Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.3-4 Pretoria mar./abr. 2020
http://dx.doi.org/10.17159/sajs.2020/6822
RESEARCH ARTICLE
Sciarid pests (Diptera: Sciaridae) from undercover crop production in South Africa
Agil KatumanyaneI; Aquillah M. KanziII; Antoinette R MalanI
IDepartment of Conservation Ecology and Entomology, Stellenbosch University, Stellenbosch, South Africa
IIDepartment of Biochemistry Genetics and Microbiology, Forestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa
ABSTRACT
Fungus gnats (sciarids) are among the most important pests in undercover crop production. They cause direct physical damage to plant roots, transfer fungal pathogens and create entry points for soil-borne plant pathogens. In 2007, Bradysia impatiens, an important fungus gnat pest was found in association with major tree nursery beds in the Mpumalanga and KwaZulu-Natal Rrovinces of South Africa and was considered invasive. In this study, eight greenhouses were surveyed in the Western Cape Province and B. impatiens was found to be present in all the greenhouses. Similar to the results of the previous studies, a high haplotype diversity was identified for B. impatiens, which may indicate multiple strain introductions into South Africa. Two other fungus gnat species, Lycoriella sativae and Lycoriella ingenua - globally important sciarid pests of mushroom cultures - were identified as new from South Africa. Through a laboratory culture, the life cycle of B. impatiens was observed to be approximately 21 days at 25 °C. Females laid between 100 and 250 eggs. Rossible introduction sources include contaminated vegetative material and growth media, thus there maybe need to revise the importation restrictions on these commodities. The identification of two novel species of sciarid pests that have only previously been identified in the Holarctic region could further emphasise this need. However, the recent discovery of such high sciarid diversity could also be due to only a few studies having been previously done on sciarid pests in South Africa.
SIGNIFICANCE:
• The fungus gnat species Bradysia impatiens is reported as present in several undercover crops in the Western Cape Province of South Africa, including cucumbers, tomatoes, chrysanthemums, mushrooms, blueberries and various herbs.
• Lycoriella ingenua and Lycoriella sativae were also found to be present in mushroom gardens - the first report of Lycoriella from the Afro-tropical region.
• The three fungus gnat species are some of the most important sciarid pests in undercover crop production worldwide. This study highlights the need for more studies on the distribution of these sciarids and possible invasion history.
Keywords: Afrotropical diptera, Bradysia impatiens, invasive species, Lycoriella ingenua, Lycoriella sativae
Introduction
Dark-winged fungus gnats (Diptera: Sciaridae) are some of the major insect pests in undercover crop production and are found in greenhouses, nursery bed crops, house plants and mushroom farms, among others. They are known to cause economic losses through direct feeding on the roots, contamination of vegetative material and marketing problems.1,2 Their secondary effects include the transmission of fungal spores3-5, and the creation of entry points for soil-borne plant pathogens, which have in the past tended to be overlooked1,6,7.
Fungus gnats are a problem, primarily under conditions of excessive moisture, which commonly occur during propagation8, at which time cuttings and plugs are developing young root systems. The larvae use their prominent chewing mouth parts to feed on most plant parts, especially on young and developing plant root systems, tender roots and root hairs. Fungus gnat larval feeding results in significant physical damage to the roots and a decrease in the plant root biomass.9 Adult flies have also been reported to transfer fungal pathogens3,5 when they fly from one plant to another. Additionally, they cause discomfort to farm labourers,10 which may reduce worker productivity.
The symptoms presented by the affected plants include wilting, loss of vigour, reduced vegetative development, and loss of leaves.3 The roots generally appear to be abraded with small brown lesions.1 The effects concerned may result in the death of the plant, especially in cases of heavy infestation, as have been reported by Springer1 and Mansilla and Rastoriza6, whereas the physical damage results in the weakening of the plants involved, which reduces their marketability.9 The combined effects of pests not only reduce the resulting yield, but also to a large extent increase the production costs per hectare.11
Bradysia impatiens Johannsen is one of the most prominent sciarid pests in greenhouses in the world and has recently been identified in South African pine tree nursery beds as an introduced species.12,13 In this study, B. impatiens is reported as a present pest in several other crops in the Western Cape Province. Other than B. impatiens, this study reports Lycoriella ingenua Dufour and Lycoriella sativae Johannsen as pests of mushrooms from South Africa and the Afro-tropical region for the first time. Lycoriella ingenua and L. sativae are the most important sciarid pests in mushrooms worldwide.14,15 An overview of the current Afro-tropical fungus gnats can be found in Menzel and Smith16.
Recently, fungus gnats were reported as concerning by many greenhouse farmers in the Western Cape Province of South Africa. This study was then conducted to determine their diversity and biology. Fungus gnat samples were collected from eight targeted greenhouses. The identification and life cycle of B. impatiens and its culture on artificial media, under laboratory conditions, were also investigated.
Materials and methods
Insect collection
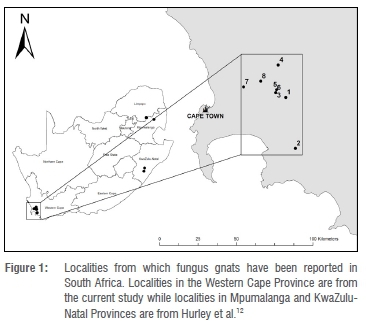
Adult fungus gnats and larvae were collected from eight greenhouses located in the Western Cape Province of South Africa (Figure 1, Table 1), after they had been reported as concerning pests by greenhouse growers. The various crops grown in these greenhouses included cucumbers, tomatoes, different herbs, blueberries, mushrooms and chrysanthemums. The cucumber farm was the most heavily infested and was the principal target for this study.
Infested plant parts, as well as the organic growing medium were randomly collected and brought to the laboratory. The mixture of plant parts and growing media was placed in closed plastic containers that were lined with moist paper towels. The containers were kept in a growth chamber (MRC 358, Labotec) at 25 °C, and the emerging adult fungus gnats were collected and used for identification. The collections from the plant greenhouses were made between February and May 2016 while the collections from the mushroom farms where made in April 2017.
Identification of the Bradysia species
Adults, and in some cases first-generation larvae, were used in the molecular identification of the species by means of the cytochrome oxidase 1 (COI) gene. DNA was separately extracted from the intact larvae and adults using the column-based QIAamp® DNA micro-extraction kit. The following Folmer primer set was used in the polymerase chain reaction (PCR): (LCO1490) 5'-GGTCAACAAATCATAAAGATATTGG-3' and (HCO2198) 5'-TAAACTTCAGGGTGACCAAAAAATCA-3'. The primer sets are used to amplify a 658-bp fragment of the COI gene in a wide range of invertebrate taxa.17 The resulting DNA sequences were edited using CLC Main Workbench (ver. 7.7.3) and blasted in on the US National Center for Biotechnology Information (NCBI)'s GenBank to determine the species identity. To determine to which clades these samples belonged, a neighbour joining tree was generated using MEGA V 6.18 Additional sequences of fungus gnats were obtained from GenBank and added into the analysis. These sequences included COI sequences of B. impatiens (MG295935.1, KR756595.1, EU450797.1, DQ060500.1), Bradysia nomica (KY846435.1), Bradysia aprica (JX418164.1), Bradysia longimentula (JN378636.1), Bradysia pallipes (MG295853.1), Bradysia hilaris (MG159303.1), Bradysia ocellaris (MG155760.1), Bradysia japponica MG157878.1 and Masakimyia pustulae (JQ613784.1). A distance-based phylogeny was generated using the neighbour-joining method.19 The test of phylogeny was performed using the bootstrap method and 1000 replicates. Sequence polymorphism was analysed using DnaSP v.6.20 The p-distance was used as the substitution model with transition and transversion included. Specimens were further morphologically verified by Hans-Georg Rudzinski (Entomo-graphisches Studio, Schwanewede, Germany) and Kai Heller (independent researcher, Quickborn, Germany).
Bradysia impatiens rearing on artificial media
The fungus gnats that were collected from the cucumber greenhouse were used to establish a culture in the laboratory. The method used to raise the fungus gnat culture was a combination of modified protocols employed by Cloyd and Dickinson21 and Lee et al.22 Greenhouse media, which consisted of partially composted, 3-mm pine wood chips (sawdust), were sterilised at 40 °C overnight, and then allowed to cool to room temperature. Sterilising the substrates at this temperature ensured the death of contaminating micro- and macro-organisms, but allowed survival of the fungal spores. The substrate was then mixed with soy meal and cornmeal, in a ratio of 3:1:1, respectively. The mixture was used as the oviposition and growth medium throughout the experiments.
Glass Petri dishes (100 x 20 mm) were lined with moist filter paper (Whatman No. 1, 90 mm), filled with moist growth media, then covered and left for 24-48 h in the growth chamber, to allow for fungal colonisation. The Petri dishes were then placed in a Perspex box, along with other Petri dishes containing adult fungus gnats. The new Petri dishes were kept moist, to attract the female fungus gnats for egg laying. After 24 h, the Petri dishes (now containing eggs), were removed, covered and transferred to a growth chamber, where they were kept at a temperature of 25 °C. The Petri dish covers were fitted with thin tissue paper to prevent the accumulation of condensed moisture on top, opened frequently for aeration, and a few drops of water were added daily until the onset of pupation. Food, consisting of cornmeal and soy meal, was sprinkled on top of the Petri dishes every second or third day, depending on the needs of the larvae population involved. As soon as the adults emerged, the original Petri dishes were transferred to the Perspex box, where they were left open for the adults to emerge, mate and fly to lay eggs in new Petri dishes.
The cycle was maintained for more than 10 generations. A new container was used for each generation, to prevent contamination of the later generations, especially in respect to mites. However, controlling all types of contamination, especially mites, in the medium was difficult, as the adult fungus gnats were observed to carry mites on their bodies as they flew to new growth media. An extra effort to reduce the mite population was made through establishing cultures using fungus gnat larvae rinsed in distilled water. Even then, mites were still a constant problem in the growth medium. The Perspex box containing the fly cultures was kept at room temperature.
Life cycle of Bradysia impatiens under laboratory conditions
To observe the life cycle of B. impatiens, an oviposition and growth medium was prepared as described above; however, finer wood chips of about 500 microns were used. The finer wood chips were obtained by means of drying out the pine sawdust at 40 °C overnight, and by blending the dry sawdust using a commercial bar blender (stainless steel; HBB250SR). The resulting powder was then sieved through a 500-μm sieve. The smaller pine wood chips permitted easy observation and the counting of the eggs laid by each female fungus gnat. The smaller cell culture dishes (35 x 10 mm CELLSTAR®) that were used for the purpose were lined with 3-cm moist filter papers (Whatman®). Freshly emerged (neonate) adult fungus gnats (1 female and 2 males) were aspirated into the growth media, where they could mate and oviposit. After the death of the females, the eggs were counted using a light microscope, and oviposition behaviour was observed. It was important to count the eggs immediately, because fungi colonisation in the media - resulting from the dead adults - would make it difficult to observe the egg-laying patterns or to count the eggs later. The Petri dishes were then fitted with fine tissue paper on the Petri dish cover, and transferred to the growth chamber to allow for the emergence and growth of the fungus gnat larvae. Individuals from each growth stage - eggs, different larval instars, pupae and adults - were randomly removed from the medium and imaged using a Zeiss Stereo Discovery.V8 microscope fitted with an Axiocam ERc 5s (n=20). The ZEISS Labscope App for iPad was then used to measure the length and width of each selected individual.
Results
Insect collection
All insects collected were from greenhouses in the Western Cape Province, South Africa (Figure 1, Table 1). The different host crops include chrysanthemums, herbs, cucumbers, tomatoes, blueberries and mushrooms.
Identification of Bradysia and Lycoriella species
Figure 2 shows the morphology of the three identified species. The specimens were identified morphologically using the male genitalia and the length of the antennae. Male B. impatiens are characterised by a comb-like row of bristles on the fore tibia and the antennal segments are more compact. Lycoriella ingenua is easily recognised by the conspicuous bristles on the basal lobe of male genitalia, while Lycoriella sativae is characterised by a fuscous, darker hypopygium and longer antennae.

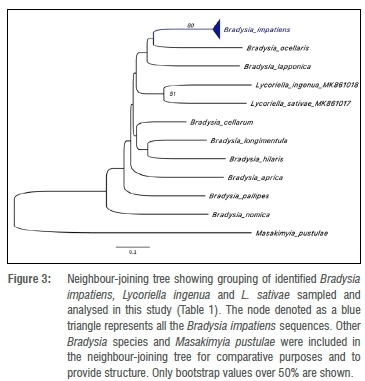
For molecular identification, the resulting DNA sequences were blasted on GenBank which resulted in a 100% match with B. impatiens, L. sativae and L. ingenua. These sequences were submitted in the NCBI GenBank database (Table 1). The results from the neighbour-joining tree showed that the B. impatiens grouped together with other Bradysia species. It seems that there are variations in the sequences as observed in the neighbour-joining tree, which shows sub-groups within the B. impatiens clade, although unsupported by bootstrap support values. Analysis of sequence polymorphism revealed eight polymorphic sites (Table 2).
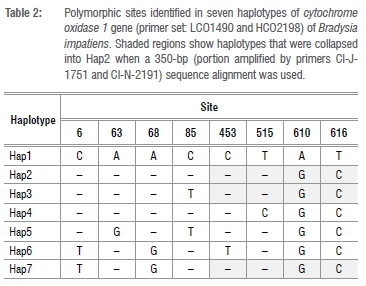
Based on these polymorphisms, these sequences were grouped into seven haplotypes: Hap1 (B_imp1-10), Hap2 (B_imp11-23), Hap3 (B_imp24-25), Hap4 (B_imp36), Hap5 (B_imp37), Hap6 (B_imp38-44) and Hap7 (B_imp45). The haplotype (gene) diversity was 0.788 and the nucleotide diversity was 0.00325. Sequences homologous to L. sativae and L. ingenua grouped together, but separately from the Bradysia species (Figure 3). Haplotype analysis using a portion of sequence (350 bp) amplified by the primers CI-J-1751 and CI-N-2191 used in a previous study by Hurley et al.23 to analyse a population of B. difformis, revealed four haplotypes including Hap1 (B_imp1-10), Hap2 (B_imp11-35,37,45), Hap3 (B_imp36) and Hap4 (B_imp38-44). In this data set, the haplotype diversity was 0.579 while the nucleotide diversity was 0.00258.
Rearing of Bradysia impatiens on artificial media
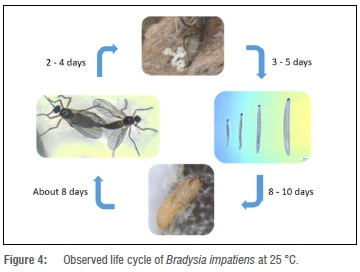
The culture medium used served as an ideal oviposition and growth medium for the fungus gnats. Up to 10 generations of fungus gnats were maintained on the same constitution of the culturing medium, without any changes being noticed in the behaviour of the fungus gnat colony. A diagram of the observed life cycle is shown in Figure 4.
Life cycle of Bradysia impatiens under laboratory conditions
The adults
Newly emerged adults had a pale neonate body with wings that barely covered the abdomen. The wings attained full length in under 30 min after emergence. The emerged adults were very active. Within a few hours after emergence, the adults had a dark-brown or dark body colour and dark wings. Mating occurred within the first few hours after emergence. The body size of the adults was 2-3 mm, with a wingspan of about 7 mm. Males were slightly smaller than females. The males hovered on top of the medium, with a bent abdomen, as they waited to mate with the emerging females. After mating, the females lived for approximately 2-4 days, after which they laid eggs and died, either immediately after or during the process of egg laying. They lowered their abdomens into crevices to lay their eggs on top of the culturing medium, or just below the moist wooden chips. If the medium particles were small enough, as was the case with the 500-μm wood chips, the females lowered their abdomens into the medium, thus completely covering the eggs.
Eggs
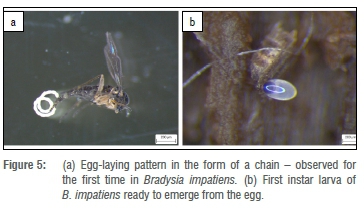
Egg laying was variable among females, which tended to lay eggs either in groups of variable numbers or singly, while moving on top of the medium, or at once, either in clusters or in chains, as shown in Figure 5a. The eggs were oval, with a shiny, semi-transparent light-yellowish colour, as soon as they were laid, but changed to colourless towards hatching. Before hatching, the larvae, with their prominent black heads, could be seen actively moving around and eating through the eggshell (Figure 5b). The eggs seemed to turn more yellow only under moisture stress. The eggs measured about 0.25 x 0.15 mm. The number of eggs laid by the females was variable and ranged between 100 and 250 (n=20).
First instar larvae
After eating through the eggshell, the first instar became active almost immediately. Their body was transparent, while their head was shiny, black and chitinised. The food and its colour could be seen through the semi-transparent abdomen. The larvae quickly moved to the bottom of the medium, becoming active feeders. They were capable of surviving completely submerged in water, but showed a high level of susceptibility to moisture stress. The first instar larvae measured about 1.2-2.5 mm in length and 0.2-0.3 mm in width (n=20). The first instar stage lasted 2 days, after which the larvae hatched into second instar larvae.
Second instar larvae
The second instar larvae were not behaviourally different from the first instars, but differed in size: they measured about 2.6-4.5 mm in length, and 0.3-0.45 mm in width. The second instar larval stage lasted about 2 days, after which they hatched into third instar larvae.
Third instar larvae
The third instar larvae were more easily visible to the naked eye than were the earlier larval instars, and they could be seen eating through the medium. Raw food substances could also be seen inside their abdomen, thus the larvae sometimes took on the colour of the food. They measured about 4.6-6.5 mm in length, and about 0.46-0.65 mm in diameter (n=20).
Fourth instar larvae
The fourth instars were thicker than the previous instars, and contained almost no raw food substances, as they prepared to pupate. Thus, they appeared whiter than the previously described instars. They measured about 6.5-7.2 mm in length and ranged from 0.65 to 0.75 mm in diameter (n = 20). The fourth instar larvae migrated to the topmost layer of the medium, with their body continuously shortening, and thereafter pupated. This stage lasted approximately 2 days. As the larvae moved, they left behind a characteristically slimy, shiny translucent gel. All the larvae were sensitive to light, and tended to hide under the medium when they were suddenly exposed to the microscope light.
Pupa
The pupae measured about 2-3 mm in length, and 0.6-0.8 mm in diameter (n=20). The pupae cocooned within the top layers of the medium, and on approximately the third day, the adult fungus gnats emerged. The pupae were yellowish-brown in colour, but they turned dark towards emergence as adults.
Discussion
A recent study by Lee et al.22 clarified that a few fungus gnat species, belonging to the subfamily Megalosphyinae (genus Phytosciara sensu lato, and part of the genus Bradysia sensu lato), have more frequently had their larvae associated with living plants than has any other Sciaridae group. The larvae of these fungus gnat species are capable of mining into the roots, stems and leaves of living plants. Some sciarid species are important pests of certain crops that are of global agricultural importance, especially crops under cover. Bradysia impatiens24, which is a major crop pest, has a global distribution. The species has previously been identified in South Africa in association with major forestry nurseries12,13 where the effects of these species still tend to go unnoticed and unreported by many farmers. Only a few studies have been undertaken thus far for the purpose of identification, and this study is the first on the biology of the species in South Africa. Further studies are needed on the pest status and possible management strategies of the species.
During the course of this study, B. impatiens was identified as present and in concerning numbers on cucumber plants in a commercial greenhouse farm in the Western Cape Province of South Africa. Bradysia impatiens was also identified as present in the rest of the sampled greenhouses in which tomatoes, mushrooms, blueberries, herbs and chrysanthemums were grown. Even though fungus gnats have previously been identified in South Africa in forestry nursery beds12,13, the current study presents the first report of fungus gnats on a crop other than pine nursery beds. This study also reports L. sativae and L. ingenua as present on mushrooms, for the first time from South Africa. Lycoriella sativae is a well-known mushroom pest which has to date been identified to be well distributed in the Holarctic region and reported to have been distributed by humans to Central America and the sub-Antarctic islands.24 Although L. ingenua has been determined to be a more important pest species in mushrooms, L. sativae is known to be an agrarian species and the most abundant species of Sciaridae on fields.15,24
The biology of B. impatiens is known to differ according to different environmental conditions. The duration of the life cycle of sciarids has been regarded as distinctive by a variety of researchers. Such duration has been attributed mainly to the variable environmental temperatures, with shorter life cycles at relatively high environmental temperatures.6,25-27 The optimum temperature for their growth has been determined to be 30 °C.2 In the current study, the duration of the life cycle of a laboratory culture of B. impatiens was found to be 2-3 weeks at 25 °C. On observing the egg-laying habits of the species, apart from their commonly observed habits, a new phenomenon was detected, that is, females were observed to lay their eggs in the form of a chain.
Different larval stage feeds have been described for the rearing of Bradysia spp. in the laboratory. Some of such feeds include the use of the fungal culture of Pleurotus astreatus Kumm 1871 grown on potato dextrose agar28; a mixture of moist coconut coir dust, commercial rabbit food and brewer's yeast; peat8; potato agar6; bacto-agar; and brewer's yeast29. In all the combinations mentioned, fungi make up a basic element in the feed for fungus gnats. Upon death, the adult flies were observed to act as a primary source of fungi in the media as they deteriorated. This observation suggests that, even without a fungal culture, one could easily rely on the adult fungus gnats for the introduction of fungi into the new medium. The culture medium that was used in the current study, consisting of a 3:1:1 mixture of pine sawdust, soy meal and cornmeal, gave satisfactory results for more than 10 generations. Because some fungus gnat species have been shown to prefer laying eggs on cut planes, rather than on whole stems,22 the use of pine sawdust (blended) provided an ideal medium for egg laying.
During culturing, moisture was an important aspect in the growth of B. impatiens, especially in the case of the larval instars, with the first instars in the current study being observed to be the most susceptible to moisture stress. Consequently, water was added to the mixture more regularly during the larval stages. Aeration of the medium is equally important, with fungus gnats having been observed to not survive under anaerobic conditions. In the present experiment, the Petri dish cover was fitted with tissue paper to prevent the accumulation of condensed moisture on the upper lid, which otherwise was observed to be capable of creating anaerobic conditions if the condensed water created a water film on the edges of the Petri dish cover.
The fungus gnat species responsible for damage on a commercial cucumber farm was identified as B. impatiens. Under laboratory conditions, the life cycle of the species concerned was determined to be 2-3 weeks at 25 °C. This report is also the first of fungus gnats laying eggs in the form of a chain, as well as the first report of B. impatiens being a pest on any other crops than tree nursery beds in South Africa. The number of eggs laid by each single female, and their short life cycle, show that large numbers of fungus gnats can easily build up in a greenhouse if control measures are not taken.
Bradysia impatiens has been reported to be an introduced species to South Africa. A study by Hurley et al.23 suggested that multiple strains of the species had been introduced into the country. Our results corroborate the results of this study where high haplotype diversity and comparable nucleotide diversity in the sequences analysed was observed. Multiple strain introductions could possibly have been caused by the human importation of contaminated material such as substrates and ornamental plants, among others. These findings emphasise the need for more stringent restrictions on the importation of vegetative material, and for constant monitoring of such crop pests.
From the results of the current study, as well as on the basis of the findings of previous studies, B. impatiens can be concluded to be a well-established pest of protected crops in South Africa. However, the current study only targeted specific greenhouses to survey fungus gnats. Such a limitation implies that more surveys are required to establish the existence of the species in other regions of South Africa, and the extent to which the species is a problem to South African growers. The question remains if these are truly introduced species or if they are South African species that have only been identified recently. If they are introduced species, it would be paramount to look into the invasion biology of these species in South Africa, as B. impatiens is already known to have a wide distribution in South Africa, since its first report in 2007. There is also a need to look into the management practices for fungus gnats as they are polyphagous and economic pests in undercover production. More important still, is the need for studies regarding the sustainable management of the pests concerned, because they attack crops to which the application of chemicals is relatively inappropriate. Current efforts for South Africa have included a review on the potential for biological control of fungus gnats using entomopathogenic nematodes as well as laboratory and field trials to test for the potential of South African entomopathogenic nematodes to control fungus gnats.30,31
Acknowledgements
We thank Hans-Georg Rudzinski (Entomo-graphisches Studio, Schwanewede, Germany) and Kai Heller (Quickborn, Germany) for assistance in the identification of fungus gnats. The financial support of NemaBio (Pty) (Ltd) and the National Research Foundation of South Africa (THRIP TP14062571871) are greatly appreciated. We also thank Sheila Storey of Nemlab for assistance during field work and Francois Bekker for drawing the map.
Authors' contributions
A.K.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - the initial draft; writing - revisions. A.M.K.: Molecular data analysis. A.M.: Conceptualisation; writing - revisions; student supervision; project leadership; project management; and funding acquisition.
Data availability
All sequences used in this study are available by searching the GenBank accession numbers provided in Table 1 on the US National Center for Biotechnology Information (NCBI) GenBank database (https://www.ncbi.nlm.nih.gov/). The sequence alignments and phylogenetic tree are available upon request.
References
1. Springer TL. Fungus gnat (Diptera: Sciaridae) feeding damage to legume seedlings. J Kans Entomol Soc. 1995;68(2):240-242. http://www.jstor.org/stable/25085588 [ Links ]
2. Chandler D, Hri W, Prince G, Bennison J. New approaches to microbial control of insect pests in protected crops and their interaction with waste-based growing media. PC 283. Coventry: The University of Warwick; 2010. [ Links ]
3. Pundt L. Fungus gnats are serious pests. Yankee Grower. 1999; September/October. [ Links ]
4. Ludwig SW, Oetting RD. Evaluation of medium treatments for management of Frankliniella occidentalis (Thripidae: Thysanoptera) and Bradysia coprophila (Diptera: Sciaridae). Pest Manag Sci. 2001;57(12):1114-1118. https://doi.org/10.1002/ps.404 [ Links ]
5. Scarlett K, Tesoriero R, Daniel R, Guest D. Sciarid and shore flies as aerial vectors of Fusarium oxysporum f. sp. cucumerinum in greenhouse in cucumbers. J Appl Entomol. 2014(5);138:368-377. https://doi.org/10.1111/jen.12098 [ Links ]
6. Mansilla JP Pastoriza MI. Estudio sobre la biologia y control de Bradysia paupera Tuomikoski (= Bradysia difformis Frey, Diptera : Sciaridae) [Study on the biology and control of Bradysia paupera Tuomikoski (= Bradysia difformis Frey, (Diptera: Sciaridae)]. Bol Sanid \feg Plagas. 2001;27:411-417. Spanish. [ Links ]
7. Vänninen I. Control of sciarid flies with Steinernema feltiae in poinsettia cutting production. Int J Pest Manag. 2003;49(2):95-104. https://doi.org/10.1080/0967087021000034139 [ Links ]
8. Jagdale GB, Casey ML, Grewal PS, Lindquist RK. Application rate and timing, potting medium, and host plant effects on the efficacy of Steinernema feltiae against the fungus gnat, Bradysia coprophila, in floriculture. Biol Control. 2004;29(2):296-305. https://doi.org/10.1016/S1049-9644(03)00164-6 [ Links ]
9. Cloyd RA, Zaborski ER. Fungus gnats, Bradysia spp. (Diptera: Sciaridae), and other arthropods in commercial bagged soilless growing media and rooted plant plugs. J Econ Entomol. 2004;97(2) 503-510. https://doi.org/10.1603/0022-0493-97.2.503 [ Links ]
10. Schuhli GSE, Penteado SDC, Reis FW, Amorim DS. Sciarid fungus-gnats as nuisance factor in Pinus timber yards. Pesq flor bras. 2014;34(80):455-457. https://doi.org/10.4336/2014.pfb.34.80.732 [ Links ]
11. Popp J, Hantos K. The impact of crop protection on agricultural production. Stud Agric Econ. 2011;113:47-66. https://doi.org/10.7896/j.1003 [ Links ]
12. Hurley BP Govender P Coutinho TA, Wingfield BD, Wingfield MJ. Fungus gnats and other Diptera in South African forestry nurseries and their possible association with the pitch canker fungus. S Afr J Sci. 2007;103(1):43-46. [ Links ]
13. Hurley BP Slippers B, Coutinho TA, Wingfield BD, Govender P Wingfield MJ. Molecular detection of fungi carried by Bradysia difformis (Sciaridae: Diptera) in South African forestry nurseries. South Hemisphere For J. 2007;69:103-109. https://doi.org/10.2989/SHFJ.2007.69.2.5.291 [ Links ]
14. Chidziya E, Mutangadura D, Jere J, Siziba L. A comparative evaluation of locally available substrates for rearing and studying biology of sciarid fly, Lycoriella mali. Afr J Biotechnol. 2013;1(4):57-61. http://dx.doi.org/10.15413/ajb.2013.0108 [ Links ]
15. Menzel F, Smith JE, Colauto NB. Bradysia difformis Frey and Bradysia ocellaris (Comstock): Two additional neotropical species of black fungus gnats (Diptera: Sciaridae) of economic importance: A redescription and review. Ann Entomol Soc Am. 2003;96(4):448-457. https://doi.org/10.1603/0013-8746(2003)096[0448:BDFABO]2.0.CO;2 [ Links ]
16. Menzel F, Smith JE. Sciaridae (black fungus gnats). In: Kirk-Spriggs AH, Sinclair BJ, editors. Manual of Afro-tropical Diptera. Pretoria; South African National Biodiversity Institute; 2017. p. 557-580. [ Links ]
17. Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol.1994;3(5):294-299. [ Links ]
18. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725-2729. https://doi.org/10.1093/molbev/mst197 [ Links ]
19. Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406-425. https://doi.org/10.1093/oxfordjournals.molbev.a040454 [ Links ]
20. Rozas J, Ferrer-Mata A, Sánchez-Delbarrio JC, Guirao-Rico S, Librado P Ramos-Onsins SE, et al. DnaSP 6: DNA sequence polymorphism analysis of large data sets. Mol Biol Evol. 2017;34(12):3299-3302. https://doi.org/10.1093/molbev/msx248 [ Links ]
21. Cloyd RA, Dickinson A. Effect of Bacillus thuringiensis subsp. israelensis and neonicotinoid insecticides on the fungus gnat Bradysia sp. nr. coprophila (Lintner) (Diptera: Sciaridae). Pest Manag Sci. 2006;62(2):171-177. https://doi.org/10.1002/ps.1143 [ Links ]
22. Lee H, Shin SJ, Hong GT, Ahn JY Cho HD. Biological characteristics of the ginseng stem fungus gnat (Phytosciara procera) and its environmental-friendly control using modified topping of ginseng peduncles. J Ginseng Res. 2010;34(1):23-29. https://doi.org/10.5142/JGR.2010.34.L023 [ Links ]
23. Hurley BP Slippers B, Coutinho TA, Wingfield BD, Govender P Wingfield MJ. Genetic diversity of Bradysia difformis (Sciaridae: Diptera) populations reflects movement of an invasive insect between forestry nurseries. Biol Invasions. 2009;12(4):729-733. https://doi.org/10.1007/s10530-009-9509-1 [ Links ]
24. Mohrig W, Heller K, Hippa H, Vilkamaa P Menzel F. Revision of black fungus gnats (Diptera: Sciaridae) of North America. Stud Dipterol. 2013;19:141-286. https://doi.org/10.11646/zootaxa.4150.4.3 [ Links ]
25. Kennedy MK. Scientific notes: A culture method for Bradysia impatiens (Diptera: Sciaridae). Ann Entomol Soc Am. 1973;66(5):1163-1164. https://doi.org/10.1093/aesa/66.5.1163 [ Links ]
26. Nielsen GR. Fungus gnats EL 50 [webpage on the Internet]. c1997 [cited 2019 Nov 25]. Available from: http://pss.uvm.edu/ppp/pubs/el50.htm [ Links ]
27. Villanueva-Sánchez E, Ibánez-Bernal S, Lomeli-Flores JR, Valdez-Carrasco J. Identificación y caracterización de la mosca en el cultivo de nochebuena (Euphorbia pulcherrima) en el centro de México [Identification and characterization of the fly in the poinsettia (Euphorbia pulcherrima) crop in central Mexico]. Acta Zool Mex. 2013;29:363-375. Spanish. [ Links ]
28. Kim HH, Choo HY, Kaya HK, Lee DW, Lee SM, Jeon HY. Steinernema carpocapsae (Rhabditida: Steinernematidae) as a biological control agent against the fungus gnat Bradysia agrestis (Diptera: Sciaridae) in propagation houses. Bio-control Sci Technol. 2004;14(2):171-183. https://doi.org/10.1080/09583150310001655693 [ Links ]
29. Katumanyane A, Ferreira T, Malan AP. Bradysia species (Diptera: Sciaridae) as pests of covered crops, with special reference to biological control using entomopathogenic nematodes. Afr Entomol 2018;26(1):1-13. https://doi.org/10.4001/003.026.0001 [ Links ]
30. Katumanyane A, Ferreira T, Malan AP. Greenhouse application of Steinernema yirgalemense to control fungus gnats, Bradysia impatiens (Diptera: Scaridae). BioContr 2018;63(5):729-738. https://doi.org/10.1007/s10526-018-9895-3 [ Links ]
31. Katumanyane A, Ferreira T, Malan AP. Potential use of local entomopathogenic nematodes to control Bradysia impatiens (Diptera: Sciaridae) under laboratory conditions. Afr Entomol. 2018;26(2):337-349. https://doi.org/10.4001/003.026.0337 [ Links ]
 Correspondence:
Correspondence:
Agil Katumanyane
agil.katumanyane@fabi.up.ac.za
Received: 15 Aug. 2019
Revised: 25 Nov. 2019
Accepted: 10 Dec. 2019
Published: 26 Mar. 2020
Editors: Teresa Coutinho; Salmina Mokgehle
Funding: NemaBio (Rty) (Ltd); National Research Foundation














