Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.115 n.11-12 Pretoria Nov./Dec. 2019
http://dx.doi.org/10.17159/sajs.2019/5972
RESEARCH ARTICLE
Antibiotic sensitivity of bacteria isolated from the oral cavities of live white sharks (Carcharodon carcharias) in South African waters
Enrico GennariI, II, III; Alison A. KockII, IV, V; Malcolm J. SmaleVI; Alison TownerIII; Nasreen KhanVII; Linda A. BesterVIII; Ryan JohnsonIX; Chris FischerX; Michael MeÿerXI; Peter MorseI
IOceans Research, Mossel Bay, South Africa
IISouth African Institute for Aquatic Biodiversity, Makhanda, South Africa
IIIDepartment of Ichthyology and Fisheries Science, Rhodes University, Makhanda, South Africa
IVCape Research Centre, South African National Parks, Cape Town, South Africa
VInstitute for Communities and Wildlife in Africa, Department of Biological Sciences, University of Cape Town, Cape Town, South Africa
VIInstitute for Coastal and Marine Research, Nelson Mandela University, Port Elizabeth, South Africa
VIIIsland Conservation Society, Victoria, Mahé, Seychelles
VIIIBiomedical Resource Unit, School of Laboratory Medicine and Medical Sciences, University of KwaZulu-Natal, Durban, South Africa
IXBlue Wilderness Research Unit, Scottburgh, South Africa
XOCEARCH, Park City, Utah, USA
XIDepartment of Environmental Affairs, Cape Town, South Africa
ABSTRACT
The white shark (Carcharodon carcharias) is responsible for 49% of shark-related injuries in South Africa, yet no information currently exists on the composition or antibiotic resistance of bacteria hosted by these apex predators in South African waters. This study aimed to address this gap by sampling the bacteria present in the oral cavities of 28 live C. carcharias along South Africa's southern coastline. The antibiotic resistance of the range of microbiota was also assessed using antibiotic disc diffusion tests. A total of 51 strains from at least 20 species of bacteria were isolated from the oral cavities of C. carcharias. Of these strains, the most common bacteria present were Serratia spp., Proteus vulgaris and Vibrio alginolyticus. The overall antibiotic resistance was relatively higher in this study than that reported for bacterial microbiota sampled from other shark species. Results indicate that the combination therapy of imipenem (carbapenem antibiotic) and vancomycin (glycopeptide antibiotic) might be the most parsimonious option to effectively treat infections resulting from white shark bites, particularly in South Africa. It is hoped that, in addition to assisting medical professionals to treat shark bite victims, these findings enhance the understanding of the microbial communities present in large coastal predators and their surrounding environments.
SIGNIFICANCE:
•Overall antibiotic resistance of bacteria in the oral cavities of C. carcharias was relatively high.
•Combination therapy of imipenem (carbapenem antibiotic) and vancomycin (glycopeptide antibiotic) is recommended for the treatment of white shark bites, particularly in South Africa.
•The findings add to understanding of the microbial communities present in large coastal predators and their surrounding environments
Keywords: antibiotic resistance, antimicrobial agents, apex predator, emergency medicine, marine microbiology
Introduction
The oral cavity of sharks, like many fauna, is host to a wide range of bacteria.1-4 Therefore, victims of shark-related injuries involving shark bites require treatment for the prevention of infections caused by the transfer of pathogenic bacteria.5,6 A review of 11 recent shark-related injuries in the USA indicated that only three of the reviewed patients received an appropriate selection of antibiotics for treatment of infection (using ciprofloxacin), and none of the reviewed patients received dual antibiotic therapy.5 An earlier review of 83 shark-related injuries in South African waters could only confirm that 18 of the reviewed patients received any antibiotic treatment (using a variety of different antibiotics), and three of these patients continued to develop septic complications that required further surgical intervention.6 Currently, there is no consensus on the most appropriate antibiotics to be used in treating bacterial infections resulting from shark-related injuries due to large differences in the composition of bacterial microbiota present among different shark species and their geographical locations.4,5 Therefore, further information is needed on the antibiotic resistance of bacteria present in the oral cavities of different shark species from different regions of the world that might be responsible for shark-related injuries in humans.
There have been increasing reports of antibiotic resistance among bacteria hosted by marine predators3,4,7, possibly because of the increased use of broad-spectrum antibiotics in humans and their subsequent entry as contaminants into coastal waters7. A study of bacteria found post-mortem in the oral cavities of bull sharks (Carcharhinus leucas) and tiger sharks (Galeocerdo cuvier) off Recife (Brazil) reported high levels of antibiotic resistance among several of the 81 isolated bacterial strains.3 In particular, that study found a 20% resistance among Proteus mirabilis strains to imipenem, a broad-spectrum antibiotic commonly used to treat Gram-negative nosocomial infections.8 Another study on bacteria isolated from cloacal swabs of C. leucas, blacktip sharks (Carcharhinus limbatus), nurse sharks (Ginglymostoma cirratum) and lemon sharks (Negaprion brevirostris) from Belize and the US East Coast, reported multidrug resistance in bacteria tested from all sampled shark species.7 A comprehensive study undertaken by Unger et al.4 of bacteria isolated from the oral cavities of adult, live C. limbatus in Florida (USA) found that the three primary bacterial types present were Vibrio spp., Staphylococcus spp. and Pasteurella spp. Unger et al.4 additionally reported that 43% of isolated bacteria showed resistance to at least one antibiotic, and the overall resistance rate of all antibiotics they tested was 12%. Despite the prevalence of antibiotic resistance, these authors concluded that the best antibiotic selection for treating infections resulting from shark-related injuries involving C. limbatus near Florida was either a broad-spectrum flouroquinolone, or a dual antibiotic treatment using a third-generation cephalosporin and doxycycline.4
South Africa has the third highest incidence (after the USA and Australia) of human/shark encounters from 1580 to the present.9 Within South Africa, white sharks (Carcharodon carcharias) account for 49% of reported shark-related injuries.6 However, data on the bacterial microbiota present in C. carcharias oral cavities are limited to one sample that was taken port-mortem from a harpooned shark in northeast USA over two decades ago.2 At present, very little is known about the full composition of bacterial strains present in C. carcharias oral cavities, or what the current resistance of these bacteria might be to available antibiotics. This study aimed to address this informational gap in order to assist medical professionals in making informed decisions when administering treatment for bacterial infections in C. carcharias bite victims. Specifically, this study aimed to identify bacterial species that are present in the oral cavities of live C. carcharias along the southern coastline of South Africa, and to assess the sensitivity of these bacteria to 16 commonly used antibiotics. It is intended that these results might help in selecting the most appropriate antibiotic(s) for this category of injury, and also enable alternatives to broad-spectrum antibiotics that continue to raise resistance levels of pathogenic microbiota.
Methods
Animal capture and sample preparation
A total of 28 C. carcharias were caught using circle hooks tethered to floating buoys from four sample sites along South Africa's southern coastline (False Bay, Gansbaai, Mossel Bay and Algoa Bay) and sampled for this study (Figure 1). Sharks were led to a submerged platform, which was then lifted out of the water to minimise cross-contamination while sampling. Water supply to the gills was achieved via pumping seawater through a rigid pipe inserted into the mouth of the shark. Sterile cotton swabs were used to collect microbial samples from sharks' oral cavities. To ensure comprehensive sampling, separate swabs were taken of sharks' teeth, gums and tongue. However, these data were compiled together into a single sample category (oral cavity) due to likely cross-contamination. Additionally, attention was given to avoiding contact with the rigid pipe while sampling in order to minimise the chance of cross-contamination among individuals. All sharks were handled for a maximum of 15 min, after which they were released and monitored by a veterinarian to ensure no external signs of stress and/or capture myopathy. All interactions were approved by the South African Department of Environmental Affairs (reference RES2012/OCEARCH/JOHNSON).

Processing and identification of bacteria
All swabs were transported to the Ampath pathology laboratory in George (Western Cape, South Africa) within a week of sampling, where bacterial samples were each plated onto three replicates of Nutrient Agar® and streaked for single colonies. They were then incubated for 24 h at 37 °C in an aerobic atmosphere and stored in Tryptone Soya Broth®. Presumptive isolates from the Nutrient Agar® replicates were recovered from all plates, sub-cultured for purity and stored in Tryptone Soya Broth® plus 10% glycerol within microbank cryovial systems at -20 °C until further processing. Initial screening of presumptive isolates began with examining for Gram status, lactose fermentation, as well as oxidase and catalase reactions. Identifications of Gram-negative bacteria were confirmed using bioMérieux® API® ٢٠E systems. Where possible, Gram-negative isolates were identified to species level, but in some cases could only be identified to genus level. Inconclusive Gram-negative isolates were reported as 'Gram-negative bacilli'. Gram-positive isolates were identified using Biorad Pastorex Staph Plus® and Remel® Streptex® kits.
Determining presence of beta-lactamase production
All confirmed bacterial strains were additionally examined for chromosomal beta-lactamase (Amp-C) production and extended beta-lactamase (ESBL) production at the Ampath pathology laboratory, as bacterial strains that can produce either type of these enzymes are likely to have strong resistance to all beta-lactam type antibiotics.10 Amp-C production was assessed by observing a flattening of the cefotaxime zone on the side of the microbe adjacent to imipenem, as well as a flattening of the ceftazidime and/or cefotaxime zone on the side of the microbe adjacent to cefoxitin. Determining the presence of ESBL production among bacterial strains was performed by observing distortions of the inhibition zones around ceftazidime and/or cefepime, in the areas adjacent to the amoxicillin/clavulanate disc. Distortions indicating ESBL production took the form of (1) an increased radius of the inhibition zone for the cephalosporin(s) or (2) the presence of a lens-shaped inhibition zone between the cephalosporin and amoxicillin-clavulanic discs either when there was no inhibition zone around the cephalosporin disc or if the inhibition zone was very narrow.
For bacterial strains in which Amp-C production was present, resistance to ampicillin; amoxicillin/clavulanic acid; piperacillin/tazobactam; first-, second- and third-generation cephalosporins; and cefoxitin was reported, regardless of zone size or further testing. For any bacterial strains for which ESBL production was confirmed, resistance to ampicillin, first-, second-, third- and fourth-generation cephalosporins, piperacillin/tazobactam, amoxicillin/clavulanic acid, as well as sensitivity to cefoxitin was reported. The susceptibilities of these strains to other antibiotics, not listed above, were reported according to results of the further testing outlined below.
Assessing bacterial sensitivity to antibiotics
All bacterial strains isolated from oral cavity samples of C. carcharias were tested for susceptibility to a panel of 16 antibiotics (summarised in Table 1) using antibiotic disc diffusion tests according to the Kirby-Bauer methodology outlined in the guidelines of the US Clinical and Laboratory Standards Institute.11
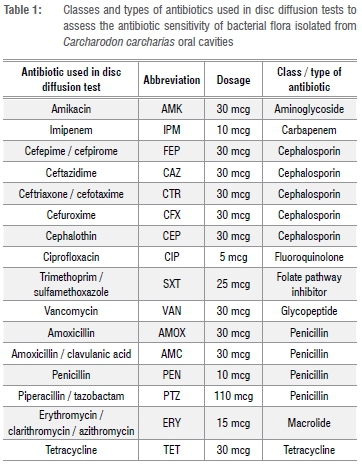
Bacteria were reported as sensitive to an antibiotic if all strains of those bacteria indicated sensitivity to the drug. Bacteria were reported as resistant to an antibiotic if all of its strains were resistant to the drug. If different strains of the same type of bacteria showed conflicting susceptibility to an antibiotic it was reported in the results as 'R*', but was considered resistant for the sake of calculating relative percentages of antibiotic effectiveness. If an antibiotic is not routinely used on an isolate, is not used for treatment of a given strain, or if there was no available information on the zone or the minimum inhibitory concentration breakpoint of the bacteria or antibiotic11, then this combination was reported in the results as 'not applicable'.
Results
Bacterial species present
A total of 51 bacterial strains were isolated from oral cavities of 28 live C. carcharias (Table 2). Among these strains, there were at least 20 species of bacteria present. An additional five strains which could not be identified were grouped into the category 'Gram-negative bacilli' (Table 2). The three most common bacterial strains identified from the oral cavities (in order of frequency) were: Serratia spp., Proteus vulgaris and Vibrio alginolyticus. Aeromonas hydrophyla, Enterococcus faecalis and Staphylococcus spp. were also present in at least 10% of all sharks sampled (Table 2).
Assessment of Amp-C and ESBL production among isolated strains
Among the 51 bacterial strains isolated from samples, 6 isolates tested positive for Amp-C production. These isolates were: E. cloacae (n=2), Klebsiella pneumoniae (n=1), Proteus mirabilis (n=1), P. vulgaris (n=1) and Serratia spp. (n=1). All strains tested negative for ESBL production.
Bacterial sensitivity to antibiotics
All strains of bacteria isolated from the oral cavities of live C. carcharias in this study displayed sensitivity to a panel of at least four types of antibiotics (Table 3). Antibiotic resistance varied greatly among bacterial strains, but was observably higher in E. cloacae, K. pneumoniae, P. mirabilis, P. vulgaris and Serratia spp. which were among the strains that tested positive for Amp-C production. Of the 16 antibiotics that were tested, ciprofloxacin, amikacin and imipenem yielded the highest sensitivity among bacterial strains (Table 3). Specifically, these three antibiotics were effective in treating each of the Amp-C positive bacterial strains mentioned above. Trimethoprim-sulfamethoxazole and tetracycline followed closely in overall effectiveness; however, several of the Amp-C positive bacteria were resistant to both these antibiotics (Table 3). Isolated bacterial strains showed the highest resistance to amoxicillin, piperacillin-tazobactam and the cephalosporins (cephalothin and cefuroxime). No single antibiotic tested could effectively treat all the bacterial strains on its own (Table 3), indicating that multiple antibiotics would be necessary in treating exposure to the full panel of bacteria isolated from C. carcharias oral cavities in this study.
Discussion
Among the 16 antibiotics tested, both imipenem and ciprofloxacin were the only two that effectively treated each of the Gram-negative bacteria found in the oral cavities of C. carcharias in this study (Table 3). The Gram-positive bacteria isolated from C. carcharias oral cavities included Enterococcus spp., Staphylococcus spp. and Streptococcus spp. and these strains showed consistent sensitivity to both vancomycin and penicillin during disc diffusion tests (Table 3). Infection with these Gram-positive bacteria may also be effectively treated with imipenem; however, at the time of writing, there was no available information on the zone or minimum inhibitory concentration breakpoint of enterococci, staphylococci or streptococci with imipenem11, and so this combination was not assessed in the disc diffusion test in the present study. Therefore, these findings indicate that treatment with imipenem, and possibly a combined regime of vancomycin would be the most effective option for preventing and/or treating infections resulting from white shark bites in South Africa. Based on the results, ciprofloxacin and penicillin, in place of imipenem and vancomycin respectively, also have potential as treatment options for white shark bite patients (Table 3). However, fluoroquinolone antibiotics, to which class ciprofloxacin belongs, often show an antagonistic effect in antibiotic combination therapies, as can the doubling up of two beta-lactam antibiotics12 (e.g imipenem and penicillin). Nevertheless, Al-Hasan et al.13 demonstrated that fluoroquinolones combined with beta-lactam antibiotics can contribute to a positive treatment outcome when suspecting Gram-negative bacilli. That said, combination therapy between fluoroquinolones and beta-lactam antibiotics should be considered carefully as the synergistic significance is not clear and clinical outcomes are conflicting.14
The overall composition of bacterial microbiota identified in the oral cavities of live C. carcharias in this study was similar to microbial presence reported from other shark species.3,4 Specifically, the prevalence of Enterobacter spp. and Proteus spp. coincided with common bacteria sampled from C. leucas and G. cuvier in Recife, Brazil.3 The occurrence of Vibrio spp. and Staphylococcus spp. strains mirrored findings from live C. limbatus oral cavities in Florida, USA.4 Additionally, the presence of Vibrio spp. and Shewanella putrefaciens was consistent with bacterial microbiota previously reported from the teeth of C. carcharias.2 However, findings from the present study also indicate a high incidence of many other bacterial microbiota, specifically Enterococcus spp. and Serratia spp. in the oral cavities of C. carcharias in South African waters. It is additionally worth noting that the delay of up to 1 week for transporting some of the swab samples to the laboratory may have biased our findings towards the presence of faster-growing and/or persistent bacterial species. Nonetheless, at least 20 distinct species of bacteria were identified from the oral cavities of live sharks in this study, all of which could pose risk of pathogenesis to patients with shark-related injuries.
The overall antibiotic resistance observed in this study was relatively higher than that reported in other shark species.3,4,7 Specifically, no single antibiotic tested in this study would be capable of effectively treating all pathogenic bacteria presently reported from C. carcharias oral cavities. This is in contrast to studies of other shark species, which have suggested that a single agent fluoroquinolone (ciprofloxacin or levofloxacin) could be used for treatment of infections in C. leucas, C. limbatus and G. cuvier bite victims.3,4 Buck et al.2 who assessed the antibiotic resistance of four bacterial strains isolated from the teeth of one C. carcharias sampled from northeast USA, reported that effective treatment of these bacteria could be achieved using aminoglycosides, tetracycline or several cephalosporin class antibiotics. These differences in bacterial composition and resistance levels reflect the dynamic nature of bacteria over space, time and host species7,15, as well as highlight the need for species and geographic-specific microbial assessments.
Due to the limited sample size and sampling region, comparison of C. carcharias bacterial compositions related to geography, host age, maturity and diet were outside the scope of this study. Nonetheless, these factors are likely to have strong impacts on the presence and antibiotic resistance of pathogenic bacteria affecting shark-related injury victims.4,7 Preliminary studies have indicated no discernible relationship between the composition of oral bacteria in C. carcharias and either their gut contents or sampling location in South Africa.16 These patterns suggest that differences in feeding ecology and fine-scale geography might have minimal impact on the bacterial compositions hosted by these highly migratory17 apex predators in South African waters. However, it is highly advocated that further microbial studies addressing the sharks' sex, age and maturity stage, as well as movement patterns over a global scale, would be very useful for making inferences between the life history of this species and the microbiota that it hosts.
Conclusion
This study presented the first assessment of antibiotic resistance among bacterial strains isolated from the oral cavities of live C. carcharias in South Africa. Bacteria identified in this study include pathogenic bacteria previously unreported for shark species occurring in South Africa. It is hoped that the data presented here can enable medical professionals to make more informed, and thus more effective decisions when administering antibiotic treatment to shark bite victims, and provide an increased understanding of the microbial communities present in large coastal predators and their surrounding environments. The antibiotic resistance reported here of bacterial microbiota hosted by these top predators will additionally serve as baseline information toward future studies and management processes serving human public health.
Acknowledgements
We thank Madie Calitz of Ampath (George, South Africa) for invaluable assistance in the lab analysis. We acknowledge Dr Sanil Singh, Marian Bezuidenhout, Ritta Radebe and the rest of the staff of the Biomedical Resource Unit (University of KwaZulu-Natal, South Africa). We also thank Dr Pieter Koen for his assistance in the field; the South African Department of Environmental Affairs for issuing the permit to conduct this research; and OCEARCH for providing the funds and logistical support that enabled this project.
Authors' contributions
This study was conceived and designed by E.G. Data were collected by E.G., A.A.K., M.J.S., A.T., C.F., R.J. and M.M. Data were validated by L.A.B. and curated by P.M., N.K., L.A.B. and E.G. An initial draft of this manuscript was produced by E.G., N.K. and L.A.B. Funding was acquired by C.F. and R.J. This project was overseen and managed by E.G. P.M. interpreted the data and was primary author of the final manuscript. All authors contributed to revisions of the final draft of the manuscript.
References
1.Abrahamian FM, Goldstein EJ. Microbiology of animal bite wound infections. Clin Microbiol Rev. 2011;24(2):231-246. https://doi.org/10.1128/cmr.00041-10 [ Links ]
2.Buck JD, Spotte S, Gadbaw J. Bacteriology of the teeth from a great white shark: Potential medical implications for shark bite victims. J Clin Microbiol. 1984;20(5):849-851. [ Links ]
3.Interaminense JD, Nascimento R, Ventura J, Batista M, Souza F, Hazin N, et al. Recovery and screening for antibiotic susceptibility of potential bacterial pathogens from the oral cavity of shark species involved in attacks on humans in Recife, Brazil. J Med Microbiol. 2010;59(8):941-947. https://doi.org/10.1099/jmm.0.020453-0 [ Links ]
4.Unger NR, Ritter E, Borrego R, Goodman J, Osiyemi OO. Antibiotic susceptibilities of bacteria isolated within the oral flora of Florida blacktip sharks: Guidance for empiric antibiotic therapy. PLoS ONE. 2014;9(8), e104577, 10 pages. https://doi.org/10.1371/journal.pone.0104577 [ Links ]
5.Tomberg RJ, Cachaper GA, Weingart GS. Shark related injuries: A case series of emergency department patients. Am J Emerg Med. 2018;36(9):1645-1649. https://doi.org/10.1016/j.ajem.2018.06.059. [ Links ]
6.Woolgar JD, Cliff G, Nair R, Hafez H, Robbs JV. Shark attack: Review of 86 consecutive cases. J Trauma Acute Care Surg. 2001;50(5):87-891. https://doi.org/10.1097/00005373-200105000-00019 [ Links ]
7.Blackburn JK, Mitchell MA, Blackburn M-CH, Curtis A, Thompson BA. Evidence of antibiotic resistance in free-swimming, top-level marine predatory fishes. J Zoo Wildl Med. 2010;41(1):7-16. https://doi.org/10.1638/2007-0061.1 [ Links ]
8.Zanetti G, Bally F, Greub G, Garbino J, Kinge T, Lew D, et al. Cefepime versus imipenem-cilastatin for treatment of nosocomial pneumonia in intensive care unit patients: A multicenter, evaluator-blind, prospective, randomized study. Antimicrob Agents Chemother. 2003;47(11):3442-3447. https://doi.org/10.1128/aac.47.11.3442-3447.2003 [ Links ]
9.International Shark Attack File [webpage on the Internet]. c2018 [cited 2018 Sep 03]. Available from: https://www.floridamuseum.ufl.edu/shark-attacks/maps/world/ [ Links ]
10.Taneja N, Rao P, Arora J, Dogra A. Occurrence of ESBL & Amp-C [beta]-lactamases & susceptibility to newer antimicrobial agents in complicated UTI. Indian J Med Res. 2008;127(1):85. [ Links ]
11.US Clinical and Laboratory Standards Institute. M100 Performance standards for antimicrobial susceptibility testing. 28th ed. In: Approved standards CLSI. Wayne, PA: Clinical and Laboratory Standards Institute; 2018. https://doi.org/10.1201/9781420014495.ch1 [ Links ]
12.Gutmann L, Williamson R, Kitzis M, Acar J. Synergism and antagonism in double beta-lactam antibiotic combinations. Am J Med. 1986;80(5C):21-29. [ Links ]
13.Al-Hasan MN, Wilson JW, Lahr BD, Thomsen KM, Eckel-Passow JE, Vetter EA. β-lactam and fluoroquinolone combination antibiotic therapy for bacteremia caused by Gram-negative bacilli. Antimicrob Agents Chemother. 2009;53(4):1386-1394. https://doi.org/10.1128/aac.01231-08 [ Links ]
14.Tamma PD, Cosgrove SE, Maragakis LL. Combination therapy for treatment of infections with Gram-negative bacteria. Clin Microbiol Rev. 2012;25(3):450-470. https://doi.org/10.1128/cmr.05041-11 [ Links ]
15.Forde SE, Thompson JN, Bohannan BJ. Adaptation varies through space and time in a coevolving host-parasitoid interaction. Nature. 2004;431(7010):841. https://doi.org/10.1038/nature02906 [ Links ]
16.Khan N. An Investigation of the bacterial profile recovered from the oral cavity of sharks, on the coast of KwaZulu-Natal, South Africa [MSc thesis]. Durban: University of KwaZulu-Natal; 2016. [ Links ]
17.Bonfil R, Meÿer M, Scholl MC, Johnson R, O'Brien S, Oosthuizen H, et al. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science. 2005;310(5745):100-103. https://doi.org/10.1126/science.1114898 [ Links ]
 Correspondence:
Correspondence:
Peter Morse
Peter.Morse@my.jcu.edu.au
Received: 23 Jan. 2019
Revised: 09 Aug. 2019
Accepted: 20 Aug. 2019
Published: 27 Nov. 2019
Editor: John Butler-Adam
Funding: OCEARCH














