Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.9-10 Pretoria sep./oct. 2019
http://dx.doi.org/10.17159/sajs.2019/6221
RESEARCH ARTICLES
Agricultural practices and their potential role in mycotoxin contamination of maize and groundnut subsistence farming
Sylvia PhokaneI, II; Bradley C. FlettI, III; Edson NcubeI; John P. RheederIV; Lindy J. RoseII
IGrain Crops Institute, Agricultural Research Council, Potchefstroom, South Africa
IIDepartment of Plant Pathology, Stellenbosch University, Stellenbosch, South Africa
IIIUnit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
IVInstitute of Biomedical and Microbial Biotechnology, Cape Peninsula University of Technology, Cape Town, South Africa
ABSTRACT
Mycotoxigenic fungi are common pathogens of maize and groundnuts; they produce mycotoxins which reduce the yield and quality of these grain crops. Numerous agricultural practices including crop rotation and storage methods have been shown to impact mycotoxin accumulation. Therefore, the farming and storage practices in maize and groundnut subsistence farming systems in Pongola, Vryheid, Jozini, Manguzi and Mbazwana Districts of northern KwaZulu-Natal (South Africa) were surveyed to determine their potential role in promoting or mitigating mycotoxin contamination. A questionnaire about agricultural farming practices and storage facilities was presented to 65 subsistence maize and/or groundnut farmers. At least 90% of the farmers surveyed were not aware of mycotoxins and their consequences to animal and human health. The majority of the farmers did not practise crop rotation. However, they practised intercropping and sorted damaged and mouldy grain (maize and groundnuts) before storage. The damaged or mouldy grain was largely used as animal feed, thereby exposing animals to an increased risk of mycotoxicoses. Metal tanks and inqolobane (a type of wooden structure) were identified as the most common storage structures. Harvested homegrown maize was mostly used for the farmers' own consumption but also sometimes sold to the local community. The implementation of mycotoxin awareness campaigns is necessary, particularly in these districts. The storage facilities used by the subsistence farmers allowed increased moisture and insect invasion. The need for the surveillance of mycotoxins in subsistence-farmed food crops is vital.
SIGNIFICANCE:
•The main finding of this study is the extent of post-harvest losses and mycotoxin contamination of maize produced by smallholder farmers in South Africa.
•We further identify methods to manage the risk of mycotoxin exposure to smallholder farmers and their communities as well as reduce post-harvest losses
Keywords: mycotoxigenic fungi, storage, mycotoxins, survey, South Africa
Introduction
Maize (Zea mays L.) and groundnuts (Arachis hypogaea L.) are produced by subsistence farmers, particularly in the northern KwaZulu-Natal Province of South Africa.1,2 Maize is an important staple food and groundnuts serve as a protein and fat supplement for subsistence farmers.3,4 Both maize and groundnut may be contaminated with mycotoxins, produced by fungi, prior to and after harvesting.5-7 Mycotoxin contamination follows infection by mycotoxigenic fungi, of which the most common are Fusarium and Aspergillus species1,2,8 that can contaminate maize and groundnut with fumonisins and aflatoxins, respectively. Ingestion of mycotoxin-contaminated food and feed can cause mycotoxicoses in humans and animals.9,10 Fumonisins have been associated with a high incidence of oesophageal cancer in rural areas in South Africa due to the preference for mouldy kernels to produce traditional umqombothi beer.11 Mycotoxicoses may also develop in cattle that consume contaminated feed.12,13 During 2011, an estimated 100 dogs died in South Africa's Gauteng Province due to the ingestion of aflatoxin-contaminated feed.14
Agricultural practices such as crop rotation, irrigation, early planting and use of transgenic hybrids are employed by commercial farmers to reduce mycotoxin contamination of crops.15 Moreover, some subsistence farmers in Tanzania and Zimbabwe recently applied these agricultural practices and a reduction in mycotoxin contamination was reported.16,17 Hand sorting of maize before storage was also reported as a good measure to reduce fungal infection and subsequent mycotoxin contamination at storage.18,19 Unlike in a commercial setting, many subsistence farmers do not apply these agronomic practices, concentrating only on sorting their grain after harvest into visually healthy and mouldy grain.20,21 In areas in the Eastern Cape and Limpopo Provinces, mouldy grain is not discarded but used for traditional beer, thereby posing a risk of mycotoxin contamination.3,22
Limited information exists on storage of these crops by subsistence farmers and the associated mycotoxin risks. Storage of improperly dried grain, accompanied by high temperatures, causes rapid proliferation of mycotoxigenic fungi which results in reduced quality, nutrition and dry matter and higher mycotoxin levels.23-25 Contamination at storage by fungi can occur when the moisture content is above 13% and temperatures are between 10 °C and 40 °C.26 Therefore, the use of ventilated storage systems to reduce mycotoxin contamination is recommended, together with appropriate post-harvest control technologies to minimise mycotoxin contamination in the food chain.27-29 For example, storage in moisture-free, dry wooden pallets, ventilated drying on polythene sheets and hand sorting led to a decrease in aflatoxin contamination of kernels at storage.30 Storage facilities are some of the control points that have to be re-evaluated in the value chain; good storage facilities will lead to good marketable agricultural products.
Subsistence farmers incur economic losses due to pre-harvest and post-harvest contamination of grain crops caused by fungal species and insect pests. The present work continues earlier studies1,2 that identified hotspots for fumonisin and aflatoxin contamination. Good farming practices and proper pre-harvest handling of maize and groundnuts, together with good storage practices, have been demonstrated to minimise the risk of fungal contamination. Hence, we aimed to identify pre- and post-harvest practices that could potentially contribute to mycotoxin contamination of maize and groundnuts produced in KwaZulu-Natal.
Materials and methods
Geographical areas surveyed
Agricultural extension officers from the South African Department of Agriculture and Rural Development assisted with the selection of five districts in northern KwaZulu-Natal and identification of households within districts where maize and groundnuts were planted. Global Positioning System (GPS) was used to detect and mark different localities within the districts. Subsistence farmers growing maize or groundnuts were interviewed in all five districts: Jozini (n=7), Manguzi (n=17), Mbazwana (n=13), Pongola (n=17) and Vryheid (n=11). All farmers in all five districts planted maize and all farmers in Jozini, Manguzi and Mbazwana planted groundnuts as well. No farmer in Vryheid planted groundnuts and there was only one identified groundnut farmer in Pongola.
Questionnaires
The agricultural farming practices, storage facilities and grain consumption for each farmer were determined through a survey. Questionnaires were drafted in English and translated into isiZulu, the predominant local language. These questionnaires were approved by the South African Medical Research Council. Both closed- and open-ended questions were asked randomly to ensure adequacy of the questionnaire. The questions were on the awareness of mycotoxins, crop rotation, intercropping, residue removal, sorting of damaged and mouldy grain, end result of the sorted grain, types of storage facilities, consumption and trading of homegrown maize and groundnut. An awareness of mycotoxins, nematodes and fungal pathogens was determined as well as whether participants were aware of negative health implications caused by fungal pathogens. Additional explanations of questions were provided when needed and included non-scientific descriptions such as mould growth for fungal infection and mycotoxin contamination. All the farmers were also informed that fungal infection may be associated with mycotoxin contamination.
Interviews
Before the interviews, the farmers were informed about the significance of the survey. The first author interviewed each farmer according to the questions stated on the questionnaire. Gathering of information was done in collaboration with local extension officers. An opportunity was granted for questions after the interviews and appropriate management strategies were discussed with the farmers and local extension officers.
Statistical analyses
The data obtained from the questionnaires were analysed using a chi-square test for independence. One-way analysis of variance (ANOVA) was used to test only the numerical entries. The significance level for both tests was set at a 95% confidence level with p<0.05 indicating a significant difference. The tested null hypothesis (Ho) for the chi-square test was that the factor evaluated is independent of the different districts surveyed. Conversely, the alternative hypothesis (Ha) was that the factor is dependent on the different districts surveyed. The null hypothesis was accepted if p>0.05 and rejected if p<0.05.31
Results
Mycotoxin awareness
None of the farmers in Jozini, Manguzi and Mbazwana were aware of mycotoxins (Figure 1).
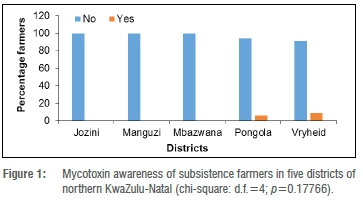
Only 6% and 9% of farmers in Pongola and Vryheid, respectively, had an idea of what mycotoxins could be, but did not know the cause of these mycotoxins and their implications on animal and human health (Figure 1). Mycotoxin awareness and maize districts were therefore independent (p=0.1766).
Residue removal
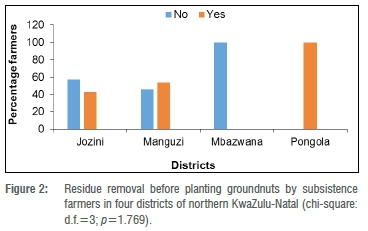
Of the farmers in Jozini, 43% removed residues from the soil before planting their groundnuts, 54% of the farmers in Manguzi did so, while all of the farmers in Pongola but none of the farmers in Mbazwana removed crop residues before planting groundnuts (Figure 2). Residue removal and groundnut districts were therefore independent (p=1.769)
Crop rotation and intercropping
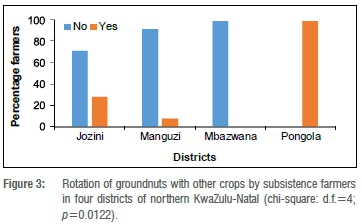
The majority of groundnut farmers did not practise crop rotation: 71%, 92% and 100% in Jozini, Manguzi and Mbazwana, respectively, did not rotate their groundnuts with any other crop (Figure 3). Only farmers in Pongola (100%) practised crop rotation (Figure 3) and therefore crop rotation and groundnut-farming districts were dependent on each other (p=0.0122). Farmers in all districts did not rotate maize with other crops (data not shown), but a variety of crops including beans, groundnuts and pumpkins were intercropped with maize. Maize was widely intercropped with groundnut in the Manguzi and Mbazwana Districts by 53% and 92% of farmers, respectively. Some farmers in all surveyed maize districts only planted maize (data not shown). Intercropping and the districts in which maize farmers were surveyed were, therefore, dependent on each other (p<0.001) (data not shown). Only farmers in the Pongola District did not intercrop groundnuts with other crops, whereas farmers in the other districts intercropped with crops such as spinach (Spinacia oleracea L.) and cowpeas (Vigna unguiculata L.) (data not shown). Therefore, intercropping was dependent on the groundnut-farming districts surveyed (p=0.0071) (data not shown).
Grain sorting before storage
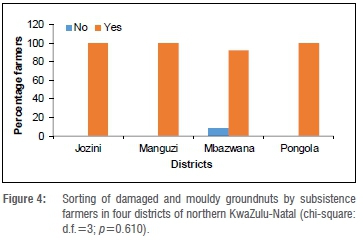
All the maize farmers in all districts surveyed sorted their maize into apparently healthy, mouldy and damaged maize before storage (results not shown). All the groundnut farmers in Jozini and Manguzi and 10% in Mbazwana also sorted their groundnuts into apparently healthy, mouldy and damaged groundnuts before storage (Figure 4). Sorting and groundnut districts surveyed are therefore independent variables (p=0.610).
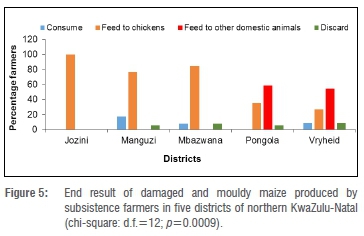
End result of mouldy and damaged grain
All the farmers in Jozini fed the mouldy and damaged maize kernels to chickens (Gallus gallus domesticus) only. Some farmers in the other four districts used the mouldy and damaged maize as chicken feed, but also discarded the grain. Additionally, 59% of farmers in Pongola and 55% of farmers in Vryheid fed the mouldy and damaged grain to other domestic animals such as pigs (Sus domesticus), cattle (Bos taurus) and goats (Capra aegagrus hircus). Furthermore, 18%, 8% and 9% of the farmers in Manguzi, Mbazwana and Vryheid, respectively, consumed the mouldy and damaged maize (Figure 5). The end-users of mouldy and damaged maize kernels and the maize districts surveyed were, therefore, dependent (p=0.0009). For groundnuts, all the farmers in Pongola and some farmers in other districts fed the mouldy and damaged groundnuts to chickens only. Less than 30% of farmers in Manguzi and Mbazwana discarded the mouldy and damaged groundnuts. In contrast with maize farmers, more groundnut farmers in Manguzi (50%) consumed the mouldy and damaged groundnuts. Also, 60% of groundnut farmers in Jozini consumed mouldy and damaged groundnuts (data not shown). The end-users of mouldy and damaged groundnuts and groundnut districts surveyed were also dependent variables (p=0.0396) (data not shown).
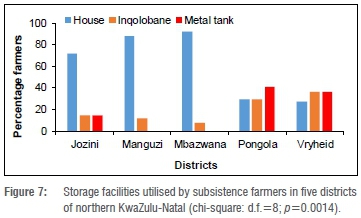
Storage facilities
A type of storage facility widely used in all the northern KwaZulu-Natal districts surveyed was an inqolobane, which is the isiZulu name for a widely ventilated wooden storage facility (Figure 6a). Metal drums were used by maize farmers only; some metal drums were ventilated and others unventilated (Figure 6b). Groundnuts were planted in small quantities in comparison to maize, hence groundnuts were easily and most commonly placed in bags which were stored in the farmers' homes (Figure 6c). In fact, in all the groundnut-farming districts (Jozini, Manguzi and Mbazwana), farmers stored groundnuts in their homes only (data not shown). Metal tanks were used to store maize by some subsistence farmers in Jozini, Pongola and Vryheid (Figure 7). The storage facilities and maize districts surveyed were dependent variables (p=0.0014).

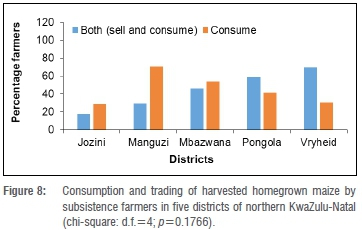
Consumption and trading of grain
Farmers from all districts either only consumed or both consumed and sold their homegrown maize (Figure 8). Consumption with trading of homegrown maize and maize-farming districts were independent (p=0.1766). Half the farmers in Mbazwana only consumed their homegrown groundnuts and the other half both sold and consumed their homegrown groundnuts. Over 60% of farmers in Jozini and Manguzi only consumed their homegrown groundnuts (data not shown). Consumption with trading of homegrown groundnuts and groundnut-farming districts were also independent (p=0.635) (data not shown). All the farmers in Jozini and Manguzi only sold their homegrown maize to the local community; farmers in Mbazwana, Pongola and Vryheid also sold their homegrown maize to the nearest markets (data not shown). Maize trading areas and maize districts were dependent on each other (p=0.0046) (data not shown).
Discussion
Numerous crop production and post-harvest practices have been found to influence mycotoxin accumulation in grain crops. In this study, the majority of groundnut farmers and all the maize farmers surveyed did not practise crop rotation. Furthermore, nearly half of the groundnut farmers did not remove plant residues before planting. Crop rotation can help reduce available inoculum for subsequent infection when non-host crops are employed.32 In a recent study, conservation agriculture - commonly described as practices that maintain permanent soil cover (no removal of plant residues) and minimum soil disturbance - did not increase the risk of maize ear rots and mycotoxin production.33 The storage facilities used by both maize and groundnut farmers favour fungal entry which increases the risk for mycotoxin contamination. Improving maize and groundnut subsistence farming and grain storage is crucial in mitigating the risk of mycotoxin contamination within the particular communities surveyed. Good-quality maize-based and groundnut-based products are not only necessary for consumption but also for trade. Hence, it was important to conduct a survey on the current farming practices in order to determine which potentially contribute to increased risk of mycotoxin contamination. This information will help to determine possible intervention strategies that could cause a reduction in the risk of mycotoxin contamination.
The lack of mycotoxin awareness in these districts indicates that humans and livestock may be consuming mycotoxin-contaminated maize and groundnuts daily which places them at a high health risk. Incidentally, some agricultural practices used by subsistence farmers, such as sorting of damaged and mouldy grain from storage, may have assisted in limiting mycotoxin exposure. Crop residues also harbour mycotoxigenic fungi,34 hence it is vital to remove crop residues before planting so that they do not serve as an inoculum source. Destruction or removal of infected crop residues from the field has been found to reduce fungal inoculum.35
The practice of rotating maize and groundnuts with other crops may be associated with the variation in soil types of the districts surveyed as this directly determines the crops that can be successfully cultivated. For example, the Manguzi and Mbazwana Districts had sandy soil types which mostly favour the cultivation of groundnuts over maize. Light-textured soils which include deep, well-drained sandy and loamy sand soils at a pH between 5.3 and 7.3 favour significant groundnut yields.36 The majority of farmers do not employ crop rotation, possibly because of a lack of knowledge of the advantages. Pest and disease cycles are broken by crop rotation, thereby reducing fungal infestation and subsequent mycotoxin contamination in the field.37 Farmers prefer to grow the same crop throughout, especially when it can be sustainably produced under prevailing conditions. However, rotating crops potentially increases crop yield and the root system health is maintained by the reduced inoculum potential of soil-borne pathogens.38 Intercropping has also been shown to reduce contamination of maize with mycotoxins.39
The manner in which farmers sorted groundnuts was determined by the quantity of groundnuts harvested and/or whether this would be kept for household consumption or sold for additional income. Mycotoxin contamination was reduced in the former Transkei region by sorting damaged/mouldy grain from apparently healthy grain.40 This study reported that fumonisin concentration decreased by 71% after removing highly infected maize kernels. Also, washing and sorting of maize kernels was found to reduce fumonisin contamination by 84%.8 In the Rombo District of Tanzania the sorting of maize also led to a reduction in fumonisin contamination.18 Therefore, it is good practice that the majority of the farmers in the northern KwaZulu-Natal sort their maize and groundnut to decrease contamination at storage. Mouldy and damaged maize was used to feed domestic livestock while most farmers across all districts fed the mouldy and damaged maize to chickens. Mycotoxin-contaminated feed generally affects the growth of chickens.41
Farmer preference dictated the use of specific storage facilities in the different districts. The choice of a storage facility may be due to problems experienced at storage relating to the different districts; for example, the use of tanks and drums to prevent mice damage specifically. Storage facilities used by farmers in the surveyed districts in northern KwaZulu-Natal are the same as those used by other farmers in sub-Saharan African countries30 and some of these storage facilities do not promote proper drying of maize and thus enhance interaction with insects, thereby promoting fungal infection and mycotoxin production30. The application of a pesticide to control stored-maize insect pests was proved to be an ineffective method compared to other post-harvest methods.42
Most farmers use wooden granaries for storage; these structures are widely used, possibly because of the ease of construction and for drying maize ears. However, this structure allows invasion by insect pests and rodents as it is not covered on top. Insects damage maize ears during feeding, thereby facilitating fungal invasion and infection.43 Therefore, maize cannot be stored for prolonged periods under such conditions. Farmers could be advised to use metal silos44,45 and hermetic storage containers46; these storage structures are airtight and, therefore, prevent any pathogen or pest from invading the stored maize42. Subsistence farmers prefer the traditional storage systems as they are cheaper to construct and maintain, although their use can cause high post-harvest losses.45 The specific storage practices employed were dictated by the quantity of maize produced. For instance, in high maize production areas such as Vryheid and Pongola, maize was predominantly stored in tanks.
Subsistence farmers consume high quantities of homegrown maize, as much as 300 g per person per day,47 and also sell the homegrown maize and groundnuts to the local community. Hence their exposure to mycotoxins is potentially higher than that of consumers in cities and towns. Furthermore, subsistence farmers have to contend with supermarkets present in local communities and small towns, which sell their good-quality products, especially maize meal and bread, at reduced costs.48 Also, pressure is placed on subsistence farmers to produce safe and healthy food due to new regulations for deoxynivalenol and fumonisin B1 and B2 limits in maize. The South African government implemented new regulations, setting maximum levels of 2000 µg/kg for deoxynivalenol and 4000 µg/kg for fumonisin B1 and B2.49 Subsistence farmers were not aware of these regulations. The monitoring of these regulations in an informal environment is unclear and possibly impractical; however, the supply chain will need to be regulated for quality and safety10 considering the potential for trade between subsistence farmers. Therefore, there is a need to determine the extent of mycotoxin contamination of these crops. Additionally, limited information is available on control methods to reduce the risk of mycotoxin contamination of food crops.
Conclusion
Mycotoxin contamination of maize and groundnuts produced through subsistence farming systems can be reduced by following good agricultural farming and storage practices such as crop rotation and sorting before storage, respectively, thus, improving the health and economic status of subsistence farmers and the communities involved. The implementation of good farming practices can be effortless; however, access to adequate storage facilities may not be feasible. Therefore, support in this regard is of utmost importance in subsistence farming. Furthermore, it is vital, to minimise mycotoxin contamination, that knowledge of good agricultural practices be transferred to subsistence farmers as well as agricultural extension officers. This knowledge transfer can form part of mycotoxin awareness campaigns to inform farmers of the threats and effects of mycotoxins on humans and animals. Additional surveillance is required to continuously monitor and advise on mycotoxin contamination and potential exposure in subsistence farming.
Acknowledgements
We acknowledge the financial support received from The Maize Trust, National Research Foundation of South Africa (Thuthuka grant no. 84162) and the Agricultural Research Council of South Africa. We thank the Department of Agriculture and Environmental Affairs in the KwaZulu-Natal Province for their collaboration and support provided; Ms Gugu Khali and Ms Yvonne Maila for assisting with sample collection in all the districts; and Ms Nicolene Thiebaut for statistical analysis of the results.
Authors' contributions
S.P.: Research design; fieldwork and sample collection; laboratory analysis of samples; data analysis; and writing article drafts. B.C.F.: Research design; research supervision; reviewing article drafts. E.N.: Obtaining funding; research design; research supervision; reviewing article drafts. J.P.R.: Obtaining funding; research design; reviewing article drafts. L.J.R.: Research design; research supervision; reviewing article drafts.
References
1.Ncube E, Flett BC, Waalwijk C, Viljoen A. Fusarium spp. and levels of fumonisins in maize produced by subsistence farmers in South Africa. S Afr J Sci. 2011;107:33-39. http://dx.doi.org/10.4102/sajs.v107i1/2.367 [ Links ]
2.Ncube E, Flett BC, Waalwijk C, Viljoen A. Occurrence of aflatoxins and aflatoxin-producing Aspergillus spp. associated with groundnut production in subsistence farming systems in South Africa. S Afr J Plant Soil. 2010;27:195-198. [ Links ]
3.Shephard GS, Burger H-M, Gambacorta L, Krska R, Powers SP, Rheeder JP, et al. Mycological analysis and multimycotoxins in maize from rural subsistence farmers in the former Transkei, South Africa. J Agric Food Chem. 2013;61:8232-8240. http://dx.doi.org/10.1021/jf4021762 [ Links ]
4.Sarvamangala C, Gowda MVC, Varshney RK. Identification of quantitative trait loci for protein content, oil content and oil quality for groundnut (Arachis hypogaea L.). Field Crop Res. 2011;122:49-59. http://dx.doi.org/10.1016/j.fcr.2011.02.010 [ Links ]
5.Beukes I, Rose LJ, Shephard GS, Flett BC, Viljoen A. Mycotoxigenic Fusarium species associated with grain crops in South Africa - A review. S Afr J Sci. 2017;113, Art. #2016-0121, 12 pages. http://dx.doi.org/10.17159/sajs.2017/20160121 [ Links ]
6.Njoroge SMC, Matumba L, Kanenga K, Siambi M, Waliyar F, Maruwo J, et al. Aflatoxin B1 levels in groundnut products from local markets in Zambia. Mycotoxin Res. 2017;33(2):113-119. http://dx.doi.org/10.1007/s12550-017-0270-5 [ Links ]
7.Mupunga I, Lebelo SL, Mngqawa P, Rheeder JP, Katerere DR. Natural occurrence of aflatoxins in peanuts and peanut butter from Bulawayo, Zimbabwe. J Food Prot. 2014;77:1814-1818. http://dx.doi.org/10.4315/0362-028X.JFP-14-129 [ Links ]
8.Van der Westhuizen L, Shephard GS, Rheeder JP, Burger H-M, Gelderblom WCA, Wild CP, et al. Simple intervention method to reduce fumonisin exposure in a subsistence maize-farming community in South Africa. Food Add Contam. 2010;27:1582-1588. http://dx.doi.org/10.1080/19440049.2010.508050 [ Links ]
9.Gelderblom WCA, Jaskiewicz R, Marasas WFO, Thiel PG, Horak RM, Vleggaar R, et al. Fumonisins-novel mycotoxins with cancer-promoting activity produced by Fusarium moniliforme. Appl Environ Microbiol. 1988;54:1806-1811. [ Links ]
10.Stoev SD. Food safety and increasing hazard of mycotoxin occurrence in foods and feeds. Crit Rev Food Sci Nutr. 2013;53:887-901. http://dx.doi.org/10.1080/10408398.2011.571800 [ Links ]
11.Shephard GS, Van der Westhuizen L, Gatveni PM, Somdyala, NM, Vismer HF, Marasas WFO. Fumonisin mycotoxins in traditional Xhosa maize beer in South Africa. J Agric Food Chem. 2005;53:9634-9637. http://dx.doi.org/10.1021/jf0516080 [ Links ]
12.Njobeh PB, Dutton MF, Ǻberg AT, Haggblom P. Estimation of multi-mycotoxin contamination in South African compound feeds. Toxins. 2012;4:836-848. http://dx.doi.org/10.3390/toxins4100836 [ Links ]
13.Marasas W, Gelderblom W, Shephard G, Vismer H. Mycotoxicological research in South Africa 1910-2011. World Mycotoxin J. 2011;5:89-102. http://dx.doi.org/10.3920/WMJ2011.1322 [ Links ]
14.Arnot LF, Duncan NM, Coetzer H, Botha CJ. An outbreak of canine aflatoxicosis in Gauteng province, South Africa. J S Afr Vet Assoc. 2012;83:2-4. http://dx.doi.org/10.4102/jsava.v83i1.2 [ Links ]
15.Bruns HA. Controlling aflatoxin and fumonisin in maize by crop management. J Toxicol. 2003;22:153-173. http://dx.doi.org/10.1081/TXR-120024090 [ Links ]
16.Nyangi C, Beed F, Mugula JK, Boni S, Koyano E, Mahuku G, et al. Assessment of pre-harvest aflatoxin and fumonisin contamination of maize in Babati District, Tanzania. Afr J Food Agric Nutr Dev. 2016;16:11039-11053. http://dx.doi.org/10.18697/ajfand.75.ILRI06 [ Links ]
17.Ndemera M, Landschoot S, De Broeve M, Nyanga LK, De Saeger S. Effect of agronomic practices and weather conditions on mycotoxins in maize: A case study of subsistence farming households in Zimbabwe. World Mycotoxin J. 2018;11:421-436. http://dx.doi.org/10.3920/WMJ2017.2227 [ Links ]
18.Kimanya ME, De Meulenaer B, Tiisekwa B, Ugullum C, Devlieghere F, Van Camp J, et al. Fumonisins exposure from freshly harvested and stored maize and its relationship with traditional agronomic practices in Rombo district, Tanzania. Food Add Contam A. 2009;26:1199-1208. http://dx.doi.org/10.1080/02652030902922784 [ Links ]
19.Odongo N. Sorting is an affordable technology that can reduce mycotoxin contamination to safe levels. Afr J Food Agric Nutr Dev. 2016;16:1-2. [ Links ]
20.Van der Westhuizen L, Shephard GS, Rheeder JP, Burger H-M, Gelderblom WCA, Wild CP, et al. Optimising sorting and washing of home-grown maize to reduce fumonisin contamination under laboratory-controlled conditions. Food Control. 2011;22:396-400. http://dx.doi.org/10.1016/j.foodcont.2010.09.009 [ Links ]
21.Matumba L, Van Poucke C, Ediage EN, Jacobs B, De Saeger S. Effectiveness of hand sorting, flotation/washing, dehulling and combinations thereof on the decontamination of mycotoxin contaminated white maize. Food Add Contam. 2015;32:960-969. http://dx.doi.org/10.1080/19440049.2015.1029535 [ Links ]
22.Phoku JZ, Dutton MF, Njobeh PB, Mwanza M, Egbuta MA, Chilaka CA. Fusarium infection of maize and maize-based products and exposure of a rural population to fumonisin B1 in Limpopo Province, South Africa. Food Add Contam A. 2012;29:1743-1751. http://dx.doi.org/10.1080/19440049.2012.708671 [ Links ]
23.Mylona K, Sulyok M, Magan N. Relationship between environmental factors, dry matter loss and mycotoxin levels in stored wheat and maize infected with Fusarium species. Food Add Contam. 2012;29:1118-1128. http://dx.doi.org/10.1080/19440049.2012.672340 [ Links ]
24.Janse van Rensburg B, McLaren NW, Flett BC. Grain colonization by fumonisin-producing Fusarium spp. and fumonisin synthesis in South African commercial maize in relation to prevailing weather conditions. Crop Prot. 2017;102:129-136. http://dx.doi.org/10.1016/j.cropro.2017.08.019 [ Links ]
25.Di Domenico AS, Christ D, Hashimoto EH, Busso C, Coelho SRM. Evaluation of quality attributes and the incidence of Fusarium sp. and Aspergillus sp. in different types of maize storage. J Stored Prod Res. 2015;61:59-64. http://dx.doi.org/10.1016/j.jspr.2014.12.001 [ Links ]
26.Atanda SA, Pessu PO, Agoda S, Isong IU, Adekalu OA, Echendu MA, et al. Fungi and mycotoxins in stored foods. Afr J Microbiol Res. 2011;5:4373-4382. [ Links ]
27.Mutegi C, Wagacha M, Kimani J, Otieno G, Wanyama R, Hell K, et al. Incidence of aflatoxin in peanuts (Arachis Hypogaea Linnaeus) from markets in Western, Nyanza and Nairobi Provinces of Kenya and related market traits. J Stored Prod Res. 2013;52:118-127. http://dx.doi.org/10.1016/j.jspr.2012.10.002 [ Links ]
28.Atukwase A, Kaaya AN, Muyanja C. Dynamics of Fusarium and fumonisins in maize during storage - A case study of the traditional storage structures commonly used in Uganda. Food Control. 2012;26:200-205. http://dx.doi.org/10.1016/j.foodcont.2012.01.016 [ Links ]
29.Waliyar F, Osiru M, Ntare BR, Kumar KVK, Sudini H, Traore A, et al. Post-harvest management of aflatoxin contamination in groundnut. World Mycotoxin J. 2014;8:245-252. http://dx.doi.org/10.3920/WMJ2014.1766 [ Links ]
30.Seetha A, Munthali W, Msere HW, Swai E, Muzanila Y, Sichone E, et al. Occurrence of aflatoxins and its management in diverse cropping systems of central Tanzania. Mycotoxin Res. 2017;33:323-331. http://dx.doi.org/10.1007/s12550-017-0286-x [ Links ]
31.Lombaard C, Van der Merwe L, Kele T, Mouton S. Elementary statistics for business and economics. Cape Town: Pearson Education South Africa; 2011. p. 57-361. [ Links ]
32.Marocco A, Gavazzi C, Pietri A, Tabaglio V. On fumonisin incidence in monoculture maize under no-till, conventional tillage and two nitrogen fertilisation levels. J Sci Food Agric. 2008;88:1217-1221. http://dx.doi.org/10.1002/jsfa.3205 [ Links ]
33.Mabuza LM, Janse van Rensburg B, Flett BC, Rose LJ. Accumulation of toxigenic Fusarium species and Stenocarpella maydis in maize grain grown under different cropping systems. Eur J Plant Pathol. 2018;152:297-308. http://dx.doi.org/10.1007/s10658-018-1475-y [ Links ]
34.Munkvold GP. Cultural and genetic approaches to managing mycotoxins in maize. Annu Rev Phytopathol. 2003;41:99-116. http://dx.doi.org/10.1146/annurev.phyto.41.052002.095510 [ Links ]
35.Wambacq E, Vanhoutte I, Audenaert K, De Gelder L, Haesaert G. Occurrence, prevention and remediation of toxigenic fungi and mycotoxins in silage: A review. J Sci Food Agric. 2016;96:2284-2302. http://dx.doi.org/10.1002/jsfa.7565 [ Links ]
36.Okello DK, Kaaya AN, Bisikwa J, Were M, Oloka HK. Management of aflatoxins in groundnuts: A manual for farmers, processors, traders and consumers in Uganda. Entebbe: National Agricultural Research Organisation; 2010. p.1-38. [ Links ]
37.TerAvest D, Carpenter-Boggs L, Thierfelder C, Reganold JP. Crop production and soil water management in conservation agriculture, no-till, and conventional tillage systems in Malawi. Agric Ecosyst Environ. 2015;212:285-296. http://dx.doi.org/10.1016/j.agee.2015.07.011 [ Links ]
38.Nel AA, Lamprecht SC. Crop rotational effects on irrigated winter and summer grain crops at Vaalharts. S Afr J Plant Soil. 2011;28:127-133. http://dx.doi.org/10.1080/02571862.2011.10640023 [ Links ]
39.Van Asselt ED, Azambuja W, Moretti A, Kastelein P, De Rijk TC, Stratakou I, et al. A Dutch field survey on fungal infection and mycotoxin concentrations in maize. Food Add Contam A. 2012;29:1556-1565. http://dx.doi.org/10.1080/19440049.2012.689997 [ Links ]
40.Mogensen JM, Sørensen SM, Sulyok M, Van der Westhuizen L, Shepherd GS, Frisvad JC, et al. Single-kernel analysis of fumonisins and other fungal metabolites in maize from South African subsistence farmers. Food Add Contam A. 2011;28:1724-1734. http://dx.doi.org/10.1080/19440049.2011.611823 [ Links ]
41.Smith EE, Kubena LF, Braithwaite RB, Harvey RB, Phillips TD, Reine AH. Toxicological evaluation of aflatoxin and cyclopiazonic acid in broiler chickens. Poult Sci. 1992;71:1136-1144. http://dx.doi.org/10.3382/ps.0711136 [ Links ]
42.Chigoverah AA, Mvumi BM. Efficacy of metal silos and hermetic bags against stored-maize insect pests under simulated smallholder farmer conditions. J Stored Prod Res. 2016;69:179-189. http://dx.doi.org/10.1016/j.jspr.2016.08.004 [ Links ]
43.Dafoe NJ, Thomas JD, Shirk PD, Legaspi ME, Vaughan MM, Huffaker A, et al. European corn borer (Ostrinia nubilalis) induced responses enhance susceptibility in maize. PLoS ONE. 2013;8(9), e73394, 18 pages. https://doi.org/10.1371/journal.pone.0073394 [ Links ]
44.Tefera T, Kanampiu F, De Groote H, Hellin J, Mugo S, Kimenju S, et al. The metal silo: An effective grain storage technology for reducing post-harvest insect and pathogen losses in maize while improving smallholder farmers' food security in developing countries. Crop Prot. 2011;30:240-245. http://dx.doi.org/10.1016/j.cropro.2010.11.015 [ Links ]
45.Gitonga ZM, De Groete H, Kassie M, Tefera T. Impact of metal silos on households' maize storage, storage losses and food security: An application of a propensity score matching. Food Policy. 2013;43:44-55. http://dx.doi.org/10.1016/j.foodpol.2013.08.005 [ Links ]
46.Murashiki TC, Chidewe C, Benhura MA, Manema LR, Mvumi BM, Nyanga LK. Effectiveness of hermetic storage technologies in limiting aflatoxin B1 and fumonisin B1 contamination of stored maize grain under smallholder conditions in Zimbabwe. World Mycotoxin J. 2018;11:459-469. http://dx.doi.org/10.3920/WMJ2017.2288 [ Links ]
47.Shephard GS, Marasas WFO, Burger H-M, Somdyala NIM, Rheeder JP, Van der Westhuizen L, et al. Exposure assessment for fumonisins in the former Transkei region of South Africa. Food Add Contam. 2007;24:62-1629. http://dx.doi.org/10.1080/02652030601101136 [ Links ]
48.D'Haese M, Van Huylenbroeck G. The rise of supermarkets and changing expenditure patterns of poor rural households case study in the Transkei area, South Africa. Food Policy. 2005;30:97-113. https://dx.doi.org/10.1016/j.foodpol.2005.01.001 [ Links ]
49.South African Department of Health. Foodstuffs, Cosmetics and Disinfectants Act, 1972 (Act 54 of 1972) - Regulations Governing Tolerances for Fungus-produced Toxins in Foodstuffs: Amendment. Government Gazette no. 40250 [document on the Internet]. 05 September 2016 [cited 2017 Feb 08]. Available from: https://www.gov.za/sites/default/files/gcis_document/201609/40250gon987.pdf [ Links ]
 Correspondence:
Correspondence:
Edson Ncube
NcubeE@arc.agric.za
Received: 04 Apr. 2019
Revised: 13 June 2019
Accepted: 15 July 2019
Published: 26 Sep. 2019
EDITOR: Teresa Coutinho
FUNDING: National Research Foundation (South Africa); The Maize Trust (South Africa); Agricultural Research Council (South Africa)














