Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.5-6 Pretoria may./jun. 2020
http://dx.doi.org/10.17159/sajs.2020/6700
RESEARCH ARTICLE
Lead and strontium isotopes as palaeodietary indicators in the Western Cape of South Africa
Mari ScottI; Petrus le RouxI; Judith SealyII; Robyn PickeringI, III
IDepartment of Geological Sciences, University of Cape Town, Cape Town, South Africa
IIDepartment of Archaeology, University of Cape Town, Cape Town, South Africa
IIIHuman Evolution Research Institute, University of Cape Town, Cape Town, South Africa
ABSTRACT
We analysed the isotopic compositions of bioavailable strontium (Sr) and lead (Pb) in 47 samples of animals and plants derived from the various geological substrates of southwestern South Africa, to explore the utility of these isotope systems as dietary tracers. Measurements were made using high-resolution multi-collector inductively-coupled-plasma mass spectrometry (MC-ICP-MS). 87Sr/86Sr could efficiently discriminate between geologically recent sediments of marine origin in near-coastal environments and older geologies further inland. However, 87Sr/86Sr was not able to distinguish between the Cape Granite Suite and the Cape System (Table Mountain sandstones), whereas Pb isotopes could, demonstrating the utility of this hitherto underused isotope system. Bioavailable 87Sr/86Sr in near-coastal terrestrial environments is influenced by marine input, whereas Pb isotopic ratios are not, because of low concentrations of Pb in seawater. There is considerable potential to use Pb isotopes as a dietary and palaeodietary tracer in near-coastal systems in fields as diverse as archaeology, palaeontology, wildlife ecology and forensics.
SIGNIFICANCE:
• This study is the first investigation of the potential of Pb isotopes as a dietary tracer in southwestern South Africa.
• Pb isotopes are a valuable dietary tracer; used in combination with 87Sr/86Sr, they can extend our knowledge of landscape usage in coastal-marine environments.
• Pb isotopes have also shown to be useful in samples from the 1980s, collected during the time when leaded petrol was in use in South Africa; however, these samples were from remote areas with low motor vehicle emissions.
Keywords: Sr isotopes, Pb isotopes, palaeodiet, archaeology, southwestern South Africa
Introduction
We examined strontium (87Sr/86Sr) and lead (206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb) isotopes as biogeochemical tracers for studying diet and landscape usage in the (semi-)arid, coastal regions of southwestern South Africa, with application in both contemporary and ancient (archaeological and palaeontological) contexts. Consumer body tissues record the isotopic composition of food and water ingested in life.1 Where these isotopes vary across the landscape, they provide a natural tracer of diet and migration. We measured Sr and Pb concentrations and isotopic compositions in animals and isotope compositions in plants collected from the major geological substrates of southwestern South Africa (shales, sandstones, granites and recent marine-derived sands), ranging in age from pre-Cambrian to Quaternary (Figure 1). We were thus able to characterise isotope ratios of bioavailable Sr and Pb for each substrate. Our work expands on previous studies of 87Sr/86Sr isotopes as a (palaeo)dietary indicator in this region2-5; however, this study is the first to investigate Pb isotopes for this purpose. In addition, we aimed to determine the utility of Pb isotope measurements on archival samples that were collected during the time when leaded petrol was in use in South Africa. This is important because there is a large body of materials in museum and other collections that can be drawn from in future studies.
Sr isotopes in the geosphere and biosphere
Sr2+ substitutes for Ca2+ in minerals including plagioclase feldspar, calcite, dolomite, aragonite, gypsum and, most importantly regarding archaeological materials, apatite in bones and teeth. 87Sr/86Sr in biological materials is increasingly widely used to track animal migrations1, in forensics6, and in archaeology and palaeontology7. 87Sr is radiogenic (87Rb - 87Sr, t1/2 = 88x1010 years), whereas 86Sr is not.8,987Sr/86Sr therefore increases gradually through time and is highest in geologically ancient rocks, and those with high Rb contents relative to Sr.9,10 Sr is released from rocks through chemical weathering and moves (without fractionation of 87Sr/86Sr) from the source rock into the soils and groundwater.9,11,12 Different components of rocks with different 87Sr/86Sr may weather at different rates, so bioavailable 87Sr/86Sr may differ from the average underlying bedrock.13 Measurement of local animals and plants is the best way to characterise bioavailable 87Sr/86Sr13,14 because Sr passes through the food chain from plants to animals and humans without significant fractionation of its isotopes7,11,15.
87Sr/86Sr in soil and water may be altered by admixing of non-local Sr from rivers flowing through different geologies, precipitation and wind-blown dust.16 Sr is homogeneously distributed in the ocean, with a residence time of 2x107 years and a concentration of 7.62 ppm.12 An important limitation of the Sr isotope system worldwide is the tendency of coastal terrestrial areas to have 87Sr/86Sr values reflecting the composition of present-day seawater at 0.709241±0.000032.17 This is due to the presence of geologically recent marine-derived calcareous sediments with high fractions of shell3,4, and Sr contributed by sea spray and mists16.
Pb isotopes in the geosphere and biosphere
Pb has four stable, naturally occurring isotopes, of which 206Pb (238U ->206Pb, t1/2 = 4.47x109 years)9, 207Pb (235U -> 207Pb, t1/2 = 0.70x109 years)9, and 208Pb (232Th -> 208Pb, t = 14.01x109 years)9 are all radiogenic. 204Pb is not radiogenic and is therefore a good reference isotope. 206Pb/204Pb, 207Pb/204Pb and 208Pb/204Pb can increase over geological timescales and are highest in geologically ancient rocks, and those with high elemental U and Th content relative to Pb.18204Pb may suffer isobaric interference from 204Hg which, if not corrected for, can pose a problem in inductively-coupled-plasma mass spectrometry (ICP-MS). That being the case, Pb isotopic ratios over 206Pb are often used.
Like Sr, bioavailable Pb moves through the food chain without significant fractionation of its isotopes19,20 from the source bedrock via chemical weathering to soils and groundwater, and is then taken up by plants through their roots21. Industrial discharges and atmospheric transport and deposition of airborne Pb increase the Pb levels in soils, surface waters and the food chain.22 Pb in modern rainwater seems to be mainly from airborne particles derived from industrial sources, most of which appears to be taken up by surface soils.23 The introduction of alkyl-lead as an antiknock agent in petrol resulted in raised atmospheric Pb levels worldwide.24 In South Africa, leaded petrol reached its peak between the 1980s and 1990s.25 Since 1996, unleaded petrol has been available to motorists and its use gradually increased until 2006, when all leaded petrol was phased out and only lead-free petrol was available in South Africa.
In the oceans, Pb is not homogeneously distributed and has a much shorter residence time (8O-1O0 years)26 than Sr (2x107 years)12, resulting in Pb fluctuating with time as well as space. When comparing the Pb concentration and isotopic ratios of current surface waters in the South Atlantic Ocean to those measured in the 1990s, a decrease in the Pb concentration can be observed from 29 pmol/kg in 199027 to 17.7 pmol/kg in 201028. Also, the 206Pb/204Pb ratio has decreased from 18.3800 in May/June 199629 to 18.0730 in October 201028. Conversely, the 208Pb/206Pb ratio has increased from 2.0900 in May/June 199629 to 2.1040 in October 201028.
Pb and Sr as palaeodietary tracers in southwestern South Africa
A number of archaeological and palaeontological studies have analysed Pb isotopes in bones and teeth for examining past mobility and geographical origins of archaeological specimens.3033 Progressively more studies are comparing Pb with Sr isotopes, and concluding that whilst Pb and Sr isotopic systems alone can provide valuable information, a combination of the two techniques is a very powerful tool.30,32,33
Extensive research has been done on the bioavailable and whole-rock 87Sr/86Sr in southwestern South Africa (Table 1). Allsopp and Kolbe34 analysed whole-rock 87Sr/86Sr from the Malmesbury shale (0.7208-0.7873) and Cape Granite (0.7701-1.1602) for geological age determination. Sealy et al.2 analysed animal bones to estimate bioavailable 87Sr/86Sr. They reported values for shales (0.7178-0.7179) and sandstones (0.7154-0.7175) based on a limited number of samples from carefully chosen sites some distance from the coast, where the soils derived from the underlying geological formations. As a result, this study showed a clear separation between the values for shales and sandstones, and those for near-coastal marine sands (0.7094-0.7117).2 Copeland et al.4 and Lehmann et al.5 also assessed bioavailable 87Sr/86Sr by analysing plants from the south coast and animal bone and teeth samples from the west coast of southern Africa. They employed much wider-ranging sampling strategies and included samples from shales, granites and sandstones near the coast, with significant marine Sr input. This is reflected in the very broad 87Sr/86Sr ranges, with marine Sr input contributing to the lower extremes: 0.7095-0.7204 for the Malmesbury shales, 0.7095-0.7236 for the Cape Granite Suite and 0.7092-0.7237 for the Cape Supergroup.
Radloff et al.3 reported bioavailable 87Sr/86Sr values of different geological substrates in the De Hoop Nature Reserve in southwestern South Africa, measured on modern rodent teeth. There is a distinction between higher values for shales (0.7101-0.7104) and sandstones (0.7098-0.7100) and lower values for the coastal sands (0.7092-0.7093) and limestones (0.7091-0.7099)3, but the values for shales and sandstones are very low, reflecting a substantial contribution from seawater-derived Sr.
Limited research has been done on whole-rock or bioavailable Pb isotopic ratios in southwestern South Africa. Soderberg and Compton35 reported 206Pb/207Pb (1.141±0.008) and 208Pb/207Pb (2.404±0.017) for a protea sample derived from the Table Mountain substrate of the Cape Floristic Region.
Methods
Sample collection
The details of the samples analysed are given in Supplementary table 1. Those collected specifically for this project comprise a variety of bones and teeth from animals that had recently died natural deaths, as well as some plants. As the goal of this study was to characterise bioavailable Pb and Sr isotopic ratios, diversity in the plant and animal species is irrelevant. It is, however, important to avoid cultivated areas where artificial fertilisers may have been used. Most samples were collected in the last few years, and therefore date from the post-2006 era of unleaded petrol. The sample set includes a few samples from the 1980s, when leaded petrol was still in use in South Africa, but these samples are from remote areas where there is likely to have been little influence from motor vehicle emissions. Small mammals from De Hoop Nature Reserve were trapped and euthanised in 2010 for a previous study.3 The set of samples was derived from all of the major geological substrates of the Western Cape. Figure 1 is a geological map showing the sample collection locations.
Sample preparation
Bones and teeth were lightly sanded to remove superficial contamination. Pieces weighing approximately 50 mg were placed in vials filled with MilliQ-water in an ultrasonic bath for about 10 min, then left to dry on watch glasses in an oven at 40 °C overnight, after which they were ready for chemical processing. As most teeth were from small animals, they were processed as 'whole-tooth' samples. In only two cases (both antelope teeth) were dentine and enamel separated and processed individually.
Plant samples were placed in quartz crucibles (uncovered) in a muffle furnace at an initial temperature of 300 °C and the temperature was increased by 100 °C every hour until a temperature of 650 °C was reached; thereafter the samples were left overnight. Possible Pb loss through volatilisation was minimised by increasing the temperature of the furnace gradually and keeping it well below the boiling point of Pb (1749 °C). The resulting ashed samples were ground to a fine powder using a mortar and pestle. Approximately 50 mg of each ash was weighed out (masses were recorded) and placed in a 7-mL Teflon vial.
The combined Sr-Pb elemental separation method used in this study is based on that of Pin et al.36, with minor modifications (see supplementary material for laboratory protocol). Sr and Pb, present in only trace amounts, were concentrated and matrix elements were removed by passing the samples through Savillex Teflon columns filled with Sr.Spec resin (Eichrom), using 0.05 M HNO3. Samples were processed in batches of eight, along with a total procedural blank and a reference material (NM95 in-house carbonate standard for the bone and tooth samples, and ALR33G in-house basalt standard for the plant-derived mineral ash samples).
Measuring Sr and Pb concentrations and isotope ratios
Elemental concentrations of Sr and Pb were determined on a Thermo X-series II quadrupole ICP-MS, to assess the quantity of sample required for isotopic analysis. Because there is no published Sr or Pb concentration data for NM95, the in-house standard solutions were run as unknowns to assess accuracy. Calibration curves were obtained using artificial multielement standards, from which standard solutions were made.
Isotopic ratios of Sr and Pb were determined on a NuPlasma HR multi-collector (MC)-ICP-MS from Nu Instruments. Samples were introduced into the MC-ICP-MS as solutions, using the Nu Instruments DSN-100 desolvating nebuliser. Solution analysis typically requires at least 50 ng of the element of interest, achieved through Sr-Pb elemental separation chemistry as described above.
The separated Sr fraction for each sample, dissolved in 2 mL 0.2% HNO3, was diluted to 200 ppb Sr for isotope analysis. Analyses were referenced to bracketing analyses of NIST SRM987, using an 87Sr/86Sr reference value of 0.710255. All Sr isotope data were corrected for Rb interference using the measured signal for 85Rb and the natural 85Rb/87Rb ratio. Instrumental mass fractionation was corrected using the measured 86Sr/88Sr ratio, the exponential law, and a true 86Sr/88Sr value of 0.1194. Analytical error associated with measurements by solution is ±0.000020 (2 a). Sr isotope results for repeat analyses of the in-house reference materials agreed well with long-term results obtained in this facility. NM95 for this study: 87Sr/86Sr=0.708938±0.000022 (n=5) and long-term values: 87Sr/86Sr=0.708911 ±0.000040 (n=414). ALR33G for this study: 87Sr/86Sr=0.704890±0.000014 (n=1) and long-term values: 87Sr/86Sr=0.704901 ±0.000040 (n=72).
The separated Pb fraction, dissolved in 1 mL 2% HNO3, was diluted to 50 ppb for isotope analysis. NIST SRM997 Tl (thallium) was added to all standards and samples to give a Pb:Tl ratio of approximately 10:1. NIST SRM981 was used as the reference standard, with 208Pb/204Pb, 207Pb/204Pb and 206Pb/204Pb normalising values of 36.7219, 15.4963 and 16.9405, respectively.37 All Pb isotope data were corrected for Hg isobaric interference by subtraction of on-peak background measurements. Instrumental mass fractionation was corrected using the exponential law, and a 205Tl/203Tl value of 2.3889. Lead isotope results for repeat analyses of the in-house reference materials agreed well with long-term results obtained in this facility. NM95 for this study: 208Pb/204Pb=38.1897±0.2386 (n=5), 207Pb/204Pb=15.7898±0.0319 (n=5), 206Pb/204Pb=20.5553±0.5133 (n=5) and long-term values: 208Pb/204Pb=38.2295±0.0436 (n=11), 207Pb/204Pb=15.7892±0.0360 (n=11), 206Pb/204Pb=20.6682±0.1521 (n=11). ALR33G for this study: 208Pb/204Pb=38.8608±0.0028 (n=1), 207Pb/204Pb=15.6174±0.0010 (n=1), 206Pb/204Pb=18.4264±0.0010 (n=1) and long-term values: 208Pb/204Pb=38.8510±0.0140 (n=16), 207Pb/204Pb=15.6152±0.0031 (n=16), 206Pb/204Pb=18.4248±0.0069 (n=16).
Results and discussion
Elemental concentrations and isotopic ratios of Sr and Pb for all samples in this study are listed in Supplementary table 1.
Sr and Pb concentrations
Sr concentrations of samples analysed here were in the range of 111-1862 ppm, while Pb concentrations were in the range of 0.012- 2.30 ppm. As shown in Figure 2, all bone samples had Sr concentrations below 900 ppm and Pb concentrations below 1 ppm, while the whole-tooth samples had Sr concentrations up to about 1900 ppm with Pb concentrations below 0.8 ppm. This finding is as expected, given that whole-tooth samples consist largely of enamel, with a much higher mineral content than bone. The 10 samples with [Sr]>1000 ppm were rock hyrax (dassie) whole-tooth samples from the Cape Supergroup, Karoo sediments and Namaqua-Natal metamorphic province, as well as the vlei rat tooth from Bredasdorp sediments. Of the entire sample set, only seven samples had Pb concentrations above 0.5 ppm. In the two cases in which the dentine and enamel of the tooth were separated, Pb concentrations were higher in dentine than in enamel, as seen in previous studies.30 In addition, the Pb concentrations were higher in the dentine compared with the individual's bone. Sr and Pb concentrations were patterned by geological substrate. All samples from regions underlain by Karoo sediments had Pb concentrations below 0.4 ppm, with Sr concentrations ranging from 111 ppm for bone samples to 1862 ppm for the tooth samples. The samples from regions underlain by Bredasdorp limestones and coastal terrestrial substrates had relatively low Pb (<0.7 ppm) and moderate Sr concentrations (<700 ppm), compared with the rest of the sample set.
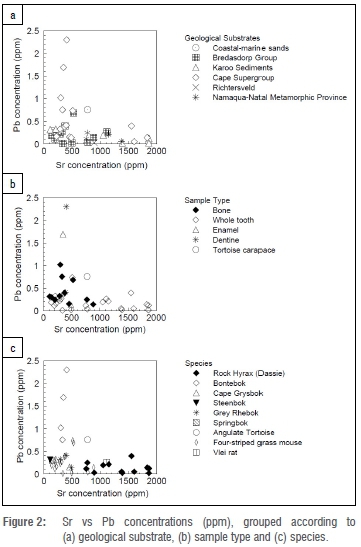
Sr isotopic ratios
Figure 3 illustrates the new bioavailable 87Sr/86Sr values obtained for the major geological substrates of southwestern South Africa. The bioavailable 87Sr/86Sr ranges for each geological substrate were as follows (from youngest to oldest geological age): 0.709282-0.709483 for the Cenozoic coastal sands, 0.709141-0.709942 for the Bredasdorp limestones, 0.715184-0.718972 for the Late Carboniferous Karoo sediments, 0.709925-0.713088 for the Ordovician Table Mountain sandstones of the Cape Supergroup, and 0.711469-0.714618 for the Late Precambrian to Early Cambrian granitoids of the Cape Granite Suite.

Samples from coastal marine sands ('Cenozoic deposits' in Figure 1) had 87Sr/86Sr close to the marine value of 0.709217, reflecting the marine-shell-rich coastal sands and the influence of sea spray. It is clear from Figure 4 that the 87Sr/86Sr values of the samples from the Table Mountain sandstones in De Hoop Nature Reserve (closed symbols at 2.5-5 km from coastline) are much lower than those from Gifberg and Bontebok National Park (40-50 km from coast). Copeland et al.4 also reported lower 87Sr/86Sr values for samples collected near the coast (as shown in their Figure 5). They did not, however, distinguish different geologies. Plotting 87Sr/86Sr of both their and our samples from Table Mountain sandstones against distance from the coast shows increasing 87Sr/86Sr as one moves further inland (Figure 4), i.e. falling off of marine-derived Sr. The effect of marine-derived Sr appears to extend as far as 40 km inland. Similar results have been reported by other researchers38,39; the magnitude of the effect depends on atmospheric circulation and is greater in soils with low Sr concentrations. Setting aside samples from older substrates close to the coast (e.g. Cape granites at Vredenburg Peninsula), the older substrates (Cape granites, Table Mountain sandstones, Malmesbury shales) show higher 87Sr/86Sr than the Cenozoic sands and Bredasdorp formation, which have values closer to seawater.

Figure 5 shows the ranges of bioavailable 87Sr/86Sr found in this study compared with those found in previous studies. We report much narrower Sr isotopic ranges for each substrate than Copeland et al.4 and Lehman et al.5, although the same analytical methods were applied using the same analytical facility. In this study, the ranges of the Cape and Karoo geologies are distinct, whereas Copeland et al.4 found them to overlap. This difference may in part be a sample population effect, as sample populations in this study (n=8 for the Karoo, 15 for Cape Supergroup) were smaller than those of Copeland et al.4 (50 and 35 respectively).
The bioavailable 87Sr/86Sr ranges for the older geological substrates from northwestern South Africa were as follows: 0.734004-0.755445 for the Namaqua-Natal metamorphic province and 0.726132 for the sample from the Richtersveld. High values are consistent with the underlying older Mesoproterozoic rocks, comprising highly deformed ultrametamorphic rocks, gneisses and migmatites.40
Pb isotopic ratios
The new bioavailable 208Pb/204Pb, 207Pb/204Pb and 206Pb/204Pb ranges for the geological substrates from the Western Cape (from youngest to oldest geological age) are given in Table 2.
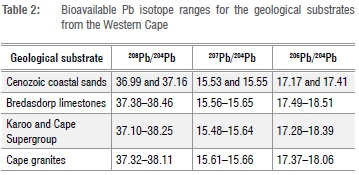
The Cape granites at Rooiheuwel farm have narrower bioavailable Pb isotopic ranges compared with the Karoo and Cape samples and show a slight offset from the rest of the samples. This offset is only seen in Figure 6a (207Pb/204Pb vs 206Pb/204P) and not in Figure 6b (208Pb/204Pb vs 206Pb/204Pb). This result is not unexpected, as the Cape granites are known to have high concentrations of U and Th relative to Pb.41,42 The half-life of 235U - 207Pb (0.70x109 years) is much shorter than that of 238u - 206Pb (4.47x109 years) and 232Th - 208Pb (14.01x109 years), therefore the initial production of 207Pb is much more rapid than 206Pb and 208Pb.43 This results in the initial rapid increase in the 207Pb/204Pb ratio of a geological system, as observed here for the Cape granites. For the Namaqua-Natal metamorphic province, the two samples from the granites on Dabidas farm plot between the Cape granites and the rest of the samples, while the two samples from the ultrametamorphic rocks of the Namaqua National Park had very different Pb isotopic ratios from the rest of the samples (Figure 6).
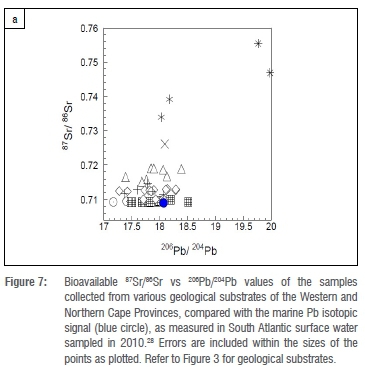
Figure 6 presents the new bioavailable Pb isotopic data of samples collected from various geological substrates of the Western and Northern Cape Provinces compared with the marine Pb isotopic signal, as measured in South Atlantic surface water sampled in 2010.28 Figure 7 compares the bioavailable 87Sr/86Sr and 206Pb/204Pb values with the respective marine values. Although samples from the Cenozoic coastal marine sands exhibited strongly marine Sr signals, their Pb isotopic ratios were quite unlike the marine Pb signal. The 206Pb/204Pb values for the Bredasdorp limestones are more varied, ranging between 17.49 and 18.51, compared with their 87Sr/86Sr values which range between 0.709141 and 0.709942. The marine contribution to bioavailable Sr in the terrestrial environment is much greater than the contribution to bioavailable Pb, due to the higher concentration of Sr (7.62 ppm)12 compared with Pb (5.22x10-6 ppm) in seawater.28
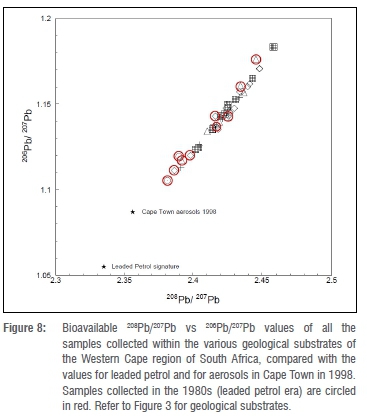
Some samples in this study were collected during the 1980s, when leaded petrol was still in use (it was phased out in 2006). The 208Pb/207Pb (2.353-2.358) and 206Pb/207Pb (1.085-1.090) ratios of Cape Town aerosols in 199824 were somewhat higher than the leaded petrol signature of 2.335 and 1.05524, respectively (Figure 8). The 1980s samples had 208Pb/207Pb and 206Pb/207Pb values distinctly higher than Cape Town's aerosols in 1998 and even higher than the leaded petrol signature. The lowest 208Pb/207Pb (2.3812) and 206Pb/207Pb (1.1054) values were measured in a tortoise from Koeberg (coastal terrestrial substrate), collected in 1982. Figure 8 shows that the red circled points (samples from the 1980s leaded petrol era) cover the same range as the non-circled points, so there appears to be no contribution from leaded petrol. These 1980s samples were collected from national parks in the Western Cape, or in coastal areas where emissions from motor vehicles are much lower than in urban areas.
Conclusions
This study has added to our database of measurements of bioavailable 87Sr/86Sr from the Western Cape Province of South Africa. 87Sr/86Sr can efficiently discriminate between coastal-marine environments and older geological substrates lying further inland. Organisms living on older geological substrates close to the coast have lowered 87Sr/86Sr as a result of marine Sr input. This decreases with increasing distance from the coast; the effects may be seen up to 40 km inland.487Sr/86Sr measurements alone cannot distinguish between the Cape Granite Suite and the Cape Supergroup (Table Mountain sandstones), whereas Pb isotopes can, as shown in this study. Pb isotopic ratios of terrestrial plants and animals living close to the coast are distinct from seawater values. There does not appear to be significant alteration from marine-derived Pb in sea spray or similar sources. Pb is much less abundant in seawater than Sr12,28, which could explain why the marine contribution to bioavailable Sr in the terrestrial environment is much greater than the contribution to bioavailable Pb. Ultimately, Pb isotope data can give valuable information on palaeolandscape usage, and can be used as an additional isotope system to extend interpretations based solely on Sr isotopes.
Samples collected from relatively remote localities in the 1980s had Pb isotope ratios similar to those of more recent samples from the same geologies, and distinct from leaded petrol. They do not appear to be compromised by contamination from leaded petrol. It should therefore be possible to use historical samples, e.g. from museum collections, in studies of this kind.
In conclusion, we have demonstrated the value of using a combination of both 87Sr/86Sr and Pb isotope systems in coastal terrestrial environments to trace mobility or migration and landscape usage. This use has applications in archaeology, palaeontology, studies of animal migration, wildlife forensics and more.
Acknowledgements
We thank the South African Research Chairs Initiative of the Department of Science and Innovation and the National Research Foundation of South Africa (grant no. 84407) for their generous bursary support which made this project possible. We also thank Fayrooza Rawoot, Kerryn Gray and Christel Tinguely of the Department of Geological Sciences at UCT, for their assistance in preparing and analysing the samples. Lastly, we thank Mr Danie Kotzé from Rooiheuwel farm, who allowed us to collect necessary samples on his farm, as well as Frans Radloff for making available samples from his earlier studies. This publication is an output of the Biogeochemistry Research Infrastructure Platform of the Department of Science and Innovation of South Africa.
Authors' contributions
M.S.: Methodology; data collection; sample collection; data analysis; validation; data curation; writing - the initial draft; writing - revisions; project leadership and management. Pl.R.: Conceptualisation; methodology; validation; data curation; writing - revisions; student supervision; project leadership and management. J.S.: Conceptualisation; data curation; writing - revisions; student supervision; project leadership and management. R.P: Data curation; writing - revisions; student supervision; project leadership and management.
References
1. Hobson KA, Barnett-Johnson R, Cerling T. Using isoscapes to track animal migration. In: West J, Bowen G, Dawson T, Tu K, editors. Isoscapes. Dordrecht: Springer; 2010. p. 273-298. https://doi.org/10.1007/978-90-481-3354-3_13 [ Links ]
2. Sealy JC, Van der Merwe NJ, Sillen A, Kruger FJ, Krueger HW. 87Sr/86Sr as a dietary indicator in modern and archaeological bone. J Archaeol Sci. 1991;18(3):399-416. https://doi.org/10.1016/0305-4403(91)90074-Y [ Links ]
3. Radloff FGT, Mucina L, Bond WJ, Le Roux PJ. Strontium isotope analyses of large herbivore habitat use in the Cape Fynbos region of South Africa. Oecologia. 2010;164:567-578. https://doi.org/10.1007/s00442-010-1731-0 [ Links ]
4. Copeland SR, Cawthra HC, Fisher EC, Lee-Thorp JA, Cowling RM, Le Roux PJ, et al. Strontium isotope investigation of ungulate movement patterns on the Pleistocene Paleo-Agulhas Plain of the Greater Cape Floristic Region, South Africa. Quat Sci Rev. 2016;141:65-84. https://doi.org/10.1016/j.quascirev.2016.04.002 [ Links ]
5. Lehmann SB, Levin NE, Braun DR, Stynder DD, Zhu M, Le Roux PJ, et al. Environmental and ecological implications of strontium isotope ratios in mid-Pleistocene fossil teeth from Elandsfontein, South Africa. Palaeogeogr Palaeoclimatol Palaeoecol. 2018;490:84-94. https://doi.org/10.1016/j.palaeo.2017.10.008 [ Links ]
6. Aggarwal J, Habicht-Mauche J, Juarez C. Application of heavy stable isotopes in forensic isotope geochemistry: A review. Appl Geochem. 2008;23(9):2658-2666. https://doi.org/10.1016/j.apgeochem.2008.05.016 [ Links ]
7. Bentley RA. Strontium isotopes from the earth to the archaeological skeleton: A review. J Archaeol Method Theory. 2006;13(3):135-187. https://doi.org/10.1007/s10816-006-9009-x [ Links ]
8. Rankama K. Isotope geology. London: Pergamon Press; 1954. [ Links ]
9. Faure G. Principles of isotope geology. 2nd ed. New York: John Wiley & Sons Inc.; 1986. https://doi.org/10.1017/S0016756800017453 [ Links ]
10. Pollard M, Batt C, Stern B, Young SMM. Analytical chemistry in archaeology. New York: Cambridge University Press; 2007. https://doi.org/10.1017/CBO9780511607431 [ Links ]
11. Graustein WC. 87Sr/86Sr ratios measure the sources and flow of strontium in terrestrial ecosystems. In: Rundel PW, Ehleringer JR, Nagy KA, editors. Stable isotopes in ecological research. New York: Springer, 1989 p. 491-512. https://doi.org/10.1007/978-1-4612-3498-2_28 [ Links ]
12. Capo RC, Steward BW, Chadwick OA. Strontium isotopes as tracers of ecosystem processes: Theory and methods. Geoderma. 1998;82(1-3):197-225. https://doi.org/10.1016/S0016-7061(97)00102-X [ Links ]
13. Sillen A, Hall G, Richardson S, Armstrong R. 87Sr/86Sr ratios in modern and fossil food-webs of the Sterkfontein Valley: Implications for early hominid habitat preference. Geochim Cosmochim Acta. 1998;62(14):2463-2473. https://doi.org/10.1016/S0016-7037(98)00182-3 [ Links ]
14. Price TD, Burton JH, Bentley RA. The characterization of biologically available strontium isotope ratios for the study of prehistoric migration. Archaeometry. 2002;44(1):117-135. https://doi.org/10.1111/1475-4754.00047 [ Links ]
15. Ericson JE. Strontium isotope characterization in the study of prehistoric human ecology. J Hum Evol. 1985;14(5):503-514. https://doi.org/10.1016/S0047-2484(85)80029-4 [ Links ]
16. Whipkey CE, Capo RC, Chadwick OA, Steward BW. The importance of sea spray to the cation budget of a coastal Hawaiian soil: A strontium isotope approach. Chem Geol. 2000;168(1-2):37-48. https://doi.org/10.1016/S0009-2541(00)00187-X [ Links ]
17. Veizer J. Strontium isotopes in seawater through time. Ann Rev Earth Planet Sci. 1989;17:141-167. https://doi.org/10.1146/annurev.ea.17.050189.001041 [ Links ]
18. Bilström K. Radiogenic isotopes and their applications within a range of scientific fields. In: Subias I, Bauluz B, editors. Instrumental techniques applied to mineralogy and geochemistry. Madrid: Spanish Society of Mineralogy; 2008. p. 111-131. [ Links ]
19. Gulson BL. Lead isotopes in mineral exploration. Amsterdam: Elsevier Science Publishers; 1986. [ Links ]
20. Hoefs J. Stable isotope geochemistry. 3rd ed. Berlin: Springer-Verlag; 1997. https://doi.org/10.1007/978-3-662-03377-7 [ Links ]
21. Adriano DC. Trace elements in the terrestrial environment. Berlin: SpringerVerlag; 1986. https://doi.org/10.1007/978-1-4757-1907-9 [ Links ]
22. Ghazi MA, Millette JR. Lead. In: Morrison RD, Murphy BL, editors. Environmental forensics. Burlington, MA: Academic Press; 2006. p. 56-74. [ Links ]
23. Bacon J, Bain D. Characterization of environmental water samples using strontium and lead stable-isotope compositions. Environ Geochem Health. 1995;17(1):39-49. https://doi.org/10.1007/BF00188631 [ Links ]
24. Bollhöfer A, Rosman KJR. Isotopic source signatures for atmospheric lead: The southern hemisphere. Geochim Cosmochim Acta. 2000;64(19):-3251-3262. https://doi.org/10.1016/S0016-7037(00)00436-1 [ Links ]
25. Mathee A, Röllin H, Von Schirnding Y Levin J, Naik I. Reductions in blood lead levels among school children following the introduction of unleaded petrol in South Africa. Environ Res. 2006;100(3):319-322. https://doi.org/10.1016/j.envres.2005.08.001 [ Links ]
26. Libes SM. An introduction to marine biochemistry. Chichester: John Wiley & Sons; 1992. [ Links ]
27. Helmers E, Van der Loeff M. Lead and aluminum in Atlantic surface waters (50°N to 50°S) reflecting anthropogenic and natural sources in the eolian transport. J Geophys Res. 1993;98(C11):20261-20273. https://doi.org/10.1029/93JC01623 [ Links ]
28. Paul M, Bridgestock L, Rehkämper M, Van DeFlierdt T, Weiss D. High-precision measurements of seawater Pb isotope compositions by double spike thermal ionization mass spectrometry. Anal Chim Acta. 2015;863:59-69. https://doi.org/10.1016/j.aca.2014.12.012 [ Links ]
29. Alleman LYChurch TM, Veron AJ, Kim G, Hamelin B, Flegal AR. Isotopic evidence of contaminant lead in the South Atlantic troposphere and surface waters. Deep Sea Res Part II. 2001;48(13):2811-2827. https://doi.org/10.1016/S0967-0645(01)00019-4 [ Links ]
30. Montgomery J. Lead and strontium isotope compositions of human dental tissues as an indicator of ancient exposure and population dynamics [PhD thesis]. Bradford: University of Bradford; 2002. https://doi.org/10.5284/1000249 [ Links ]
31. Ghazi AM. Lead in archaeological samples: An isotopic study by ICP-MS. Appl Geochem. 1994;9(6):627-636. https://doi.org/10.1016/0883-2927(94)90023-X [ Links ]
32. Shaw H, Montgomery J, Redfern R, Gowland R, Evans J. Identifying migrants in Roman London using lead and strontium stable isotopes. J Archaeol Sci. 2016;66:57-68. https://doi.org/10.1016/j.jas.2015.12.001 [ Links ]
33. Dudás FÖ, LeBlanc SA, Carter SW, Bowring SA. Pb and Sr concentrations and isotopic compositions in prehistoric North American teeth: A methodological study. Chem. Geol. 2016; 429:21-32. https://doi.org/10.1016/j.chemgeo.2016.03.003 [ Links ]
34. Allsopp HL, Kolbe P. Isotopic age determinations on the Cape granite and intruded Malmesbury sediments, Cape Peninsula, South Africa. Geochim Cosmochim Acta. 1965;29(10):1115-1130. https://doi.org/10.1016/0016-7037(65)90115-8 [ Links ]
35. Soderberg K, Compton JS. Dust as a nutrient source for fynbos ecosystems, South Africa. Ecosystems. 2007;10:550-561. https://doi.org/10.1007/s10021-007-9032-0 [ Links ]
36. Pin C, Gannoun A, Dupont A. Rapid, simultaneous separation of Sr, Pb, and Nd by extraction chromatography prior to isotope ratios determination by TIMS and MC-ICP-MS. J Anal At Spectrom. 2014;29(10):1858-1870. https://doi.org/10.1039/C4JA00169A [ Links ]
37. Galer S, Abouchami W. Practical application of lead triple spiking for correction of instrumental mass discrimination. Mineralogical Magazine. 1998;62A(1):491-492. [ Links ]
38. Quade J, Chivas AR, McCulloch MT. Strontium and carbon isotope tracers and the origins of soil carbonate in South Australia and Victoria. Palaeogeogr Palaeoclimatol Palaeoecol. 1995;113(1):103-117. https://doi.org/10.1016/0031-0182(95)00065-T [ Links ]
39. Evans J. Biosphere isotope domain map GB (V1): Strontium isotope data. British Geological Survey. 2018. http://dx.doi.org/10.5285/ba36de6f-5a20-476b-965d-48182166114a [ Links ]
40. Cornell DH, Thomas RJ, Moen HFG, Reid DL, Moore JM, Gibson RL. The Namaqua-Natal Province. In: Johnson MR, Anhaeusser CR, Thomas RJ, editors. The geology of South Africa. Johannesburg: Geological Society of South Africa and Council for Geoscience; 2016. [ Links ]
41. Schoch AE, Leygonie FE, Burger AJ. U-Pb ages for Cape granites from the Saldanha Batholith: A preliminary report. Trans Geol Soc S Afr. 1975;78(1):97-100. [ Links ]
42. Scheepers R, Poujol M. U-Pb zircon age of Cape Granite Suite ignimbrites: Characteristics of the last phases of the Saldanian magmatism. S Afr J Geol. 2002;105(2):209-224. https://doi.org/10.2113/105.2.163 [ Links ]
43. Dickin AP. Radiogenic isotope geology. Cambridge: Cambridge University Press; 1995. [ Links ]
 Correspondence:
Correspondence:
Mari Scott
mari.scott@uct.ac.za
Received: 22 July 2019
Revised: 26 Feb. 2020
Accepted: 01 Mar. 2020
Published: 27 May 2020
Editor: Priscilla Baker
Funding: National Research Foundation (South Africa) (grant no. 84407)
Supplementary Data
The supplementary data is available in pdf:[Supplementary Data]














