Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.116 no.1-2 Pretoria ene./feb. 2020
http://dx.doi.org/10.17159/sajs.2020/6443
SCIENTIFIC CORRESPONDENCE
Proposed adaptation of the KMnO4 oxidation method for determining active carbon for South African soils
Anélia MaraisI; Elmarie KotzéII; Johan LabuschagneI; Lientjie VisserIII; Craig D. MorrisIV
IDirectorate Plant Sciences, Department of Agriculture Western Cape, Elsenburg, South Africa
IISoil Crop and Climate Sciences, University of the Free State, Bloemfontein, South Africa
IIIAgricultural Research Council - Small Grain Institute, Bethlehem, South Africa
IVAgricultural Research Council - Animal Production, University of KwaZulu-Natal, Pietermaritzburg, South Africa
Keywords: soil organic carbon, shaking position, soil quality
One of the most widely acknowledged indicators of soil quality is soil organic matter and its elemental constituents, like soil organic carbon (SOC) and nitrogen.1,2 However, due to the fact that soil organic matter has no definite chemical composition, SOC is more commonly estimated and reported in scientific literature.
With an ever-increasing interest in sustainability and the subsequent quality of soils, it is of utmost importance to measure sensitive indicators of soil quality. One of these indicators is active or labile carbon. This portion of the SOC is a small but relatively labile fraction and acts as fuel for the soil food web.2 Thus the active carbon fraction could be used as an early indicator of changes in soil quality because of the influence of agricultural management practices.3 Various methods have been published whereby active carbon can be measured. A review4 on the then current methods was published in 2006, listing the advantages and disadvantages of each method.
A major advantage of the KMnO4 oxidation method is that it is easy to perform and does not use hazardous chemicals in large amounts. There are, however, many different versions of the same method in which aspects like incubation time, amount of soil used, shaking time and manner, differ.2,3,5-8
The aim of this contribution was to adapt the KMnO4 oxidation method for soils from different South African localities, because it was found that strictly following the protocol, especially with low carbon soils, resulted in low repeatability. The method described by Culman et al.7 was used and adapted as deemed necessary.
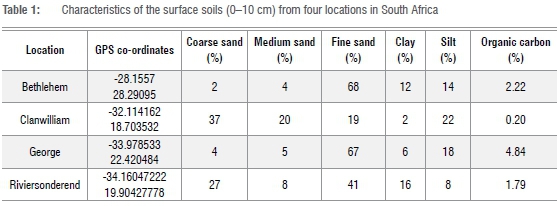
Four soils from different localities in South Africa were chosen and analysed for particle size as well as organic carbon.9 The general characteristics of these soils as analysed by the Elsenburg Analytical Laboratories are depicted in Table 1. The organic carbon varied widely between the four soil types, with Clanwilliam having the lowest (0.2%) and George the highest (4.84%).
Because it was difficult to get comparable and positive results in soils with low carbon content, such as with Clanwilliam, both 5 g of soil, as was originally suggested2, as well as 2.5 g as suggested by some other authors7,8, were tested. Most of the methods studied were unclear as to the required positon of the tubes during shaking.
The tubes with the soil samples and KMNO4 were therefore shaken either in an upright or flat position on an orbital shaker at 120 rpm for 2 min as suggested by Culman et al.7 Shaking the centrifuge tube in the flat position should result in better mixing of the KMnO4 with the soil sample, thus potentially extracting more active carbon.
Each soil sample was tested, with five repeats, with both amounts of soil (2.5 vs 5 g) in both positions (flat vs upright).
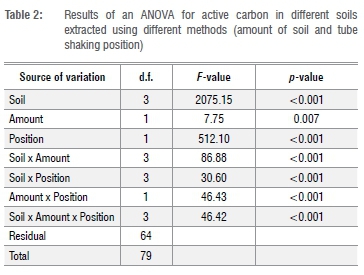
The data were subjected to a three-way factorial ANOVA (soil type (4) x amount (2) x position (2) x 5 replications) using the software program Genstat 18.10 The dependent variable (active carbon, mg/kg) was not transformed because residuals were neither skewed nor heteroscedastic.
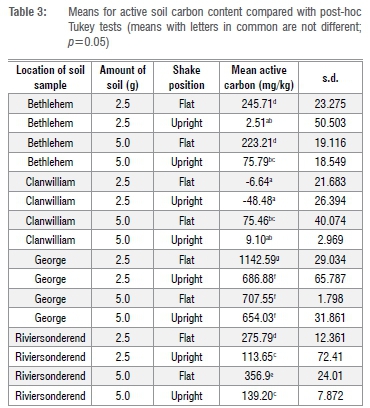
Soils differed markedly (p<0.001) in their average active carbon content (Tables 1 and 2), ranging from almost 800 g/kg for carbon-rich George soil to very low carbon sandy soil from Clanwilliam (7.4 mg/kg), with Riviersonderend (221.4 mg/kg) and Bethlehem (136.8 mg/kg) soils being of intermediate active carbon content. Generally, more active carbon (p<0.001) was extracted in the flat than the upright tube shaking position (377.6 vs 204.1 mg/kg) and when using 2.5 g rather than 5 g of soil (301.5 vs 280.2 mg/kg). However, because all two-way interactions as well as the soil x amount x position interaction were significant (p<0.001), the most effective combination of position and soil amount depended on soil type (Table 2).
For Bethlehem soil, the flat shaking position produced a similar result for both amounts of soil whereas in the flat position, 2.5 g of soil yielded 435.1 mg/kg more carbon than 5 g of soil for George soil (Table 3). Flat shaking with 5 g of soil gave better results (by 80 mg/kg) than 2.5 g for Riviersonderend soil (Table 3), although this increase was not significant. Clanwilliam's sandy soil produced very low, variable and often negative results for active carbon and no method seemed to achieve acceptable results (Table 3).
From these results, it is recommended that the tubes should preferably be shaken in the flat position in order to allow the KMnO4 to properly mix with the soil sample, using 2.5 g of soil as most of the published protocols suggested. In case of negative values obtained for a certain soil, the experiment should be redone using 5 g of soil, because it was found that increasing the amount of soil resulted in more detectable values in the low carbon soils, although not significantly so.
The final protocol that gave the best repeatable results is consistent with that of Culman et al.7, but with the tubes lying flat while being shaken. Additionally, if negative absorbance values are obtained at 550 nm, it is advised that the procedure should be repeated, using 5 g of soil and adapting the equation accordingly.
References
1.Wander MM, Drinkwater LE. Fostering soil stewardship through soil quality assessment. Appl Soil Ecol. 2000;15:61-73. https://doi.org/10.1016/S0929-1393(00)00072-X [ Links ]
2.Weil RR, Islam KR, Stine MA, Gruver JB, Samson-Liebig SE. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am J Altern. 2003;18(1):3-17. https://doi.org/10.1079/AJAA200228 [ Links ]
3.Conteh A, Blair JG, Lefroy R, Whitbread A. Labile organic carbon determined by permanganate oxidation and its relationships to other measurements of soil organic carbon. Humic Subst Environ. 1999;1:3-15. [ Links ]
4.Strosser E. Methods for determination of labile soil organic matter: An overview. J Agrobiol. 2006;27(2):49-60. https://doi.org/10.2478/s10146-009-0008-x [ Links ]
5.Blair GJ, Lefroy RDB, Lisle L. Soil carbon fractions based on their degree of oxidation and the development of a carbon management index. Aust J Agric Res. 1995;46:1459-1466. https://doi.org/10.1071/AR9951459 [ Links ]
6.Blair GJ, Lefroy R, Whitbread A, Blair N, Conteh A. The development of the KMnO4 oxidation technique to determine labile carbon in soil and its use in a carbon management index. In: Lal R, Kimble J, Follet R, Stewart B, editors. Assessment methods for soil carbon. Boca Raton, FL: Lewis Publishers; 2001. p. 323-337. [ Links ]
7.Culman SW, Snapp SS, Freeman MA, Schipanski ME, Beniston J, Lal R, et al. Permanganate oxidizable carbon reflects a processed soil fraction that is sensitive to management. Soil Sci Soc Am J. 2012;76:494-504. https://doi.org/10.2136/sssaj2011.0286 [ Links ]
8.The Non-Affiliated Soil Analyses Work Committee. Handbook of standard soil testing methods for advisory purposes. Pretoria: Soil Science Society of South Africa; 1990. [ Links ]
9.Tatzber M, Schlatter N, Baumgarten A, Dersch G, Körner R, Lehtinen T, et al. KMnO4 determination of active carbon for laboratory routines: Three long-term field experiments in Austria. Soil Res. 2015;53:190-204. https://doi.org/10.1071/SR14200 [ Links ]
10.VSN International. GenStat for Windows 18th edition. Hemel Hempstead, UK: VSN International; 2015. [ Links ]
 Correspondence:
Correspondence:
Anélia Marais
aneliam@elsenburg.com
Published: 29 January 2020














