Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.115 n.11-12 Pretoria Nov./Dec. 2019
http://dx.doi.org/10.17159/sajs.2019/6199
RESEARCH ARTICLE
Antimicrobial activity and toxicity profile of selected southern African medicinal plants against neglected gut pathogens
Hlambani ShirindaI; Carmen LeonardII; Geoffrey CandyIII; Sandy van VuurenI
IDepartment of Pharmacy and Pharmacology, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Pharmaceutical Sciences, Tshwane University of Technology, Pretoria, South Africa
IIIDepartment of Surgery, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Anaerobes outnumber aerobic bacteria in the human gut. The most commonly isolated microorganisms in intra-abdominal infections include Escherichia coli, Peptostreptococcus micros as well as Bacteroides and Clostridium species. Several studies have been undertaken on southern African medicinal plant species and their antimicrobial efficacy against pathogens such as E. coli that cause stomach ailments. However, pathogens such as Helicobacter pylori, Fusobacterium varium as well as others have been neglected in medicinal plant antimicrobial research. The aim of this study was to evaluate the antimicrobial activity of selected medicinal plants documented for stomach ailments against neglected gut pathogens. A total of 102 aqueous and organic extracts were prepared from 40 different plant species. These plant samples were screened for antimicrobial efficacy against eight anaerobes and two microaerophilic strains using the micro-dilution antimicrobial assay. Plant extracts that displayed noteworthy antimicrobial activity against Clostridium perfringens were further evaluated for antibiofilm activity using the crystal violet staining assay. The toxicity profiles of plants that displayed noteworthy antimicrobial activity were evaluated using the brine shrimp lethality assay which revealed that most of the tested plant samples were non-toxic in nature, and the aqueous extracts proved to be safer. The organic extract of Lippia javanica leaf showed the best antimicrobial activity with a minimum inhibitory concentration of 0.5 µg/mL against C. perfringens. The organic extract of Salvia africana-caerulea displayed the best antibiofilm activity overall, at cell attachment (4 h) biofilm developmental stage with inhibition percentages of 82.8%.
SIGNIFICANCE:
•L. javanica and Gunnera perpensa demonstrated the highest antimicrobial activity with minimum inhibitory concentrations of 0.5 μg/mL and 2.0 μg/mL against C. perfringens, respectively.
•Salvia africana-caerulea was the most effective plant species demonstrating biofilm attachment.
•Lowest toxic effects were observed for the organic extracts of Aloe marlothii, A. tenuior, Bridelia cathartica, G. perpensa leaf and the aqueous extracts of G. perpensa (leaf and rhizome).
•This study demonstrates, for the first time, both antimicrobial and antibiofilm activities for most of these plant species against neglected anaerobes.
•Noteworthy antimicrobial activities in many cases validate traditional use and safety
Keywords: anaerobe, biofilms, traditional medicine, intra-abdominal infections, medicinal plants, minimum inhibitory concentration
Introduction
Intra-abdominal infections are infections of the stomach and are a substantial cause of mortality and morbidity.1,2 Intra-abdominal inflictions include peritonitis, intra-abdominal abscesses, appendicitis, colorectal cancer, ulcerative colitis, food poisoning, chronic atrophic gastritis, peptic ulceration and stomach cancer.3-5 Pathogens associated with intra-abdominal infections include Escherichia coli, the Bacteroides fragilis group, and Clostridium species.6,7Bacteroides species are opportunistic bacteria that form part of the normal microbiota and are often associated with polymicrobial infections such as intra-abdominal, pelvic, genital, complicated skin and soft tissue, and bloodstream infections.6,8-10 Clostridium species are associated withpseudomembranous colitis which is triggered by the intake of broad-spectrum antibiotic therapy and may be the cause of infectious diarrhoea in hospital patients.11 Other pathogens that are isolated in intra-abdominal infections include Helicobacter pylori as well as Fusobacterium species.3,5,12Helicobacter pylori infects more than 50% of the world's population; however, only a small percentage of patients develop severe disorders.13 People that are most likely to be infected are from developing countries.14 Another bacterial species that is associated with cancer of the gut is Fusobacterium spp. These species are associated with severe infections and are often related to colorectal cancer, which is the third most common cancer worldwide.12,15
A wide range of antibiotics and treatment regimens are used for the treatment of intra-abdominal infections. Increased antibiotic resistance is the main cause of treatment failure.9,16 Phytomedicine has proved to be an alternative treatment for different diseases, including gastrointestinal disorders.14,17-19 The use of the medicinal plants selected for this study have previously been reported; however, the scientific evidence for their activity against neglected pathogens of the gut has not been adequately explored.
Globally, some antimicrobial studies have focused on evaluating the activity of traditional medicinal plants against neglected gut pathogens and have shown promising antimicrobial activities against fastidious gut pathogens.14,20,21 In southern Africa, several studies have focused on evaluating the antimicrobial efficacy of medicinal plants against commonly studied gut pathogens such as Staphylococcus aureus, Shigella flexineri, E. coli, Enterococcus faecalis and Candida albicans.22 A review from a period dating almost 20 years demonstrated that very few, if any, southern African medicinal plant studies are related to gut anaerobes.22,23 Most plant-based antimicrobial studies have focused on planktonic microorganisms, although many of the fastidious pathogens selected for this study occur not only in planktonic form but also as biofilms. Biofilms are defined as multicellular matrices of bacteria surrounded by an extracellular polysaccharide called a glycocalyx.24 The ability of bacteria to aggregate and form biofilms makes it difficult to treat bacterial infections as biofilms enhance the bacteria's ability to resist the host's immune system response, thus contributing to the development of antibiotic resistance.25,26 As far as we could ascertain, no previous study has focused on the antibiofilm activity of medicinal plants against C. perfringens and thus, this warranted attention.
Furthermore, plants commonly used in traditional medicine are often believed to be non-toxic. However, scientific research has shown that many of them can be lethal, mutagenic and carcinogenic.27,28 Thus the aim of this study was to evaluate the antimicrobial activity of selected medicinal plants documented for stomach ailments against neglected gut pathogens responsible for intra-abdominal infections and to further investigate biofilm activity (using C. perfringens as a model) and toxicity profiles of plants that demonstrated noteworthy antimicrobial activities.
Materials and methods
Ethnobotanical review, plant identification and collection
An ethnobotanical literature review was conducted to identify the southern African medicinal plants used traditionally to treat stomach ailments (Table 1). Several medicinal plant based books and scientific databases were used to search for plants that are used traditionally to treat stomach ailments.29,30-33 Approximately 155 medicinal plant species were identified. From these, medicinal plant species which could be successfully collected from various botanical gardens (with respect to cost, season, accessibility, sustainability and time) were selected for the study. The selected plant species were collected from the Walter Sisulu National Botanical Garden (Roodepoort, Gauteng, South Africa), where the chief horticulturist, Mr Andrew Hankey, granted permission and assisted in plant identification. All documents for the transfer of materials for research purposes were completed accordingly. Medicinal plant material that was not available at Walter Sisulu National Botanical Garden was purchased from Random Harvest Indigenous Nursery (Muldersdrift, Gauteng, South Africa). Following collection, voucher specimens were prepared for each species and were housed in the Department of Pharmacy and Pharmacology, University of the Witwatersrand.
The collected plant samples were left to dry at room temperature. Once completely dried, samples were separated into different plant parts, i.e. roots, leaf, fruits, bark and stems. Dried plant materials were then crushed to powder using the high-speed Fritsch Pulverisette grinder (Labotec, Johannesburg, South Africa) or using a hand-held pounder (purchased at Faraday supermarkets) for harder stems and barks.
Preparation of plant extracts
Plant powder was resuspended in 1:1 dichloromethane:methanol (Sigma-Aldrich, Johannesburg, South Africa) at a ratio of plant powder:solvent of 1:2, and then placed in the platform shaker incubator (Labcon, Johannesburg, South Africa) at 37 °C for 24 h. Thereafter, the solvent was filtered and left in a fume hood to evaporate. The samples were extracted again with fresh solvent for another 24 h. Once the solvent had evaporated, the extract was transferred into suitable amber bottles for storage at ambient temperature. Aqueous extracts were prepared by immersing plant powder material in sterile distilled water. This immersion was followed by incubation in platform shaker incubator, overnight at 30 °C. Thereafter, the liquid extracts were strained and stored at -80 °C for 24 h before lyophilisation. Aqueous extracts were lyophilised using a freeze dryer (Virtis, South Africa) for approximately 7 h or overnight. Before use, aqueous extracts were placed under ultraviolet light overnight to eliminate possible microbial contaminants. All plant samples were stored in appropriate containers at room temperature. Table 1 details the plant species collected, common names, reported traditional use, plant part used and percentage yield.
Plant sample preparation
Samples were prepared by weighing out the crude extracts and calculating the volume of solvent to be added to create a sample concentration of 32 mg/mL. Acetone (Sigma-Aldrich) was used as the solvent of choice for organic samples as it has minimal antimicrobial effects. Sterile water was used to dissolve aqueous extracts.
Test microorganisms
Test pathogens were selected according to their propensity to cause stomach ailments. Most of the selected microorganisms were obtained from the American Type Culture Collection (ATCC) and were purchased from Davies Diagnostics (Johannesburg, South Africa). Eight members of the Gram-negative anaerobic bacilli were selected. Two non-fastidious pathogens, E. coli (ATCC 8739) and E. faecalis (ATCC 29212), were included as comparators of activity (Table 2). These microorganisms were cultured in the respective media and under the incubation conditions prescribed by the Clinical Laboratory Standards Institute34, with slight modifications as described in Table 2. Two ethics waivers for the use of these microorganisms were obtained from the University of the Witwatersrand Human Research Ethics Committee (reference no. W-CBP-180509-01 for anaerobes and aerobic bacteria; and M170582 for H. pylori strains).
For H. pylori, the clinical strain was obtained from Chris Hani Baragwanath Academic Hospital (Johannesburg, South Africa). Methods as previously described35 were used to isolate the strains from patients. This isolation was achieved by obtaining biopsies from the antrum and corpus. These specimens were then placed in sterile bijou bottles containing a mixture of cysteine (200 mg/mL) and glycerol (20%) in brain heart infusion broth and transported on ice to the laboratory within 2 h of collection. Helicobacter pylori isolates were then confirmed by: polymerase chain reaction using glmM as the target gene; colony morphology and characteristic spiral morphology on Gram staining; and positive catalase, urease and oxidase tests. Confirmed isolates were suspended in 20% glycerol and stored at -80 °C in a freezer for future use. A reference strain, namely H. pylori (B8), was also tested. This strain was obtained from the Ludwig Maximilian University of Munich (Germany) medical microbiology laboratory, through the University of the Witwatersrand's Department of Surgery.
Antimicrobial analysis
Antimicrobial susceptibility was evaluated using the minimum inhibitory concentration (MIC) assay with specific modifications to facilitate fastidious growth of pathogens.34,36 Using aseptic techniques, 100 µL of broth, selected depending on the microorganism being tested, was introduced to all wells of the 96-well microtitre plates. Thereafter, 100 µL of respective plant sample to be tested was placed in the top row of the microtitre plate.
Controls (positive, negative and culture) were included in all assays. The role of the negative control was to ensure that the solvent (acetone) exerted no or minimal antimicrobial effect. Positive controls at starting concentrations of 0.01 mg/mL were used to validate the microbial susceptibility: ciprofloxacin was used for E. coli, E. faecalis, C. perfringens and Fusobacterium species; an equal ratio mix of clarithromycin and amoxicillin was used for H. pylori species; imipenem for Bacteroides species; and metronidazole for C. difficile. Ciprofloxacin was used as a broad-spectrum antibiotic. Metronidazole, imipenem, clarithromycin and amoxicillin were selected based on their antimicrobial susceptibility. A culture control was added to ensure the broth's ability to support microbial growth. Serial dilutions were then performed, and the plant extracts were diluted to concentrations of 8000, 4000, 2000, 1000, 500, 250, 130 and 60 µL/mL. A 100-µL volume of a standardised culture suspension (1 x 108 CFU/mL) prepared as a 0.5 McFarland's standard was added to all the wells of the microtitre plates. This resulted in two-fold dilutions descending along each row. Assays were undertaken at least in duplicate to ensure accuracy. The microtitre plates were incubated at optimal conditions (Table 2) without an adhesive seal film to allow the exposure of the cultures to required atmospheric conditions.
Antibiofilm analysis
Plant extracts that exhibited noteworthy activity (MIC≤160 µg/mL) against C. perfringens were selected for biofilm studies. Clostridium perfringens was also selected because it was the most susceptible of all the pathogens studied. Plant samples were immersed in sterile water and thereafter sonicated at room temperature and low speed using ultrasonic waves (SCIENTECH). The effect of plant extracts on biofilm attachment was tested using the method described by Sandasi et al.37 Using spectrophotometric methods, microbial cultures containing approximately 1x106 CFU/mL were prepared and added to the wells of a new 96-well microtitre plate, and a blank column containing sterile broth was also included. Prior to testing, the plate was incubated anaerobically for 4 h at 37 °C.
To test for the effect of plant extracts on established biofilms, the method described above was used, except stock cultures were incubated for 24h, 48h and 72h at 37 °C. After incubation, 100 μL of each plant extract was transferred to a final concentration of 1 mg/mL in the wells. Plates were incubated overnight at 37 °C, after which the crystal violet assay was performed at selected time intervals and the biofilm biomass determined. The percentage inhibition was calculated using Equation 137:

The crystal violet assay was undertaken to evaluate the ability of the extracts to prevent and inhibit the development of biofilms. This was done by washing the incubated plates with sterile water and oven drying them at 60 °C for 45 min. Once dried, all the wells were stained with 200 μL of 1% crystal violet and left at room temperature for 15 min to allow for proper absorption of the stain. This was followed by washing the plates with sterile water three times to remove the unabsorbed stain and adding 125 μL ethanol as a de-staining solution. A volume of 100 μL of the de-staining solution was transferred to a new microtitre plate and the absorbance was determined at 590 nm using a microplate reader (Universal microplate reader ELX 800). The mean absorbance of the extracts was determined prior to calculating the percentage inhibition. All tests were repeated at least in triplicate for reproducibility.
Toxicity of plant extracts
In order to hatch brine shrimp larvae, artificial seawater was prepared by dissolving 16 g of Tropic Marine® salt in 500 mL sterile water. Thereafter, 0.5 g of brine shrimp larvae (Artemia franciscana) (Ocean Nutrition) was added to the prepared seawater. Seawater was selected because it promotes the growth of brine shrimp larvae. A mixture containing the brine shrimp larvae and seawater was exposed to constant light from a light emitting diode (LED) bulb. Then larvae were aerated using a rotary pump (Kiho) to promote a better hatch. The mixture was then left at room temperature (25 °C) for 1-2 days. Toxicity was investigated for all extracts that displayed noteworthy antimicrobial activities (MIC≤160 µg/mL) against any of the tested pathogens (Table 3). Both the dichloromethane:methanol and aqueous plant extracts were prepared to a stock concentration of 2 mg/mL, and then a starting concentration of 1 mg/mL was achieved after dilution. Organic extracts were dissolved in 2% v/v dimethyl sulfoxide and aqueous extracts were dissolved in sterile water.
Hatched shrimp were transferred into a shallow, four-sided container, and then the LED study lamp was placed next to the container facing the opening of the container. This placement allowed for maximum light exposure, which in turn allowed the shrimp to gather in one place for easy collection. A volume of 400 μL seawater containing the brine shrimp (numbering 39-75) was transferred to each well of the 48-well microtitre plate. Viability of the brine shrimp was confirmed by observation under a light microscope (Olympus) prior to adding the samples. A volume of 400 μL of each organic and aqueous plant sample was added to 48-well microtitre plates. Each test was done in triplicate. Thereafter, 32 mg/mL seawater and 1.6 mg/mL potassium dichromate (Sigma) were added as positive and negative controls, respectively. All shrimp that were found dead after 24h and 48 h incubation were counted under the light microscope.
Plant extracts that displayed toxic effects were further tested at six concentrations (1000, 500, 250, 125, 63 and 31 μg/mL) to generate LC50 values that were determined using IBM® SPSS statistics and probit analysis. The LC50 value is defined as the concentration of a test material that possesses a toxic effect on half (50%) the tested shrimp. A lower LC50 value indicates a higher toxic profile of a material. Extracts with LC50 values lower than 249 μg/mL were considered highly toxic, 250 to 499 μg/mL moderately toxic, 500 to 999 μg/mL of low toxicity and values ≥1000 were considered non-toxic.38
Results and discussion
Antimicrobial analysis
The results of the antimicrobial assay expressed as MIC values are represented in Table 3. Antimicrobial activity was considered noteworthy for plant extracts when MIC values were ≤160 µg/mL. Moderate values were between 160 µg/mL and 1000 µg/mL and weak activity was classified as MICs of >1000 µg/mL. Poor activity is expressed by MICs greater than 8000 µg/mL.22,39,40 For the aqueous extracts, G. perpensa (leaf and rhizome) was the most active with a MIC of 130 µg/mL against the Clostridium species. As the organic extracts showed better activity, only these results are presented in Table 3.
Antimicrobial activity was compared for leaf and other plant parts; 7 of the 10 plants evaluated (70%) showed better activity for leaves than for other plant parts. Interestingly, none of the plant extracts displayed noteworthy antimicrobial activity against the common gut pathogens E. coli and E. faecalis.
Antimicrobial activity against Gram-positive bacteria
Gram-positive bacteria included two Clostridium species: C. perfringens and C. difficile. The Gram-positive bacteria were more vulnerable to the extracts than were the Gram-negative bacteria. Clostridium perfringens was the most susceptible. Approximately 10% of the extracts displayed noteworthy antimicrobial activity against C. difficile, whereas 39% of the extracts displayed moderate activity. Approximately 39% of the extracts displayed noteworthy activity against C. perfringens and another 39% displayed moderate activity.
The organic extracts of L. javanica leaf showed the best antimicrobial activity with an MIC of 0.5 µg/mL against C. perfringens. This value was comparable to the control antibiotic ciprofloxacin (MIC=0.2 µg/mL). The traditional use of L. javanica corroborates with the antimicrobial activity against Clostridium species, as the leaf infusion is traditionally used to treat diarrhoea, which is one of the symptoms of food poisoning or pseudomembranous colitis.41 Even though L. javanica displayed the best antimicrobial activity, to the best of our knowledge, this plant species has not been tested previously against Clostridium species. Other studies have instead focused on the antimicrobial activity of this plant species against common pathogens such as S. aureus, E. coli, E. faecalis, and Pseudomonas aeuruginosa.42
The organic extracts of G. perpensa (leaf 2 µg/mL and rhizome 130 µg/mL), as well as the leaf extracts of S. africana-caerulea (130 µg/mL and 30 µg/mL), S. africana (130 µg/mL), Syzygium cordatum (130 µg/mL) and Tetradenia riparia (130 µg/mL) displayed noteworthy antimicrobial activity against both Clostridium species. Traditionally, unspecified parts of G. perpensa are used for the treatment of stomach bleeding and the roots are used for other stomach ailments.30 To date, no previous studies have reported on the antimicrobial effectiveness of this plant on neglected pathogens. Nevertheless, findings from the current study were comparable to those reported in the literature, that is, Madikizela et al.43 reported good activity for the organic extracts of G. perpensa (leaf) against the gut pathogens Campylobacter jejuni, E. coli, S. aureus and Shigella flexineri, with MICs between 0.39 mg/mL and 0.78 mg/mL.
Traditionally, twig and leaf infusions of S. africana-caerulea are mixed with Epsom salts (magnesium sulfate) and lemon to treat stomach illnesses such as colic, diarrhoea, indigestion and stomach pain.30 To the best of our knowledge, no antimicrobial study was found with regard to S. africana-caerulea and the gut pathogens selected for this study. However, several other studies have reported on the antimicrobial activity of S. africana-caerulea against other gut microorganisms.44
Spirostachys africana is commonly known as the jumping-bean tree and it is traditionally used for the treatment of stomach ulcers, stomach pain, dysentery, acute gastritis and diarrhoea.31 The antimicrobial effects of S. africana on other pathogens has also been reported,45 with leaf and twig extracts showing good activity against S. aureus at a mean MIC value of 0.78 mg/mL.
The antimicrobial activity of S. cordatum validates the traditional use as the bark is boiled in water, then the mixture is taken orally three times a day until diarrhoea resolves.30 Mathabe et al.31 reported S. cordatum to be effective against a wide variation of diarrhoeal pathogens, including S. aureus, E. coli, S. typhimurium, Vibrio cholerae as well as Shigella species, with MIC values in the range of 0.16-0.31 mg/mL.
In previous studies, T. riparia showed good antimicrobial activity against common pathogens of the gut.28,44Tetradenia riparia is a multi-branched shrub or small tree, the leaves of which are traditionally used in infusions to treat stomach aches and diarrhoea.30 No study was found on the antimicrobial activity of T. riparia against Clostridium species. In a previous study44, T. riparia was found to be active against S. aureus with an MIC value of 0.78 mg/mL. Good antimicrobial activity of T. riparia was also noted against oral pathogens.28 Other extracts that displayed noteworthy activity against C. perfringens include Acokanthera oppositifolia (MIC=130 µg/mL), Aloe arborescens (MIC=30 µg/mL), Aloe marlothii (MIC=130 µg/mL), Aloe tenuior (MIC=2 µg/mL), Antidesma venosum (MIC=60 µg/mL), Artemisia afra (MIC=8 µg/mL), Bridelia micrantha (MIC=130 µg/mL), Polygala fruticosa (MIC=20 µg/mL), Solanum incanum (MIC=130 µg/mL) and S. cordatum (MIC=130 µg/mL).
Antimicrobial activity of organic extracts against Gram-negative bacteria
Gram-negative bacteria included eight bacterial groups which were further divided into two classes': (1) B. fragilis, B. ovatus, B. thetaiotaomicron, B. vulgatus, F. nucleatum and F. varium and (2) Gram-negative microaerophiles (H. pylori reference and the clinical strain).
Gram-negative anaerobes
Three extracts displayed noteworthy pathogen-specific activity. A total of 37 of the organic extracts displayed moderate activity against one or more Gram-negative anaerobes. The organic extracts of L. javanica (leaf) exhibited the best antimicrobial activity in this category, being active against B. fragilis and B. vulgatus, with MIC values of 20 µg/mL for both bacteria. Other plant extracts that were active in this category include P. fruticosa, which was active against B. thetaiotaomicron and F. nucleatum with an MIC value of 130 µg/mL for both bacteria. S. puniceus was active against B. thetaiotaomicron with an MIC value of 130 µg/mL. Polygala fruticosa roots are used traditionally for the management of intestinal sores.30
Gram-negative microaerophiles
Microaerophiles included the Helicobacter spp. which are a group of microorganisms that require a lower concentration of oxygen to survive.46,47 The organic extracts of A. arborescens displayed the best antimicrobial activity with an MIC value of 130 µg/mL against the reference strain. Comparative studies regarding anti-Helicobacter activities of A. arborescens were not found in the literature; however, it is not surprising that this species displayed good antimicrobial activity against H. pylori, because a decoction of the fresh leaves of Aloe species is traditionally used for management of H. pylori related infections.18
Antibiofilm assay
Results for the antibiofilm activities are categorised into four phases corresponding to biofilm developmental stages. First, the initial attachment of biofilms is represented at 4 h; biofilm formation at 24 h; and development of a mature biofilm at 48 h and 72 h. The results are presented in Table 4 and are interpreted either as weak antibiofilm activity (0-49%) or good antibiofilm activity (50-100%).48 Negative percentage inhibition denotes enhancement rather than inhibition of biofilms. Values in bold typeface denote good antibiofilm activity. At initial cell attachment stage (4 h), 19% of the extracts had antibiofilm inhibitory activity with at least 50% reduction in cell attachment. Approximately 57% of the extracts displayed good antibiofilm development (24 h) with percentage >50%. Most of the extracts had better activity than ciprofloxacin, whereas 38% of extracts displayed good antibiofilm activity and stopped the development of mature biofilms at 48 h and 72 h. With the exception of the organic extracts of A. tenuior, Bridelia cathartica and B. micrantha, all extracts displayed good antibiofilm activity for at least one stage of biofilm development.
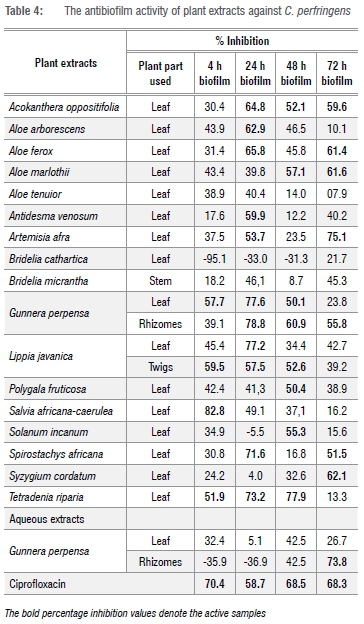
The bold percentage inhibition values denote the active samples
The organic extract of S. africana-caerulea leaf displayed the best antibiofilm activity overall, at 4 h at which it exhibited a percentage inhibition of 82.8%. The organic extracts of A. oppositifolia (leaf), G. perpensa (leaf), L. javanica (twigs) and T. riparia (leaf) displayed good antibiofilm activities for at least three biofilm developmental stages. Acokanthera oppositifolia displayed good antibiofilm activity at 24 h, 48 h and 72 h, preventing both initial biofilm formation and development of mature biofilms. Acokanthera oppositifolia displayed poor activity at 4 h. It can thus be concluded from these results that A. oppositifolia was more effective on older biofilms. At 24 h, the activity of A. oppositifolia was greater than that of ciprofloxacin (64.8% vs 58.7%). To the best of our knowledge, this study is the first antibiofilm study of A. oppositifolia.
The organic extracts of G. perpensa (leaf) were active at 4 h, 24 h and 48 h, preventing the attachment, formation and development of mature biofilms, whereas the organic extracts of G. perpensa rhizomes were active at 24 h, 48 h and 72 h. Gunnera perpensa extracts were mostly active against mature biofilms.
Lippia javanica twigs were active at 4 h, 24 h and 48 h with similar inhibition percentages. This finding suggests that the activity of L. javanica is not dependent on the incubation period and can work at any stage of biofilm development. Concerning the best activity in the MIC assay (Table 3), it is very interesting to note that the L. javanica extract was not only active against planktonic cells of C. perfringens but also displayed activity at an additional three biofilm developmental stages. These results support a previous study in which it was found that the same plant extracts that had good antibacterial activity also had good antibiofilm activity.48
The organic extracts of S. africana-caerulea leaf stood out, with the highest antibiofilm activities at 4 h. At 4 h, S. africana-caerulea reduced cell attachment with a better reduction percentage (82.79%) than that of ciprofloxacin (70.35%). The antimicrobial activity of S. africana-caerulea decreased with an increase in incubation period, thus it can be concluded that S. africana-caerulea was more effective on new biofilms.
The organic extracts of T. riparia (leaf) demonstrated notable antibiofilm activity at 4 h, 24 h and 48 h, preventing cell attachment, stopping development of biofilms and development of mature biofilms. At 72 h, T. riparia displayed poor antibiofilm activity, meaning that it is more effective on premature biofilms than on mature biofilms.
This study is the first to report on the antibiofilm activity of plant extracts on C. perfringens biofilms. Globally, most plant-based studies have focused on the antibiofilm activity of medicinal plants against biofilm formers such as E. coli, S. aureus and P. aeruginosa.49,50 For southern African plant species, studies undertaken on antibiofilm activity have been neglected. Only a few relevant studies have been investigated.22 Most of these have focused on the antibiofilm activities of southern African medicinal plants against clinically important pathogens such as Listeria monocytogenes, P. aeruginosa and C. albicans.48-52 The antibiofilm activity of southern African medicinal plants has been investigated against the oral pathogen Streptococcus mutans.28
The current study showed that some plant extracts that showed good antimicrobial activity against C. perfringens in the MIC assay are capable of inhibiting C. perfringens biofilms. Prevention of cell attachment proved to be more difficult to achieve than prevention of biofilm development in a mature biofilm. It is very surprising that many extracts displayed better activity at biofilm development stage (24 h) than at cell attachment stage (4 h), as a previous study reported that inhibiting initial cell attachment is easier than inhibiting preformed biofilms.47
Toxicity assay
The 22 medicinal plant extracts that displayed noteworthy antimicrobial activity (MIC ≤160 µg/mL) (Table 3) against neglected gut pathogens, were screened for toxicity. The results of the brine shrimp lethality assay for both organic and aqueous extracts are shown in Table 5. None of the aqueous extracts possessed toxic effects. At 24 h, none of the extracts displayed toxic effects. At 48 h, 82% of the tested extracts were non-cytotoxic and 18% of the extracts possessed toxic effects. Organic extracts of A. oppositifolia, A. venosum, L. javanica and T. riparia leaves were toxic, with percentage mortalities of 73.23%, 100%, 94.70% and 59.65%, respectively.
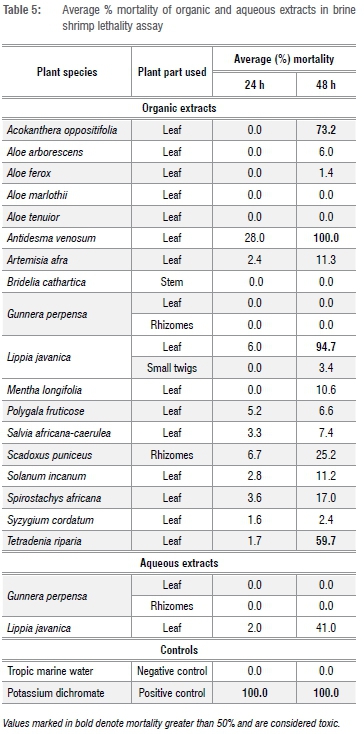
The majority of the tested plant extracts were non-toxic. The lowest toxic effects were observed for the leaf organic extracts of A. marlothii, A. tenuior, B. cathartica and G. perpensa, and the aqueous extracts of G. perpensa leaf and rhizome for which the percentage mortalities of 0% were displayed at both 24 h and 48 h. Similar conclusions were reached in a study by Gehring et al.53 They found that the dichloromethane extracts of G. perpensa rhizome had no toxic effects on brine shrimp at a concentration of 1 mg/mL.
When the LC50 values of extracts of the plants that displayed toxic effects were tested (Table 6), A. oppositifolia leaf demonstrated low toxicity on the brine shrimp with an LC50 of 984 μg/mL. Antidesma venosum leaf was moderately toxic with an LC50 of 297 μg/mL after 48 h, whereas L. javanica and T. riparia leaves were highly toxic after 48 h with LC50 values of 88 μg/mL and 77 μg/mL, respectively. These plant extracts were highly active against planktonic bacteria and biofilms, but the high toxicity demonstrates a very low therapeutic index.
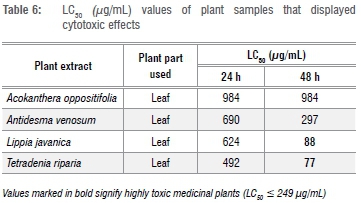
Table 7 displays a complete overview of the plant extracts that were active against at least one pathogen, displayed good antibiofilm activity at one biofilm development stage and had low cytotoxic effect. These plant species warrant further investigation.
Conclusion
The results from the MIC assay favour the traditional use of some plant extracts for intra-abdominal infections. The Gunnera perpensa organic extract was the most interesting of all the tested extracts, in that it displayed very good antimicrobial activity against Clostridium species (MIC = 2-130 µg/mL). The plant species also displayed good antibiofilm activity against new and older biofilms (average inhibition = 52.3% for leaf extract and 58.7% for rhizome), with no toxic effects (mortality = 0%). A notable result was seen in the aqueous extracts of G. perpensa (leaf and rhizomes), where noteworthy activity was observed against Clostridium species with MIC values of 130 µg/mL. In some instances, there was a direct relationship between the antimicrobial activity and the traditional use. For example, S. africana is traditionally used for diarrhoea. In the current study the organic extract of the leaf displayed noteworthy activity against C. difficile and C. perfringens. Also interesting is that none of the plant extracts displayed noteworthy activity against the common pathogens E. coli and E. faecalis. Biofilm results indicated that most of the plants that were active against C. perfringens were also effective against C. perfringens biofilms. The brine shrimp lethality assay results revealed that most of the plant samples were non-toxic to the brine shrimps. This study demonstrates that investigations should not only focus on common pathogens, but also on neglected pathogens which may yield excellent results not previously reported. This study contributes to the knowledge of the antimicrobial properties of plants commonly found in southern Africa.
Acknowledgements
We thank the National Research Foundation (South Africa) for funding the running costs of this study. We acknowledge a Postgraduate Merit Award and Faculty Research Committee Grant (both University of the Witwatersrand) for financial support. Chief horticulturist of the Walter Sisulu National Botanical Garden, Mr Andrew Hankey, is thanked for his permission and assistance in plant collection and identification.
Authors' contributions
H.S.: method development; data collection; sample analysis; data analysis; writing - the initial draft; writing - revisions. C.L.: Assisted with biofilm assay; edited final draft of manuscript. G.C.: Method development; editing drafts; student supervision; project leadership; funding acquisition. S.v.V.: Conceptualisation of project; method development; data collection; sample analysis; data analysis; editing drafts; primary student supervision; project leadership; project management; funding acquisition.
References
1.Marshall JC. Intra-abdominal infections. Microbes Infect. 2004;6(11):1015-1025. https://doi.org/10.1016/j.micinf.2004.05.017 [ Links ]
2.Sartelli M, Chichom-Mefire A, Labricciosa FM, Hardcastle T, Abu-Zidan FM, Adesunkanmi AK, et al. The management of intra-abdominal infections from a global perspective: 2017 WSES Guidelines for management of intra-abdominal infections. World J Emerg Surg. 2017;12(1), Art. #29, 34 pages. https://doi.org/10.1186/s13017-017-0141-6 [ Links ]
3.Farnbacher M, Jahns T, Willrodt D, Daniel R, Haas R, Goesmann A, et al. Sequencing, annotation, and comparative genome analysis of the gerbil-adapted Helicobacter pylori strain B8. BMC Genomics. 2010;11(1), Art. #335, 22 pages. https://doi.org/10.1186/1471-2164-11-335 [ Links ]
4.Castellarin M, Warren RL, Freeman JD, Dreolini L, Krzywinski M, Strauss J, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299-306. https://doi/10.1101/gr.126516.111 [ Links ]
5.Bosques-Padilla FJ, Remes-Troche JM, González-Huezo MS, Pérez-Pérez G, Torres-López J, Abdo-Francis JM, et al. The fourth Mexican consensus on Helicobacter pylori. Revista de Gastroenterología de México (English edition). 2018;83(3):325-341. https://doi.org/10.1016/j.rgmxen.2018.07.002 [ Links ]
6.Mazuski JE, Solomkin JS. Intra-abdominal infections. Surg Clin N Am. 2009;89(2):421-437. https://doi.org/10.1016/j.suc.2008.12.001 [ Links ]
7.Nagy E. Anaerobic infections. Drugs. 2010;70(7):841-858. [ Links ]
8.Wexler HM. Bacteroides: The good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20(4):593-621. https://doi.org/10.1128/cmr.00008-07 [ Links ]
9.Ho PL, Yau CY, Ho LY, Lai EL, Liu MC, Tse CW, et al. Antimicrobial susceptibility of Bacteroides fragilis group organisms in Hong Kong by the tentative EUCAST disc diffusion method. Anaerobe. 2017;47:51-56. https://doi.org/10.1016/j.anaerobe.2017.04.005 [ Links ]
10.Hansen KC, Schwensen SA, Henriksen DP, Justesen US, Sydenham TV. Antimicrobial resistance in the Bacteroides fragilis group in faecal samples from patients receiving broad-spectrum antibiotics. Anaerobe. 2017;1;47:79-85. https://doi.org/10.1016/j.anaerobe.2017.04.013 [ Links ]
11.Varga JJ, Therit B, Melville SB. Type IV pili and the CcpA protein are needed for maximal biofilm formation by the Gram-positive anaerobic pathogen Clostridium perfringens. Infect Immun. 2008;76(11):4944-4951. https://doi.org/10.1128/iai.00692-08 [ Links ]
12.Zhou Z, Chen J, Yao H, Hu H. Fusobacterium and colorectal cancer. Front Oncol. 2018;8, Art. #371, 11 pages. https://doi.org/10.3389/fonc.2018.00371 [ Links ]
13.Abadi AT, Yamaoka Y. Helicobacter pylori therapy and clinical perspective. J Glob Antimicrob Resist. 2018;14:111-117. https://doi.org/10.1016/j.jgar.2018.03.005 [ Links ]
14.Zaidi SF, Yamada K, Kadowaki M, Usmanghani K, Sugiyama T. Bactericidal activity of medicinal plants, employed for the treatment of gastrointestinal ailments, against Helicobacter pylori. J Ethnopharmacol. 2009;121(2):286-291. https://doi.org/10.1016/j.jep.2008.11.001 [ Links ]
15.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386. https://doi.org/10.1002/ijc.29210 [ Links ]
16.Shigefuku R, Watanabe T, Kanno Y, Ikeda H, Nakano H, Hattori N, et al. Fusobacterium nucleatum detected simultaneously in a pyogenic liver abscess and advanced sigmoid colon cancer. Anaerobe. 2017;48:144-146. https://doi.org/10.1016/j.anaerobe.2017.08.010 [ Links ]
17.Njume C, Jide AA, Ndip RN. Aqueous and organic solvent-extracts of selected South African medicinal plants possess antimicrobial activity against drug-resistant strains of Helicobacter pylori: Inhibitory and bactericidal potential. Int J Mol Sci. 2011;12(9):5652-5665. http://doi.org/10.3390/ijms12095652 [ Links ]
18.Njume C, Afolayan AJ, Ndip RN. Diversity of plants used in the treatment of Helicobacter pylori associated morbidities in the Nkonkobe Municipality of the Eastern Cape province of South Africa. J Med Plant Res. 2011;5(14):3146-3151. [ Links ]
19.McGaw LJ, Jäger AK, Van Staden J. Antibacterial, anthelmintic and anti-amoebic activity in South African medicinal plants. J Ethnopharmacol. 2000;72:247-263. https://doi.org/10.1016/S03788741(00)00269-5 [ Links ]
20.Kloucek P, Polesny Z, Svobodova B, Vlkova E, Kokoska L. Antibacterial screening of some Peruvian medicinal plants used in Calleria District. J Ethnopharmacol. 2005;99(2):309-312. https://doi.org/10.1016/j.jep.2005.01.062 [ Links ]
21.Costa ES, Hiruma-Lima CA, Lima EO, Sucupira GC, Bertolin AO, Lolis SF, et al. Antimicrobial activity of some medicinal plants of the Cerrado, Brazil. Phytother Res. 2008;22(5):705-707. https://doi.org/10.1002/ptr.2397 [ Links ]
22.Van Vuuren S, Holl D. Antimicrobial natural product research: A review from a South African perspective for the years 2009-2016. J Ethnopharmacol. 2017;17:236-252. https://doi.org/10.1016/j.jep.2017.07.011 [ Links ]
23.Van Vuuren SF. Antimicrobial activity of South African medicinal plants. J Ethnopharmacol. 2008;119(3):462-472. https://doi.org/10.1016/j.jep.2008.05.038 [ Links ]
24.Cai J, Huang H, Song W, Hu H, Chen J, Zhang L, et al. Preparation and evaluation of lipid polymer nanoparticles for eradicating H. pylori biofilm and impairing antibacterial resistance in vitro. Int J Pharm. 2015;495(2):728-737. https://doi.org/10.1016/j.ijpharm.2015.09.055 [ Links ]
25.Chen M, Yu Q, Sun H. Novel strategies for the prevention and treatment of biofilm related infections. Int J Mol Sci. 2013;14(9):18488-18501. https://doi.org/10.3390/ijms140918488 [ Links ]
26.Agarwal H, Gayathri M. Biological synthesis of nanoparticles from medicinal plants and its uses in inhibiting biofilm formation. Asian J Pharm Clin Res. 2017;10(5):64-68. https://doi.org/10.22159/ajpcr.2017.v10i5.17469 [ Links ]
27.Fennell CW, Lindsey KL, McGaw LJ, Sparg SG, Stafford GI, Elgorashi EE, et al. Assessing African medicinal plants for efficacy and safety: Pharmacological screening and toxicology. J Ethnopharmacol. 2004;94(2-3):205-217. https://doi.org/10.1016/j.jep.2004.05.012 [ Links ]
28.Akhalwaya S, Van Vuuren S, Patel M. An in vitro investigation of indigenous South African medicinal plants used to treat oral infections. J Ethnopharmacol. 2018;210:359-371. https://doi.org/10.1016/j.jep.2017.09.002 [ Links ]
29.Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of southern and eastern Africa. Edinburgh: E & S Livingstone Ltd; 1962. [ Links ]
30.Van Wyk B-E, Van Oudtshoorn BV, Gericke N. Medicinal plants of South Africa. Pretoria: Briza; 2009. https://doi.org/10.1365/s10337-010-1583-0 [ Links ]
31.Mathabe MC, Hussein AA, Nikolova RV, Basson AE, Meyer JM, Lall N. Antibacterial activities and cytotoxicity of terpenoids isolated from Spirostachys africana. J Ethnopharmacol. 2008;116(1):194-197. https://doi.org/10.1016/j.jep.2007.11.017 [ Links ]
32.Hutchings A, Scott AH, Lewis G, Cunningham AB. Zulu medicinal plants: An inventory. Pietermaritzburg: University of Natal Press; 1996. [ Links ]
33.Von Koenen EV. Medicinal, poisonous, and edible plants in Namibia. Göttingen: Klaus Hess Publishers; 2001. [ Links ]
34.Clinical and Laboratory Standards Institute (CLSI). Methods for antimicrobial susceptibility testing of anaerobic bacteria: Approved standard. 8th ed. CLSI document M11-A8. Wayne, PA: CLSI; 2012. [ Links ]
35.Tanih NF, Okeleye BI, Ndip IM, Clarke AM, Naidoo N, Mkwetshana N, et al. Helicobacter pylori prevalence in dyspeptic patients in the Eastern Cape province - Race and disease status. S Afr Med J. 2010;100(11):734-737. https://doi.org/10.7196/SAMJ.4041 [ Links ]
36.Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Plant Med. 1998;64(08):711-713. https://doi.org/10.1055/s-2006-957563 [ Links ]
37.Sandasi M, Leonard CM, Van Vuuren SF, Viljoen AM. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S Afr J Bot. 2011;77(1):80-85. https://doi.org/10.1016/j.sajb.2010.05.011 [ Links ]
38.Bussmann RW, Malca G, Glenn A, Sharon D, Nilsen B, Parris B, et al. Toxicity of medicinal plants used in traditional medicine in Northern Peru. J Ethnopharmacol. 2011;137(1):121-140. https://doi.org/10.1016/j.jep.2011.04.071 [ Links ]
39.Pauw E, Eloff JN. Which tree orders in southern Africa have the highest antimicrobial activity and selectivity against bacterial and fungal pathogens of animals? BMC Complement Alt Med. 2014;14(1), Art. #317, 12 pages. https://doi.org/10.1186/1472-6882-14-317 [ Links ]
40.Freires I, Denny C, Benso B, de Alencar S, Rosalen P. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: A systematic review. Molecules. 2015;20(4):73297-73358. https://doi.org/10.3390/molecules20047329 [ Links ]
41.Shikanga EA, Combrinck S, Regnier T. South African Lippia herbal infusions: Total phenolic content, antioxidant and antibacterial activities. S Afr J Bot. 2010;76(3):567-571. https://doi.org/10.1016/j.sajb.2010.04.010 [ Links ]
42.Madikizela B, Ndhlala AR, Rengasamy KR, McGaw LJ, Van Staden J. Pharmacological evaluation of two South African commercial herbal remedies and their plant constituents. S Afr J Bot. 2017;111:291-298. https://doi.org/10.1016/j.sajb.2017.03.038 [ Links ]
43.Madikizela B, Ndhlala AR, Rengasamy KR, McGaw LJ, Van Staden J. Pharmacological evaluation of two South African commercial herbal remedies and their plant constituents. S Afr J Bot. 2017;111:291-298. https://doi.org/10.1016/j.sajb.2017.03.038 [ Links ]
44.Kamatou GP, Makunga NP, Ramogola WP, Viljoen AM. South African Salvia species: A review of biological activities and phytochemistry. J Ethnopharmacol. 2008;119(3):664-672. https://doi.org/10.1016/j.jep.2008.06.030 [ Links ]
45.Nielsen TR, Kuete V, Jäger AK, Meyer JJ, Lall N. Antimicrobial activity of selected South African medicinal plants. BMC Complement Alt Med. 2012;12(1), Art. #74, 6 pages. https://doi.org/10.1186/1472-6882-12-74 [ Links ]
46.Prescott LM, Harley JP, Klein DA. Microbiology. 3rd ed. Chicago, IL: Wim C Brown Publishers; 1996. [ Links ]
47.Hogg S. Essential microbiology. Oxford: John Wiley & Sons; 2013. [ Links ]
48.Sandasi M, Leonard CM, Viljoen AM. The in vitro antibiofilm activity of selected culinary herbs and medicinal plants against Listeria monocytogenes. Lett Appl Microbiol. 2010;50(1):30-35. https://doi.org/10.1111/j.1472-765X.2009.02747.x. [ Links ]
49.Andriani Y, Mohamad H, Bhubalan K, Abdullah MI, Amir H. Phytochemical analyses, anti-bacterial and anti-biofilm activities of mangrove-associated Hibiscus tiliaceus extracts and fractions against Pseudomonas aeruginosa. J Sustain Sci Manage. 2017;12(2):45-51. [ Links ]
50.Costa GM, Endo EH, Cortez DA, Ueda-Nakamura T, Nakamura CV, Dias Filho BP. Effect of plant extracts on planktonic growth and biofilm of Staphylococcus aureus and Candida albicans. Int J Curr Microbiol Appl Sci. 2015;4:9081-9087. [ Links ]
51.Bazargani MM, Rohloff J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control. 2016;61:156-164. https://doi.org/10.1016/j.foodcont.2015.09.036 [ Links ]
52.Leonard CM, Virijevic S, Regnier T, Combrinck S. Bioactivity of selected essential oils and some components on Listeria monocytogenes biofilms. S Afr J Bot. 2010;76(4):676-680. https://doi.org/10.1016/j.sajb.2010.07.002 [ Links ]
53.Gehring R, Katsoulis L, Eloff JN, McGaw LJ. Is the use of Gunnera perpensa extracts in endometritis related to antibacterial activity? Onderstepoort J Vet Res. 2005;72(2):129-134. https://doi.org/10.4102/ojvr.v72i2.208 [ Links ]
 Correspondence:
Correspondence:
Sandy van Vuuren
Sandy.vanvuuren@wits.ac.za
Received: 04 Apr. 2019
Revised: 19 May 2019
Accepted: 25 July 2019
Published: 27 Nov. 2019
Editor: Pascal Bessong
Funding: University of the Witwatersrand, National Research Foundation (South Africa)














