estimates range between 404 cm3 and 415 cm3 according to the gorilla curve, 405 cm3 and 406 cm3 according to the chimpanzee curve, and 407 cm3 and 430 cm3 according to the two human curves (Table 3). The two modern human growth curves differ from each other in terms of percentage-growth change and estimated adult cranial capacities, with a more prolonged growth trajectory in brains derived from 19th-century German autopsy material46 than in a 20th-century sample of cranial capacities from an Australian research hospital47 (Table 3, Figures 1-2). Adult cranial capacity estimates for different starting cranial capacities and ages are presented in Supplementary tables 1 and 2.
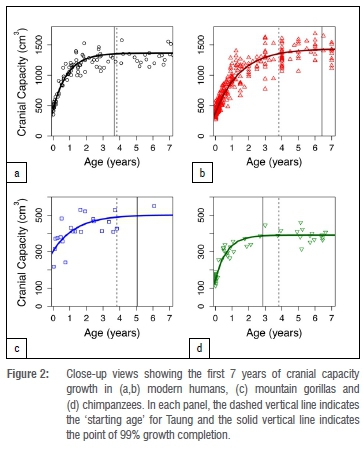
Regardless of which growth curve is used, all the adult estimates noted above place Taung at the low end of the range of adult A. africanus variation. Original estimates for A. africanus cranial capacities were fairly large (>500 cm3 in several cases), whereas revised estimates tend to be smaller. One cranium in particular, Sts 25 (350-375 cm3), does not appear in many comparative analyses of A. africanus cranial variation because it is fragmentary and still partially embedded in matrix, so its cranial capacity has been estimated by regression30 (Wolpoff M 2018, personal communication). We tested the effect of including Sts 25 in the A. africanus hypodigm on CV values by running analyses with and without this specimen. Table 4 shows CV values for the A. africanus hypodigm. Including new adult estimates for Taung in the A. africanus sample produces CV values that range between 11.8% and 13.6%, which are similar to values for the sample including Holloway's22 440-cm3 adult estimate but lower than CV values for samples that included larger adult estimates (Supplementary table 3). Coefficient of variation values for 404-440 cm3 estimates in this study and others22,28,32 can be accommodated within the range of variation for all extant samples, but larger adult estimates (>550 cm3 and >600 cm3 for samples that include and exclude Sts 25, respectively) fall outside the 95% confidence intervals for modern humans (Figure 3).
Discussion
Adult cranial capacities estimated for Taung with starting ages between 3.73 and 3.93 years (Table 3) ranged between 404 cm3 (no increase) and 430 cm3 (6.4% increase). These values are at the lower end of the adult A. africanus range of variation (Table 2). As expected, the largest of these adult estimates are produced by modern human growth curves. Estimates calculated using chimpanzee and gorilla growth curves ranged between 404 cm3 and 415 cm3 (Table 3). If A. africanus brain growth followed a chimpanzee trajectory as previously suggested12,13,15,16,18,19,24,25,31,32,39, then Taung had <1% growth remaining, so 405-406 cm3 may be the most reasonable estimate of adult cranial capacity.
Five modern estimates of Taung's adult cranial capacity are available for comparison. Based on an average value of 92% for brain growth completion prior to eruption of the first molar in hominoids19,33,34, Holloway22 added 35 cm3 to Taung's juvenile cranial capacity to predict an adult value of 440 cm3. Following the same logic we followed here, Falk31 increased Taung's juvenile estimate using a chimpanzee brain development curve, producing estimates ranging between 404 cm3 and 420 cm3. Somewhat presciently, Falk31(p,19) wrote: ''If Taung was as old as 3.7 years, … (the) curve suggests that its adult cranial capacity had already been achieved!'' Falk and Clarke28 estimated a juvenile cranial capacity of 382 cm3 based on a different reconstruction of Taung's endocast, then increased this value to 406 cm3 based on a chimpanzee growth curve. Holloway and Broadfield29 updated Falk and Clarke's28adult estimate (to 390 cm3) by setting their juvenile value to 98% growth-complete. Conroy et al.32 replaced the averaged value for hominoid brain growth completion prior to eruption of the first molar33 used by Holloway22 with a different set of values based on growth in chimpanzees, increasing Holloway's 404/405-cm3 juvenile estimate to 431 cm3 based on a mixed-sex sample, 422 cm3 based on a female sample, and 455 cm3 based on a male sample. Our new estimates for Taung based on chimpanzee growth curves - 405-406 cm3 - corroborate Falk's31 estimates using a different data set and are similar to estimates from Holloway and Broadfield29, but are lower than estimates from Holloway22, Conroy et al.32 and Falk and Clarke28.
The range of A. africanus cranial capacity variation in this study, 205 cm3 (363-568 cm3), is fairly large compared to older studies (Supplementary table 3) because of the inclusion of two small crania: Sts 2530 (350-375 cm3), a small specimen which preserves the left half of the cranial base and a partial vault still covered with breccia; and a new, smaller estimate of 391 cm3 for Sts 6023. If Sts 25 is excluded from the sample, the range drops to 177 cm3, with end-points of the range defined by crania that have been digitally reconstructed with a high degree of confidence.23 Coefficients of variation range between 11.8% and 13.6% for different iterations of the sample (Table 4), which are on par with recent studies but higher than in earlier work (Supplementary table 3).22,27,31,34 It has been recognised previously that variability between specimens can be underestimated when regression formulae are used to estimate cranial capacities as well as when complete fossil crania like Sts 5 are used as 'templates' to reconstruct less complete crania.34,55 Both of these conditions apply historically (Supplementary table 3). Coefficient of variation values for A. africanus cranial capacity (Table 4) generated in this study fall within the ranges of lowland gorilla, chimpanzee and modern human variation, but fall outside the range of mountain gorilla variation derived from limited samples (Figure 3). Adult estimates for Taung >550-600 cm3 yield CV values outside the range of modern human variation and estimates >565 cm3 yield CV values outside the range of gorilla variation (Figure 3). High CV values (>10%) support results based on craniofacial linear measurements.56
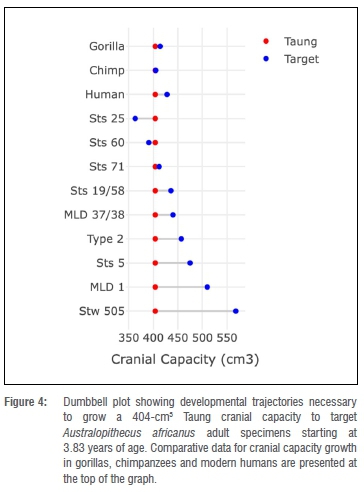
As noted above, another way to approach this problem is to estimate how much the brain would have had to grow to reach target adult A. africanus cranial capacities and to compare these values to growth data for hominoid comparative samples. Starting at 3.83 years of age with a 404 cm3 cranial capacity, Taung would have needed to grow ~2% to match Sts 71's cranial capacity (Figure 4, Table 5) - a value that falls within the range of variation of two of the comparative samples (Figure 4). Setting aside for a moment Sts 25 and Sts 60 (two small specimens with adult cranial capacities smaller than Taung's juvenile cranial capacity), Taung's cranial capacity would otherwise have had to increase 7.9-40.6% to match any of the other A. africanus specimens and 17.6% or 26.2-40.6% to match growth in male specimens. This amount of brain growth remaining at 3.83 years of age is unlikely. In fact, to match these values, Taung would have had to have been between 1.19 and 2.63 years old based on human growth curves and 0.51 and 1.45 years old based on chimpanzee curves (Table 5) - values outside the range of ages previously suggested for Taung. If Taung grew more like a chimpanzee12,13,15,16,18,19,24,25,31,39, then it would not have been possible to reach the upper echelons of A. africanus adult variation. Another line of evidence supports a small adult cranial capacity estimate for Taung. According to developmental simulations of craniofacial growth11, Taung would have grown up to resemble Sts 71, a small-brained putative female, more closely than Sts 5 (which is either a large-brained female35 or a small-brained male36) and other early hominin specimens. It is reassuring that different types of data from the brain and craniofacial region point to the same specimen as an adult target. If Taung grew according to an ape-like brain growth trajectory, then its estimated adult size and similarity to Sts 71, both in the face and neurocranium, support interpretations that Taung is a small female.24,25,57,58
In this study, we followed the logic of previous studies by using modern human and African ape growth curves to estimate Taung's adult brain size. However, Taung is one of only a few Australopithecus juveniles with a fairly secure developmental age that can be used to test hypotheses about how the pattern and rate of hominin growth compare to growth in African apes and modern humans.45,59,60 The results presented here rely on the assumption that brain growth in Australopithecus can be modelled accurately with reference to these comparative samples. We acknowledge that this does not necessarily have to be the case. It will take a combination of new fossil discoveries of juvenile specimens, new comparative data43-47, innovative analyses44,45,59,60, and reinterpretations of previous data and analyses48,49,51 to test this assumption about growth and development in the genus Australopithecus.
Conclusions
Our results support the hypothesis that Taung was female and help to clarify the lower end of the A. africanus range of variation. This study focused on brain ontogeny in Taung, which is important not only in an historical sense but also because Taung is one of only a few juvenile specimens that can shed light on brain growth in Australopithecus. It is possible to extend this type of developmental simulation to other juvenile fossil endocasts and crania, including specimens of Australopithecus afarensis, Paranthropus boisei, P. robustus, Australopithecus sediba, Homo habilis, Homo erectus and Homo neanderthalensis. Evidence for early cessation of brain growth in hominoid comparative samples published here and elsewhere42,48,49,51,59,60 brings to light the intriguing possibility that previous adult cranial capacity predictions from juvenile specimens might be overestimates.
Acknowledgements
R.C.M. acknowledges support from the Dr. Scholl Foundation and E.Z. acknowledges the support of the Benedictine University Natural Science Summer Research Program. We thank Philip Novack-Gottshall for help with R programming, Milford Wolpoff and Ralph Holloway for providing information about Sts 25 and Sts 60, and two anonymous reviewers for suggestions that helped improve the manuscript.
Authors' contributions
R.C.M. conceptualised the study, designed the methodology, validated data, performed some of the data analyses, and wrote and revised all drafts of the paper. E.Z. collated and curated data, performed data analysis, and helped revise early drafts of the paper.
Data availability
Data in this article are available as an open data set.61
References
1.Dart RA. Australopithecus africanus: The man-ape of South Africa. Nature. 1925;115(2884):195-199. https://doi.org/10.1038/115195a0 [ Links ]
2.Dart RA. Taungs and its significance. Nat Hist. 1926;26:315-327. [ Links ]
3.Dart RA. The relationships of brain size and brain pattern to human status. S Afr J Med Sci. 1956;21:23-45. [ Links ]
4.Falk D. A reanalysis of the South African australopithecine natural endocasts. Am J Phys Anthropol. 1980;53:525-539. https://doi.org/10.1002/ajpa.1330530409 [ Links ]
5.Holloway RL. Some additional morphological and metrical observations on Pan brain casts and their relevance to the Taung endocast. Am J Phys Anthropol. 1988;77:27-33. https://doi.org/10.1002/ajpa.1330770106 [ Links ]
6.Falk D, Redmond JC, Guyer J, Conroy GC, Recheis W, Weber GW, et al. Early hominid brain evolution: A new look at old endocasts. J Hum Evol. 2000;38:695-717. https://doi.org/10.1006/jhev.1999.0378 [ Links ]
7.Holloway RL, Clarke RJ, Tobias PV. Posterior lunate sulcus in Australopithecus africanus: Was Dart right? Comptes Rendus Palevol. 2004;3:287-293. https://doi.org/10.1016/j.crpv.2003.09.030 [ Links ]
8.Bromage T. Taung facial remodeling: A growth and development study. In: Tobias PV, editor. Hominid evolution: Past, present, and future. New York: Alan R. Liss; 1985. p. 239-245. [ Links ]
9.Bromage T. Ontogeny of the early hominid face. J Hum Evol. 1989;18:751-773. https://doi.org/10.1016/0047-2484(89)90088-2 [ Links ]
10.Ackermann RR, Krovitz GE. Common patterns of facial ontogeny in the hominid lineage. Anat Rec. 2002;269:142-147. https://doi.org/10.1002/ar.10119 [ Links ]
11.McNulty KP, Frost SR, Strait DS. Examining affinities of the Taung child by developmental simulation. J Hum Evol. 2006;51:274-296. https://doi.org/10.1016/j.jhevol.2006.04.005 [ Links ]
12.Bromage TG, Dean MC. Re-evaluation of the age at death of immature fossil hominids. Nature. 1985;317:525-527. https://doi.org/10.1038/317525a0 [ Links ]
13.Conroy GC, Vannier MW. Dental development of the Taung skull from computerized tomography. Nature. 1987;329:625-627. https://doi.org/10.1038/329625a0 [ Links ]
14.Mann AE. The nature of Taung dental maturation. Nature. 1988;333:123. https://doi.org/10.1038/333123a0 [ Links ]
15.Conroy GC, Vannier MW. Dental development in South African australopithecines. Part II: Dental stage assessments. Am J Phys Anthropol. 1991;86:37-156. https://doi.org/10.1002/ajpa.1330860205 [ Links ]
16.Smith BH. Life history and the evolution of human maturation. Evol Anthropol. 1992;1:134-142. https://doi.org/10.1002/evan.1360010406 [ Links ]
17.Lacruz RS, Ramirez Rossi F, Bromage TG. Dental enamel hypoplasia, age at death, and weaning in the Taung child. S Afr J Sci. 2005;101:567-569. [ Links ]
18.Dean MC. Measures of maturation in early fossil hominins: Events at the first transition from australopiths to early Homo. Phil Trans Roy Soc B. 2016;371, Art. #20150234. https://doi.org/10.1098/rstb.2015.0234 [ Links ]
19.Zuckerman S. Age changes in the chimpanzee, with special reference to growth of brain, eruption of teeth, and estimation of age; with a note on the Taungs ape. Proc Zool Soc Lond. 1928;1:1-142. https://doi.org/10.1111/j.1469-7998.1928.tb07138.x [ Links ]
20.Falk D, Zollifkofer CPE, Morimoto N, Ponce de Leon MS. Metopic suture of Taung (Australopithecus africanus) and its implications for hominin brain evolution. Proc Natl Acad Sci USA. 2012;109:8467-8470. https://doi.org/10.1073/pnas.1119752109 [ Links ]
21.Holloway RL, Broadfield DC, Carlson KJ. New high-resolution computed tomography data of the Taung partial cranium and endocast and their bearing on metopism and hominin brain evolution. Proc Natl Acad Sci USA. 2014;111:13022-13027. https://doi.org/10.1073/pnas.1402905111 [ Links ]
22.Holloway RL. Australopithecine endocast (Taung specimen, 1924): A new volume determination. Science. 1970;168:966-968. https://doi.org/10.1126/science.168.3934.966 [ Links ]
23.Neubauer S, Gunz P, Weber GH, Hublin J-J. Endocranial volume of Australopithecus africanus: New CT-based estimates and the effects of missing data and small sample size. J Hum Evol. 2012;62:498-510. https://doi.org/10.1016/j.jhevol.2012.01.005 [ Links ]
24.Keith A. The fossil anthropoid ape from Taungs. Nature. 1925;115:234-235. https://doi.org/10.1038/115234a0 [ Links ]
25.Keith A. New discoveries relating to the antiquity of man. New York: W.W. Norton and Company; 1931. [ Links ]
26.Elliot Smith G. The fossil anthropoid ape from Taungs. Nature. 1925;115:235. https://doi.org/10.1038/115234a0 [ Links ]
27.Holloway RL, Broadfield DC, Yuan MS. The human fossil record. Volume 3: Brain endocasts - the paleoneurological evidence. New York: John Wiley and Sons; 2004. https://doi.org/10.1002/0471663573 [ Links ]
28.Falk D, Clarke R. Brief communication: New reconstruction of the Taung endocast. Am J Phys Anthropol. 2007;134:529-534. https://doi.org/10.1002/ajpa.20697 [ Links ]
29.Holloway RL, Broadfield DC. Technical note: The midline and endocranial volume of the Taung endocast. Am J Phys Anthropol. 2011;146:319-322. https://doi.org/10.1002/ajpa.21570 [ Links ]
30.De Miguel C, Henneberg M. Variation in hominid brain size: How much is due to method? Homo. 2001;52:3-58. https://doi.org/10.1078/0018-442X-00019 [ Links ]
31.Falk D. Hominid paleoneurology. Ann Rev Anthropol. 1987;16:13-30. https://doi.org/10.1146/annurev.an.16.100187.000305 [ Links ]
32.Conroy GC, Falk D, Guyer J, Weber GW, Seidler H, Recheis W. Endocranial capacity in Sts 71 (Australopithecus africanus) by three-dimensional computed tomography. Anat Rec. 2000;258:391-396. https://doi.org/10.1002/(SICI)1097-0185(20000401)258:4%3C391::AID-AR7%3E3.0.CO;2-R [ Links ]
33.Ashton EH, Spence TF. Age changes in the cranial capacity and foramen magnum of hominoids. Proc Zool Soc Lond. 1958;130:169-181. https://doi.org/10.1111/j.1096-3642.1958.tb00567.x [ Links ]
34.Tobias PV. The brain in hominid evolution. New York: Columbia University Press; 1971. https://doi.org/10.5962/bhl.title.15880 [ Links ]
35.Grine FE. The alpha taxonomy of Australopithecus africanus. In: Reed KE, Fleagle JG, Leakey RE, editors. The paleobiology of Australopithecus. Vertebrate paleobiology and paleoanthropology. Dordecht: Springer; 2013. p. 73-104. https://doi.org/10.1007/978-94-007-5919-0_6 [ Links ]
36.Tawane GM, Thackeray JF. The cranium of Sts 5 ('Mrs Ples') in relation to sexual dimorphism of Australopithecus africanus. S Afr J Sci. 2018;114, Art. #a0249, 4 pages. http://dx.doi.org/10.17159/sajs.2018/a0249 [ Links ]
37.Pffister H. Die kapazität des schädels (der kopfhöhle) beim säugling und älteren kinde [The capacity of the skull (the head cavity) in infants and older children]. Monatsschr Psychiatr Neurol. 1903;13(6):577-589. German. https://doi.org/10.1159/000219469 [ Links ]
38.Berry RJA. Brain and mind: Or, the nervous system of man. New York: The MacMillan Company; 1928. [ Links ]
39.Biggerstaff RH. Time-trimmers for the Taungs child, or How old is 'Australopithecus africanus'? Am Anthropol. 1967;69:217-220. https://doi.org/10.1525/aa.1967.69.2.02a00110 [ Links ]
40.Wolpoff MH, Monge JM, Lampl M. Was Taung human or an ape? Nature. 1988;335:501. https://doi.org/10.1038/335501a0 [ Links ]
41.Mann AE. Paleodemographic aspects of the South African australopithecines. Philadelphia, PA: University of Pennsylvania; 1975. [ Links ]
42.McFarlin SC, Barks SK, Tocheri MW, Massey JS, Eriksen AB, Fawcett KA, et al. Early brain growth cessation in wild Virunga mountain gorillas (Gorilla beringei beringei). Am J Primatol. 2013;75:450-463. https://doi.org/10.1002/ajp.22100 [ Links ]
43.Herndon JG, Tigges J, Anderson DC, Klumpp SC, McClure HM. Brain weights throughout the lifespan of the chimpanzee. J Comp Neurol. 1999;409:567- 579. https://doi.org/10.1002/(SICI)1096-9861(19990712)409:4%3C567::AID-CNE4%3E3.0.CO;2-J [ Links ]
44.DeSilva JM, Lesnick JM. Chimpanzee neonatal brain size: Implications for brain growth in Homo erectus. J Hum Evol. 2006;51:207-212. https://doi.org/10.1016/j.jhevol.2006.05.006 [ Links ]
45.DeSilva JM, Lesnick JJ. Brain size at birth throughout human evolution: A new method for estimating neonatal brain size in hominins. J Hum Evol. 2008;55:1064-1074. https://doi.org/10.1016/j.jhevol.2008.07.008 [ Links ]
46.Marchand F. Ueber das Hirngewicht des Menschen [About human brain weight]. [ Links ] Leipzig: B.G. Teubner; 1902. German.
47.Abbott AH, Netherway DJ, Niemann DB, Clark B, Yamamoto M, Cole J, et al. CT-determined intracranial volume for a normal population. J Craniofac Surg. 2000;11:211-223. https://doi.10.1097/00001665-200011030-00002 [ Links ]
48.Jolicoeur P, Baron G, Cabana T. Cross-sectional growth and decline of human stature and brain weight in 19th-century Germany. Growth Dev Aging. 1988;52:201-206. [ Links ]
49.Cofran Z. Brain size growth in wild and captive chimpanzees. Am J Primatol. 2018;80(7), e22876. https://doi.org/10.1002/ajp.22876 [ Links ]
50.Rohatgi A. WebPlotDigitizer v. 4.1. 2018. Available from: https://automeris.io/WebPlotDigitizer [ Links ]
51.Cofran Z, DeSilva JM. A neonatal perspective on Homo erectus brain growth. J Hum Evol. 2015;81:41-47. https://doi.org/10.1016/j.jhevol.2015.02.011 [ Links ]
52.Kretschmann H-J, Schleicher A, Wingert F, Zilles K, Löblich H-J. Human brain growth in the 19th and 20th century. J Neurol Sci. 1979;40:169-188. https://doi.org/10.1016/0022-510X(79)90202-8 [ Links ]
53.Sokal RR, Rohlf FJ. Biometry. 4th ed. New York: W.H. Freeman and Company; 2012. [ Links ]
54.Beaudet A, Clarke RJ, De Jager EJ, Bruxelles L, Carlson KJ, Crompton R, et al. The endocast of StW 573 ('Little Foot') and hominin brain evolution. J Hum Evol. 2019;126:112-123. https://doi.org/10.1016/j.jhevol.2018.11.009 [ Links ]
55.Holloway RL. Australopithecine endocasts, brain evolution in the Hominoidea, and a model of hominid evolution. In: Tuttle R, editor. The functional and evolutionary biology of primates. Chicago, IL: Aldine Atherton; 1972. p. 185-203. http://dx.doi.org/10.4324/9781315132129-8 [ Links ]
56.Lockwood CA. Sexual dimorphism in the face of Australopithecus africanus. Am J Phys Anthropol. 1999;108:97-127. https://doi.org/10.1002/(SICI)1096-8644(199901)108:1<97::AID-AJPA6>3.0.CO;2-O [ Links ]
57.Hrdlička A. The Taungs ape. Am J Phys Anthropol. 1925;8:379-392. https://doi.org/10.1002/ajpa.1330080402 [ Links ]
58.Robinson A. The Taungs skull. Brit Med J. 1925;March 21. [ Links ]
59.Cofran Z. Brain size growth in Australopithecus. J Hum Evol. 2019;130:72-82. https://doi.org/10.1016/j.jhevol.2019.02.006 [ Links ]
60.Zollikofer CPE, Ponce de Leon MS. Pandora's growing box: Inferring the evolution and development of hominin brains from endocasts. Evol Anthropol. 2013;22:20-33. https://doi.org/10.1002/evan.21333 [ Links ]
61.McCarthy R. Revised estimates of Taung's brain size growth [data set]. [ Links ] Mendeley Data v1 2020. http://dx.doi.org/10.17632/wyfvwd4s22
 Correspondence:
Correspondence:
Robert McCarthy
Email: rmccarthy@ben.edu
Received: 31 Jan. 2019
Revised: 01 Apr. 2020
Accepted: 01 Apr. 2020
Published: 29 July 2020
Editor: Maryna Steyn
Funding: Dr. Scholl Foundation, Benedictine University Natural Science Summer Research Program
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














