Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.116 n.3-4 Pretoria Mar./Apr. 2020
http://dx.doi.org/10.17159/sajs.2020/7906
INVITED COMMENTARY
Alexander Quandt
School of Physics, University of the Witwatersrand, Johannesburg, South Africa
Keywords: materials science, multi-scale modelling, 2D materials, quasicrystals, topological materials, plasmonics
One of the oldest arts of humankind is toolmaking. Over the millennia, all civilisations have experimented with different types of raw materials. Many generations of stonemasons, blacksmiths and gaffers have invented better and better types of nuts and bolts to fix old things, and to facilitate the making of new things. These are the unknown pioneers upon whose shoulders our modern civilisation stands today, and their forgotten contributions to science and engineering also mark the origins of materials science.
If toolmaking is so deeply embedded in the DNA of our species, then our desire to understand the secrets of the universe around us appears to be another major driving force. Through this desire, we have essentially learned two important lessons. First, that we need extremely sophisticated technical instruments to explore the world around us at the smallest and the largest length scales. And second, that the knowledge gained from the study of the physical processes at these extreme scales pales in comparison to the complexity and the sophistication of even the simplest version of the molecular nanomachineries which keep us 'alive and kicking' on a day-to-day basis.
Colleagues who are not from the field of materials science usually tend to dismiss such remarks by pointing out that the complexity of living organisms stems from the peculiar nature of a handful of light elements which are at the heart of organic chemistry. Inorganic materials by contrast are generally perceived to be as boring and as well understood as rock salt, and thus so must be the whole field of materials science. Nothing could be further from the truth.
I hope that through this Commentary, and by talking about some of the highlights I have encountered during a long career in computational materials science, I will be able to convince readers that modern materials science amounts to very much more than serving as the warehouse clerks in the supply chain of industry and other scientific fields.
Nature of the chemical bond
The year 2019 marked the 150th anniversary of the periodic table of chemical elements in its most popular form, first created by Dmitri Mendeleev. The filling of the missing gaps in the periodic table was anything but a civilized and courteous endeavour - the bitter race to fame and worldwide media attention that we often witness today is obviously something that was already common in the 'good old days'.1 But despite all the battles, the discovery of the chemical elements was just the beginning of a long journey into the modern science of materials - a journey which is far from over.
One highlight provided by the periodic table was the prediction of a striking chemical similarity between some of its elements; a similarity which is based on the shell structure of the electrons in an atom - a fact that could be explained to some extent using simple quantum mechanics.2 From that point onwards, scientists knew at least something about the quantum mechanical foundations of materials science. The next major step was to find out how these various atoms would combine, which amounted to a systematic description of the atomic structure of organic and inorganic materials.
This task was taken up by eminent scientists like Linus Pauling. In his famous book3The Nature of the Chemical Bond (first published in 1939), Pauling essentially presented the first systematic description of chemical bonding in materials. His main resources were crystallographic data and a very intuitive approach to quantum mechanical many-electron systems. His elegant style of combining experimental data with basic quantum mechanical concepts had a deep impact on generations of computational materials scientists, including myself. When I first stumbled over a well-worn copy of Pauling's book as a high school student, I was astounded by the complexity of some of the inorganic materials described in his book. That was the moment when I decided to study physics and chemistry.
It was a long way from Pauling's initial ideas to the almost industrial-scale type of computational infrastructure that aids today's search for new materials. A key method that drove the development of computational materials science was density functional theory (DFT), which was developed by Walter Kohn and co-workers in the late 1960s.4 Based on this novel and groundbreaking many-particle method, it was possible to predict the key materials properties of materials with high accuracy and with reasonable numerical resources. The numerical implementation of DFT required many subtle tricks of the trade, and the article by Payne et al.5 in Reviews of Modern Physics (1992) was a milestone in this development. Almost all the popular programme packages in use today are based on at least some of the numerical methods described in this detailed review article.
One of the co-authors of this influential article is Mike Teter, who was my postdoctoral supervisor at Cornell University (USA). He taught me that computational materials science is a great deal more than pushing buttons on computational black boxes. If done properly, it also involves a constant improvement and optimisation of the underlying numerical methods. A survey of the latest many-particle simulation methods and their implementation on supercomputing facilities can be found in another monograph6, Computational Many-Particle Physics, to which I contributed two chapters. This book has been downloaded from Springer so many times that a copy of it must sit on nearly every computer running materials simulations today.
Surprises
If you are late for a conference dinner and are seated at a table with colleagues that you hardly know, you might want to break the ice by starting a typical scientist's joke: 'A crystallographer, a physicist, a chemist, a mathematician, a materials scientist and a mineralogist find themselves in a room...'. However, you would not have much success with such a joke at a typical quasicrystals conference, because such is typically the composition of these conferences. Since the discovery of quasicrystals by Dan Shechtman and co-workers in 1984,7 these materials have put into question some of the most fundamental concepts in crystallography and condensed matter physics. Among them is the question of whether a material with no obvious translational order but sharp X-ray diffraction peaks would still qualify as a crystal or not. (Answer: it does!). But sadly, some of the godfathers of modern materials science like Linus Pauling dismissed the field of quasicrystals at the outset.8
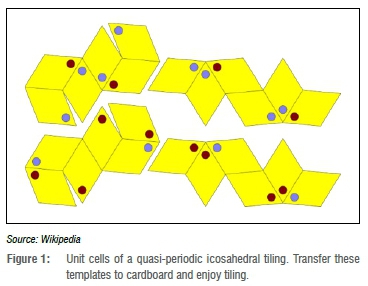
In hindsight, the borderline existence of the field of quasicrystals among the condensed matter establishment turned out to be a blessing. In the relative tranquillity of the quasicrystals conference, a very diverse scientific community met and worked out groundbreaking new ideas about the structure and the properties of complex periodic and aperiodic materials. My PhD supervisor, Peter Kramer, was among the pioneers of this field. He developed one of the first tiling models for icosahedral quasicrystals.9 In Figure 1 I have posted some instructions to fabricate paper versions of the corresponding tiles, and as this is a Commentary about toolmaking, you are invited to produce paper versions of the corresponding tiles, and try to tile space in an aperiodic fashion, but without creating holes or overlaps between those tiles.
Under the supervision of Professor Kramer I started to develop some of the first atomistic models for layered and icosahedral quasicrystals, one example being the Quandt-Elser model for AlPdMn.10 Today, the combination of experimental or crystallographic probes with DFT-based materials simulations has become mainstream in the field of quasicrystals research.
Another case in which Pauling found himself corrected was the case of boron. In his famous book3 he goes to great lengths to explain the astonishingly complex nature of the chemical bond in the elementary phases of boron and makes a strong case for boron icosahedra as the building blocks of such materials. In a collaboration with my colleague Ihsan Boustani, we took this idea very seriously and tried to develop a structure model for a one-elemental boron quasicrystals built on Pauling's ideas. To our surprise we discovered something completely different: namely that boron forms quasi-planar clusters rather than icosahedra, which gives rise to boron nanotubes, boron fullerenes and quasi-planar boron sheets (now called borophene).11 Several allotropes of borophene are shown in Figure 2.
The formulation of an Aufbau principle for boron clusters marked the birth of the field of boron nanomaterials.11 Historically, the prediction of quasi-planar boron sheets predated the discovery of graphene by several years. Unfortunately, borophene was much harder to synthesise than we originally thought it would be, whereas its cousin material, graphene, has become one of the biggest sensations in the story of materials science.12 The only materials class in which carbon cannot really compete with boron is the possible existence of boron quasicrystals.11 Up until now, however, the boron quasicrystal remains our only prediction which has not been confirmed over the years. But maybe something as simple as a pure sample of alpha-boron and a ball mill might do the job?
Applied computational materials science
One aspect of cutting-edge computational materials science is the systematic and almost industrial scale search for new materials.13 This seems to be a promising strategy, provided one is privileged enough to gain very generous access to the corresponding supercomputing infrastructure. Another promising approach to computational materials science is the modelling of possible technological devices. Note that atomistic methods like DFT can only be applied to idealised model systems comprising up to several hundred atoms. This number falls short by at least 20 orders of magnitude compared to the number of atoms forming a typical solar cell or nano-optical device. The way out of this dilemma is the use of multiscale approaches,14 in which device simulations are essentially based on phenomenological models, but the key materials parameters for these phenomenological models may be taken either from experiment or from an atomistic simulation using DFT This allows for 'in silico' development of new types of devices without ever going to the laboratory. Over the years we were able to demonstrate that this approach works extremely well in the field of photovoltaics.15
Another field in which we had explored a full suite of fundamental and phenomenological simulation tools is computational plasmonics.16 Plasmonics is a key optical technology based on surface waves, which is supposed to bridge the fields of electronics and optics. Figure 3 shows predictions of plasmonic surface waves ('plasmons') for graphene and borophene made by my colleague Robert Warmbier, which is a follow-up on our earlier work referred to above. The strongest peaks in the spectra correspond to resonances induced by surface plasmons. For graphene we notice the existence of plasmons in the UV (beyond 3 eV) and in the THz range (1-100 meV), whereas some of the strongest peaks seen in the case of borophene indicate the existence of plasmons within the optical range (1-3 eV).
Even more surprises
For a born toolmaker, even the most exotic natural phenomena can be easily understood after referring to a material or a process that the toolmaker could move with their own hand. I was rather surprised to find out from a remarkable book by Strogatz17, that undisputed millennial geniuses like Archimedes and Newton did not make their biggest mathematical discoveries based on abstract reasoning but on simple mechanical analogues.
It turns out that materials science provides us with a fascinating zoo of possible analogies to particles and processes, which are otherwise found only at some of the smallest and at some of the largest length scales in our universe. This was the topic of a rather visionary book by Volovik18. It almost appears as though materials science provides us with something like the mysterious aleph, which has so vividly been described in the famous short story by Borges19.
As it happened, Volovik was immediately vindicated by the discovery of topological insulators and related materials20, which brought into materials laboratories worldwide effects and concepts that previously were only known from field theory and high energy physics. Based on these materials analogies, we now have the unique possibility of studying model versions of black holes, magnetic monopoles and new elementary particles in a materials lab, and hopefully will learn a good deal about their fundamental properties before we start to hunt for the same physics elsewhere using giant telescopes and particle accelerators.
Summary and outlook
It is the complex nature of the chemical bond that gives rise to an amazing variety of materials, some of which have been described in this Commentary. Among them we will surely find new classes of 'wonder materials' which will drive the technologies of the future. Over time, these new materials will lead to a fundamentally different portfolio of basic industrial materials. When this happens, countries like South Africa, which have a wide range of minerals resources, will be in a very comfortable strategic position. However, these mineral-rich countries will need to use their natural resources wisely, and they must also succeed in building a strong beneficiation industry around their mining sectors and include investments in the necessary human capital.
Some steps in that direction have already been made in South Africa by funding internationally established institutions like the Centre of Excellence in Strong Materials (CoE-SM) with headquarters at the University of the Witwatersrand. Beyond these national efforts, there are also new initiatives like the Centre of Excellence in Materials, Energy and Nanotechnology (CoE-MEN), which aims to provide materials scientists in Africa with a new continent-wide research platform, and which operates under the auspices of the African Research Universities Alliance.
These initiatives will need time and continuous financial and technical support to evolve to their full potential, and one should not make the mistake of expecting that the futuristic Wakanda of the Marvel Universe will arise overnight. But in the CoE-SM and the related Materials for Energy Research Group, we have already seen many cases in which teams of researchers start from the atomistic simulation of the key materials and end up with the production of sophisticated high-tech devices.
And so, the story of toolmaking will continue, and new discoveries will surely surprise us on our long and winding road to 'Wakanda'.
Acknowledgement
I thank Prof. Jane Carruthers, Editor-in-Chief, for the opportunity to write this personal essay on materials science.
References
1. Scerri E. A tale of seven elements. Oxford: Oxford University Press; 2013. [ Links ]
2. Slater JC. The theory of complex spectra. Phys Rev. 1929;34:1293. https://doi.org/10.1103/PhysRev.34.1293 [ Links ]
3. Pauling L. The nature of the chemical bond. 3rd ed. Ithaca, NY: Cornell University Press; 1960. [ Links ]
4. Kohn W, Sham LJ. Self-consistent equations including exchange and correlation effects. Phys Rev. 1965;140:A1133. https://doi.org/10.1103/PhysRev.140.A1133 [ Links ]
5. Payne MC, Teter MP Allan DC, Arias TA, Joannopoulos JD. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev Modern Phys. 1992;64:1045. https://doi.org/10.1103/RevModPhys.64.1045 [ Links ]
6. Quandt A. Ab initio approach to the many-electron problem; ab initio methods applied to structure optimization and microscopic modelling. In: Fehske H, Schneider R, Weisse A, editors. Computational many-particle physics. Berlin: Springer; 2008. p. 415, 437. https://doi.org/10.1007/978-3-540-74686-7_15 [ Links ]
7. Shechtman D, Blech I, Gratias D, Cahn J. Metallic phase with long-range orientational order and no translational symmetry. Phys Rev Lett. 1984;53:1951. https://doi.org/10.1103/PhysRevLett.53.1951 [ Links ]
8. Cahn JW, Gratias D, Shechtman D. Pauling's model not universally accepted. Nature. 1986;319:6049. https://doi.org/10.1038/319102a0 [ Links ]
9. Kramer P Neri R. On periodic and non-periodic space fillings in E^m obtained by projection. Acta Crystallogr. 1984;A40:580. https://doi.org/10.1107/S0108767384001203 [ Links ]
10. Quandt A, Elser V. Ab initio based modeling of i-AlPdMn. Phys Rev. 2000;B61:9336. https://doi.org/10.1103/PhysRevB.61.9336 [ Links ]
11. Quandt A, Boustani I. Boron nanotubes. ChemPhysChem. 2005;6:2001. https://doi.org/10.1002/cphc.200500205 [ Links ]
12. Geim AK. Graphene: Status and prospects. Science. 2009;324:1530. https://doi.org/10.1126/science.1158877 [ Links ]
13. Saal JE, Kirklin S, Aykol M, Meredig B, Wolverton C. Materials science and discovery with high-throughput density functional theory: The open quantum materials database (oqMD). JOM. 2013;65:1501. https://doi.org/10.1007/s11837-013-0755-4 [ Links ]
14. Weinan E. Principles of multiscale modelling. Cambridge: Cambridge University Press; 2011. [ Links ]
15. Quandt A, Mokgosi I, Warmbier R. Simulations of conventional and augmented types of solar cells. In: Enrichi F, Righini G, editors. Solar cells and light management. Amsterdam: Elsevier; 2020. p. 249. https://doi.org/10.1016/B978-0-08-102762-2.00007-0 [ Links ]
16. Mohammed F, Warmbier R, Quandt A. Computational plasmonics: Theory and applications: Numerical techniques. In: Agrawal A, Benson T, De La Rue RM, Wurtz GA, editors. Recent trends in computations photonics. Springer series in optical sciences. Cham: Springer; 2017. p. 315; 341. https://doi.org/10.1007/978-3-319-55438-9_12 [ Links ]
17. Strogatz S. Infinite powers. New York: Houghton Mifflin Harcourt; 2019. [ Links ]
18. Volovik GE. The universe in a helium droplet. Oxford: Oxford University Press; 2003. [ Links ]
19. Borges JL. The aleph. London: Penguin Books, 1998. Reprint from 1949. [ Links ]
20. Hasan MZ, Kane CL. Colloquium: Topological insulators. Rev Modern Phys. 2010;82:3045. https://doi.org/10.1103/RevModPhys.82.3045 [ Links ]
 Correspondence:
Correspondence:
Alex Quandt
alex.quandt@wits.ac.za
Published: 26 March 2020
Professor Alexander Quandt is the recipient of the 2018/2019 NSTF-South32 Special Annual Theme Award: Materials for Inclusive Economic Development (in recognition of the United Nations 'International Year of the Periodic Table of Chemical Elements').














