Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.9-10 Pretoria sep./oct. 2019
http://dx.doi.org/10.17159/sajs.2019/5988
RESEARCH ARTICLE
A first assessment of glyphosate, 2,4-D and Cry proteins in surface water of South Africa
Suranie HornI; Rialet PietersI; Thomas BøhnII
IUnit for Environmental Sciences and Management, North-West University, Potchefstroom, South Africa
IIInstitute of Marine Research, Tromsø, Norway
ABSTRACT
Agriculture plays a vital role in the South African economy, as well as in the production of maize for food. Genetically modified maize is transformed to encode for crystalline (Cry) proteins found in Bacillus thuringiensis (Bt) and is referred to as Bt maize. Ingestion of specific Cry proteins causes the death of target insects that cause harm to maize plants. Bt crops, along with herbicides such as glyphosate and 2,4-dichlorophenoxyacetic acid (2,4-D), are widely adopted as part of the South African farming regime that aims to increase crop yield and reduce costs of production. As chemical compounds used in agriculture often end up in water sources, their presence should be monitored. There are many such monitoring programmes worldwide, but not in South Africa. We screened surface water sources in a maize-dominated agricultural area in the North West Province in South Africa for the presence of Cry1Ab, glyphosate and 2,4-D using enzyme-linked immunosorbent assays (ELISAs). Cry1Ab was not detected at any site; glyphosate was below the limit of detection at most of the sites but one sample had quantifiable traces of glyphosate; and 2,4-D was detected at all the sites. The concentrations of 2,4-D exceeded those for drinking water according to European guidelines, thus highlighting the need for regular monitoring of these compounds. Many people depend on untreated water resources, which may be contaminated by toxic agricultural chemicals. This report is the first on levels of these target compounds in South African water systems.
SIGNIFICANCE:
•This report is the first on the presence of glyphosate, 2,4-D and Cry1Ab in the South African aquatic environment.
•Concentrations of 2,4-D in South African surface waters exceed the European guideline for drinking water, indicating a risk to people using these water sources.
•These preliminary results highlight the need to regularly monitor for the presence of glyphosate, 2,4-D and Cry1Ab in water resources in South Africa
Keywords: GMO, Roundup, 2,4-dichloro-phenoxyacetic acid, Cry1Ab, ELISA, mixtures
Introduction
In a water-scarce country such as South Africa, water contaminated with chemicals is of even greater concern for residents dependent on untreated surface and groundwater resources because less water causes these compounds to concentrate. One sector of the economy that inadvertently contributes to water pollution is agriculture. A large portion of the South African economy is driven by the agricultural sector; maize is grown on 2.8 million hectares, with the Free State, Mpumalanga and North West Provinces accounting for approximately 84% of total maize production in the country.1 Moreover, maize serves as the staple food for the majority of South Africans. Therefore, meeting the basic needs of the population relies on successful agriculture.2
Globally, there have been major advances in the agricultural sector over the past 40 years which have increased crop yield and reduced pesticide use.3 The genes that encode for crystal (Cry) proteins, which are produced by Bacillus thuringiensis (Bt), have been incorporated into maize, thereby creating genetically modified (GM) crops. Ingestion of these proteins can be lethal for specific insect groups; for example, ingestion of Cry1Ab toxin is lethal for lepidopterans. In South Africa, Cry1Ab maize has been used with success against the stem borer Busseola fusca.4 However, resistance evolution by target pests threatens the sustainability of Bt maize in Africa5, in part because of unique challenges, such as a lack of refugia where healthy and susceptible insects can be produced6.
Cry proteins are considered to be environmentally benign with little or no effects on non-target organisms.7 However, studies on Cry in aquatic ecosystems have been scarce and recent reports indicate negative effects in mussels, some insects and other invertebrates like Daphnia magna.8 Cry1Ab proteins are not commonly found in water sources but the Cry1Ab transgene was detected in river water as far as 82 km away from an area intensively cultivated with Bt maize in Canada.9 When Cry1Ab occurs in the aquatic system, it readily partitions to clay and organic materials.10
Another genetic modification of maize makes plants tolerant to the herbicide glyphosate (the active ingredient in Roundup®). These herbicide-tolerant crops are referred to as Roundup-ready maize and can be sprayed with glyphosate-based herbicides in larger quantities and during the entire period of the growing season without causing damage to the crops.11
Glyphosate [N-(phosphonomethyl)glycine] is the most used herbicide in the world.12 It is a broad-spectrum, non-selective, post-emergent herbicide used for weed and vegetation control. Glyphosate is known to rapidly degrade and strongly adsorb to the soil.13 Glyphosate's mechanism of action is to inhibit the enzyme 5-enolpyruvyl-shikimate-3-phosphate synthase of the shikimate pathway. The shikimate (shikimic acid) pathway is responsible for the biosynthesis of folates and aromatic amino acids (phenylalanine, tyrosine and tryptophan) in plants, bacteria, fungi, algae and some protozoan parasites.14 Glyphosate is known to be non-toxic to animals and has a low ecotoxicological potential.15 However, recent evidence of more profound toxicological effects has made the use of glyphosate (Roundup products) more controversial.16 Moreover, glyphosate has been classified as a probable human carcinogen by the International Agency for Research on Cancer17, but not by the European Food Safety Authority18.
Insufficient crop management has led to glyphosate-resistant weeds.19 To address the tolerance of weeds towards glyphosate, farmers use herbicides with different mechanisms of action.20 One of the herbicides used in South Africa, against which fewer weeds have developed resistance, is 2,4-dichloro-phenoxyacetic acid (2,4-D).21,22 2,4-D is a post-emergent auxin herbicide and has been used for selective control of broadleaf weeds.
South Africa is the biggest user of pesticides in sub-Saharan Africa and has more than 500 registered active ingredients.23 The use of herbicides on GM maize - of which 80% is the Roundup-ready version - has increased drastically over past years, and further increases are expected to occur in the next few years.22 Glyphosate-based herbicides are the most used herbicides in South Africa, with an estimated 23 million litres sold in 2012. The amount of herbicides used in South Africa (with a maize production of 2 million ha) is far less than that by the top producers such as the USA (40 million ha maize production), Brazil (13 million ha maize production) and China (7 million ha maize production).24 Generally, pesticides are developed to target specific pests and to be immobile. However, run-off, leaching and spray drift occur and spread the compounds into unintended sections of the environment, and to water sources. These compounds generally occur at low concentrations and it is assumed that they would not have detrimental effects on non-target organisms. However, exposure to low levels of pesticides poses a chronic risk to human health, including endocrine disruption, immune impacts, neurotoxicity, genotoxicity, carcinogenesis and mutagenicity.25
This report is the first on the presence of the herbicides glyphosate and 2,4-D as well as Cry proteins in water sources in South Africa. In this study, the aforementioned herbicides were applied to GM maize expressing Cry1Ab proteins on two farms in South Africa. Because this was a screening survey, further studies are needed to determine how these contaminants reach the water; how long after application they remain in the aquatic environment; and how their concentrations change within and between seasons. These compounds are not regularly monitored in South Africa. However, South Africa has a target water quality guideline level for 2,4-D of 20 µg/L of water used for livestock.26 The persistence of glyphosate, 2,4-D and Cry proteins in the environment and their toxicity are still under scientific discussion worldwide. To the best of our knowledge there are no data published on environmental concentrations of these compounds for South Africa.
Materials and methods
Study area
The sampling sites were located on two farms in close proximity to the Renoster and Vaal Rivers in South Africa. Farm A is in the Free State Province and Farm B is on the border between the North West and Free State Provinces (Figure 1). Fields on Farm A were planted with Bt and Roundup-ready maize and those on Farm B were planted with Roundup-ready maize only. Farm A employed rainfed farming practices whereas Farm B used an irrigation system. On both farms, the pesticide spraying regime consisted of pre-emergent Roundup® and post-emergent Roundup® as well as 2,4-D. It was assumed that the farmers applied the herbicides according to the manufacturer's guidelines. Climatic conditions, such as rainfall, are one of the mechanisms that move these compounds from the point of application to water sources. Rainfall during the month of the sampling periods was 10-25 mm for the pre-herbicide application (October 2014), 100-200 mm for the post-herbicide application (November 2014) and 50-100 mm after the harvest (March 2015).27
Sampling
Water was sampled at different intervals during the planting season of 2014/2015 (October-May): (1) pre- and (2) post-herbicide application, as well as (3) after the harvest (Table 1). Water was sampled on Farm A from the Renoster River (A1) and from a dam on the farm (A2) and on Farm B from the Vaal River (B1), from an inflow dam on the farm where water is recycled from run-off after rainfall and irrigation (B2) and used again for irrigation, and from a dam on the farm used for recreational activities (B3). Surface water at a 30-cm depth was sampled in 250-mL high-density polyethylene bottles (Nalgene™, Rochester, NY, USA), protected from UV radiation and kept at 4 °C during transportation.
Concentrating Cry1Ab proteins from water samples
Each water sample was concentrated using an Amicon® ultracentrifugation tube (Millipore, Billerica, MA, USA) with a 30 000 molecular mass cut-off membrane. In short, a 15 mL aliquot of the sample was centrifuged at 870 g for 30 min. The eluent was discarded and another 15 mL was added and again centrifuged at 870 g for 30 min. The Amicon® tubes were subjected to a third centrifugation cycle whereafter the Cry proteins were rinsed off the membrane with 1 mL phosphate-buffered saline and Tween assay buffer. This concentrate of the samples was stored at 4 °C and quantified within 24 h.
Enzyme-linked immunosorbent assays
Over the past few years, enzyme-linked immunosorbent assays (ELISAs) have demonstrated results comparable with those of instrumental analytical methods for the quantification of contaminants in water sources. ELISA assays are therefore reliable and good substitutes for screening and monitoring such systems.28
Cry1Ab
The commercially available ELISA kit used for quantification of Cry1Ab in the water samples was obtained from Envirologix (Portland, ME, USA) (QualiPlate Kit for Cry1Ab/Cry1Ac Cat # AP003CRBS). The kit does not include a reference standard with a known concentration; the package insert advises that, if the kit is to be used for quantification purposes, a reference standard should be obtained from elsewhere. Lyophilised activated Cry1Ab toxin prepared from Cry1Ab protoxin was acquired from Marianne Pusztai-Carey at the Department of Biochemistry, Case Western University (Cleveland, OH, USA).29 The lyophilised protein was re-suspended in 10 mM CAPS buffer at pH 10.5 at a concentration of 100 µg/mL and frozen at -80 °C until use.30 The quantification of the Cry1Ab protein was determined by including two independent 12-point standard curves ranging from 0 to 3.5 μg/L. The samples, blanks and calibrators (Cry1Ab) were loaded in triplicate on the 96-well-microtitre plate pre-coated with antibodies specific for Cry1Ab/Ac and containing Cry1Ab/Ac enzyme conjugate. The plates were left to incubate for 2 h and washed four times with 300 μL wash buffer. A substrate was then added, resulting in a blue colour produced by the hydrolysis of hydrogen peroxide by peroxidase. After 20 min, the stop solution containing 1 N HCl was added and the optical density was measured at 450 nm and 650 nm (reference) using a multimode microplate reader (TriStar LB 941, Berthold, Bad Wildbad, Germany).31
Glyphosate
Glyphosate was quantified through the use of the Abraxis ELISA kit (PN 500086; Warminster, PA, USA). The method was performed according to the manufacturer's instructions. A six-point calibration curve that ranged from 0 to 4 µg/L was used to quantify the levels of glyphosate in the sample. In short, the samples, blanks and standards were derivatised and loaded into a 96-well plate coated with antibodies. A glyphosate antibody solution was added and the plates were incubated for 30 min. After incubation, the enzyme conjugate solution was added and the second incubation time was 60 min. Thereafter, the plate was washed three times with 250 µL wash buffer. A colour solution was added and after 30 min incubation, the stop solution was added. Absorbance was measured at 450 nm.28,32
2,4-D
To determine the levels of 2,4-D in the surface water, an ELISA specifically for 2,4-D (PN 54003A, Abraxis, Warminster, PA, USA) was employed. The 7-point calibration curve ranged from 0 to 80 µg/L. The water samples, standards and blanks were added to the wells on the test plate. The enzyme conjugate and antibody solution followed shortly after and the plate was incubated for 60 min. After the incubation period, the plates were washed three times using 250 µL wash buffer. After the washing step, a colour substrate was added and incubated for 30 min, after which a stop solution was added and absorbance was read at 450 nm.
Quality control
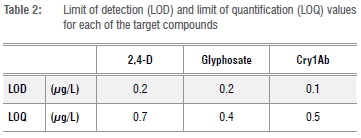
All samples were quantified in triplicate using ELISAs specific for each target compound. The mean absorbance values were calculated and the coefficient of variation was determined for each sample, requiring a coefficient of variation of <20%. The limit of detection (LOD) and limit of quantification (LOQ) were determined using a regression analysis of the calibration curves where LOD=3Sb/b and LOQ=10Sb/b with Sb=slope uncertainty and b=slope (Table 2).33 The concentrations of glyphosate, 2,4-D and Cry1Ab were determined against the linear regression line of the calibration curve, with a correlation coefficient (R2) as close as possible to 1.
Results and discussion
Concentration of the compounds in water sources
Cry1Ab
Although the water samples were concentrated 30 times, there were no detectable levels of Cry1Ab proteins in any of the water samples. It is well known that Cry1Ab proteins degrade quickly in water sources, and this was corroborated by the results of the current study (Table 1). Cry1Ab proteins break down when exposed to high temperatures (24-33 °C), thus resulting in microbial degradation. Soil type influences adsorption, making these proteins more persistent, but also decreasing their extractability. Cry1Ab has high conformational stability and retains its activity when absorbed to polar, charged surfaces in soils, which is important when assessing its potential adverse effects in agricultural systems.34 There is a lack of evidence on the bioactivity and potential health risks of Cry1Ab fragments that may be present in the environment.
In contrast to our results, Tank et al.35 detected Cry1Ab proteins in 23% of 215 water samples taken from streams near agricultural fields 6 months after harvest. They reported a mean concentration of 14 ng/L and a maximum of 32 ng/L. Whiting et al.36 detected no Cry1Ab in groundwater samples, but found concentrations of 129 ng/L in run-off water between maize fields. The same research group also analysed soil and run-off sediment, but in contrast to the high levels in water, a maximum mean concentration of only 9 ng/g was detected in soil during the pollination stage of the maize plants. Cry1Ab was detected in run-off water from a non-Bt maize field with levels from below LOD to 42 ng/L, whilst higher levels (maximum concentration of 130 ng/L) were detected from a Bt maize field.37 It should be noted that the concentrations of Cry1Ab detected in other studies were below the LOD of the current study. The ELISA method used could therefore have missed the presence of Cry1Ab at lower levels. The presence of Cry1Ab proteins in water, although at low levels, highlights the importance of investigating the potential long-term effects of these proteins on non-target organisms.
Glyphosate
The levels of glyphosate were below the LOD at most of the sites (Table 1). The water sampled from the dam (B3) on Farm B had traces of glyphosate with levels between LOD and LOQ after the spraying event. Glyphosate levels of 0.42 µg/L were detected at the in-flow dam on Farm B (B2) after the spraying event. These levels decreased to <LOD at the end of the season (Table 1). Glyphosate is very water soluble and has been found in various water sources around the world, but it also degrades quickly, which can be the reason for low detection. Some studies ascribe the lower than detection limit levels of glyphosate and its quick metabolising capability to its main metabolite aminomethylphosphonic acid (AMPA).38,39 AMPA was, however, not quantified within the scope of this study. Glyphosate concentrations are also highly influenced by precipitation and can change from year to year.40
In contrast to the current study, in other studies from all over the world, glyphosate has been detected in water sources. Sanchís et al.41 analysed 140 groundwater samples from Spain and found quantifiable levels for 41% of the samples. The mean concentration of glyphosate in Sanchís et al.'s study was 200 ng/L and the maximum concentration was 2.5 μg/L. Glyphosate concentrations of 663 ng/L were found in the Nottawasaga River watershed in Canada.42 According to Smith et al.43, 45 μg/L of glyphosate was detected in well water at the Massey Drive substation in the USA 7 weeks after spraying. This station is built on a limestone bed that has high permeability, thus emphasising that glyphosate is very mobile in water sources. In the USA, glyphosate was detected in a stream and wastewater treatment plant effluent samples in a study by Kolpin et al.44 The maximum concentration they reported was 2.2 μg/L. Also in the USA, an extensive study by Battaglin et al.39 reported glyphosate levels for different environmental matrices: 73 μg/L in streams; 2.03 μg/L in groundwater; 427 μg/L in ditches and drains; 3.08 μg/L in large rivers; 1 μg/L in soil water; 301 μg/L in wetlands, lakes, and ponds; 2.5 μg/L in precipitation; 476 μg/L in soil and sediment; and 0.3 μg/L in wastewater treatment outfall. It is evident that glyphosate ends up in water sources.
2,4-D
Most of the samples in the current study contained quantifiable levels of 2,4-D with a minimum of 0.72 µg/L and a maximum of 1.08 µg/L. Before planting, the concentrations of 2,4-D were below the LOD in both river samples and the dam on Farm A. It was also detected at low quantifiable levels before planting in both dams on Farm B. The highest concentration was detected after the spraying event and decreased towards the end of the season (Table 1).
According to Wilson et al.45, 2,4-D amine salts and 2,4-D esters are very mobile but they are not persistent under most environmental conditions. 2,4-D does not adsorb to the soil but readily moves into water resources - a finding confirmed by Mountassif et al.46 who reported that 91.7% of the applied 2,4-D eventually ends up in water, thus explaining the high levels detected in various countries.
Hernandez et al.47 detected 0.05 μg/L 2,4-D in Lake Chapala, Mexico, which is an order of magnitude lower than the levels found in the current study. The concentrations of 2,4-D found in our study are in the same range as those in two European studies: Rodil et al.48 detected levels of 0.062-0.2 μg/L 2,4-D in drinking and surface water in Spain and Tsaboula et al.49 reported 1.16 μg/L 2,4-D in the Pinios River Basin, Greece. A few US studies by Serrano and DeLorenzo50, Ensminger et al.51 and Wijnja et al.52, reported 2,4-D levels in surface water, urban run-off, a freshwater pond and Kushiwah Creek, Charleston, of 0.1 μg/L to 11.5 μg/L. Rodil et al.48 reported 2,4-D detected in drinking and surface water in Spain at concentrations ranging between 62 ng/L and 207 ng/L. The estimated recent environmental concentrations of 2,4-D in US water sources ranged from 4 μg/L to 24 μg/L.53 These concentrations are much higher than the levels obtained in the current study.
The Canadian guideline for the maximum residue limit (MRL) for any pesticide in drinking water is 280 µg/L, and for freshwater aquatic life is 65 µg/L.54 In the USA, the MRL for pesticides in drinking water is 700 µg/L54 and the maximum contaminant level - specifically for 2,4-D - is 70 µg/L55. In the European Union (EU), the MRL for pesticides in drinking water is less than 0.1 µg/L54 - a level exceeded by the 2,4-D concentrations found in the current study (Figure 2). Some of the levels of 2,4-D were an order of magnitude higher than the EU guideline (Figure 2), which could mean possible effects on human health. A Canadian study found a significantly increased risk of cancer (non-Hodgkins' disease) in men exposed to 2,4-D.56 Some studies reported that 2,4-D could reduce growth rates, induce reproductive problems, and produce changes in appearance or behaviour, or could cause death of non-target species, including plants, animals and microorganisms.57 In contrast, other studies examined the systemic toxicity, developmental neurotoxicity, developmental immunotoxicity, reproductive toxicity, endocrine modulation and thyroid effects in humans, and found that 2,4-D is unlikely to pose a significant health risk.58,59 The debate on the safety of herbicides continues as there may be unknown long-term effects on human health and the environment.60
Conclusion
South Africa relies on agriculture to supply food to the majority of its people and is the 10th largest maize producer in the world. Both small-scale subsistence farming and modern agriculture are important in the country and both sectors use transgenic insect toxins and may experience development of tolerance to herbicides. Modern agriculture increases food production but may involve excessive use of herbicides and toxins for pest control. Ideally, herbicidal compounds are developed to have a specific mechanism or mode of action to avoid toxic effects in non-target organisms. However, non-target effects need to be investigated and the risk assessed for each chemical substance in use. The first step is to monitor and determine whether herbicides and agricultural toxins used by farmers can be found in the environment. To our knowledge, this has not been done previously for Cry1Ab toxin, glyphosate and 2,4-D in South Africa, although these are dominant agrochemicals in modern South African agriculture. Thus, this report is the first investigation of the presence and concentrations of these substances in water sources in South Africa.
As Cry1Ab, glyphosate and 2,4-D are highly mobile once released into the environment, increased use will elevate the levels in the environment. We did not find Cry1Ab proteins at quantifiable levels and only one sample contained glyphosate. 2,4-D was present at quantifiable levels in more than 70% of the samples and all of these concentrations exceeded the EU guideline for drinking water. Recently, research has revealed adverse health effects of Cry1Ab, glyphosate and 2,4-D exposure to non-target organisms. These effects could also influence biodiversity; therefore, water sources should be monitored to ensure both healthy aquatic ecosystems as well as safe drinking water.
Recommendations
From the results of this first survey conducted over a single maize growing season it is recommended that follow-up studies be done which include more sampling locations across larger geographical regions in South Africa. Also, monitoring should be performed over longer periods to cover variability over seasons and between years. We recommend the use of ELISAs as a screening tool followed by confirmation of positive results using other analytical methods.
Acknowledgements
We thank Anja Greyling for creating the map and acknowledge the South African Department of Agriculture, Forestry and Fisheries as the source of the map data. We thank the National Research Foundation (NRF) of South Africa for funding (grant numbers 106242 and 103487). Opinions expressed and conclusions derived are those of the authors and are not necessarily to be attributed to the NRF.
Authors' contributions
S.H. was responsbile for conceptualisation; data collection; sample analysis; data analysis and validation; and writing of the initial draft. R.P. contributed to the conceptualisation and was responsible for sample collection; student supervision; funding; and writing revisions. T.B. contributed to the conceptualisation and was responsible for project leadership; funding; and writing revisions.
References
1.Department of Agriculture, Forestry and Fisheries (DAFF). Maize market value chain profile 2010-2011. Pretoria: DAFF; 2011. [ Links ]
2.Jury MR. Economic impacts of climate variability in South Africa and development of resource prediction models. J Appl Meteorol. 2002;41(1):46-55. https://doi.org/10.1175/1520-0450(2002)041<0046:EIOCVI>2.0.CO;2 [ Links ]
3.Gouse M, Pray CE, Kirsten J, Schimmelpfennig D. A GM subsistence crop in Africa: The case of Bt white maize in South Africa. Int J Biotechnol. 2005;7(1/2/3):84-94. [ Links ]
4.Kruger M, Van Rensburg JBJ, Van Den Berg J. Perspective on the development of stem borer resistance to Bt maize and refuge compliance at the Vaalharts irrigation scheme in South Africa. Crop Prot. 2009;28(8):684-689. https://doi.org/10.1016/j.cropro.2009.04.001 [ Links ]
5.Van den Berg J, Hilbeck A, Bøhn T. Pest resistance to Cry1Ab Bt maize: Field resistance, contributing factors and lessons from South Africa. Crop Prot. 2013;54:154-160. https://doi.org/10.1016/j.cropro.2013.08.010 [ Links ]
6.Van den Berg J. Insect resistance management in Bt maize: Wild host plants of stem borers do not serve as refuges in Africa. J Econ Entomol. 2017;110(1):221-229. [ Links ]
7.Soberón M, Gill SS, Bravo A. Signaling versus punching hole: How do Bacillus thuringiensis toxins kill insect midgut cells? Cell Mol Life Sci. 2009;66(8):1337-1349. https://doi.org/10.1007/s00018-008-8330-9 [ Links ]
8.Venter HJ, Bøhn T. Interactions between Bt crops and aquatic ecosystems: A review. Environ Toxicol Chem. 2016;35(12):2891-2902. https://doi.org/10.1002/etc.3583 [ Links ]
9.Douville M, Gagné F, Blaise C, André C. Occurrence and persistence of Bacillus thuringiensis (Bt) and transgenic Bt corn cry1Ab gene from an aquatic environment. Ecotoxicol Environ Saf. 2007;66(2):195-203. https://doi.org/10.1016/j.ecoenv.2006.01.002 [ Links ]
10.Strain KE, Whiting SA, Lydy MJ. Laboratory and field validation of a Cry1Ab protein quantitation method for water. Talanta. 2014;128:109-116. https://doi.org/10.1016/j.talanta.2014.04.036 [ Links ]
11.Benbrook CM. Impacts of genetically engineered crops on pesticide use in the U.S. - The first sixteen years. Environ Sci Eur. 2012;24(24):1-13. https://doi.org/10.1186/2190-4715-24-24 [ Links ]
12.Dai P, Hu P, Tang J, Li Y, Li C. Effect of glyphosate on reproductive organs in male rat. Acta Histochem. 2016;118(5):519-526. https://doi.org/10.1016/j.acthis.2016.05.009 [ Links ]
13.Simonsen L, Fomsgaard IS, Svensmark B, Spliid NH. Fate and availability of glyphosate and AMPA in agricultural soil. J Environ Sci Heal B. 2008;43(5):365-375. https://doi.org/10.1080/03601230802062000 [ Links ]
14.Vivancos PD, Driscoll SP, Bulman CA, Ying L, Emami K, Treumann A, et al. Perturbations of amino acid metabolism associated with glyphosate-dependent inhibition of shikimic acid metabolism affect cellular redox homeostasis and alter the abundance of proteins involved in photosynthesis and photorespiration. Plant Physiol. 2011;157(1):256-268. https://doi.org/10.1104/pp.111.181024 [ Links ]
15.Williams GM, Kroes R, Munro IC. Safety evaluation and risk assessment of the herbicide Roundup and its active ingredient, glyphosate, for humans. Regul Toxicol Pharmacol. 2000;31(2 Pt 1):117-165. https://doi.org/10.1006/rtph.1999.1371 [ Links ]
16.Cuhra M, Bohn T, Cuhra P. Glyphosate: Too much of a good thing? Front Environ Sci. 2016;4(28):1-14. https://doi.org/10.3389/fenvs.2016.00028 [ Links ]
17.International Agency for Research on Cancer (IARC). IARC Monographs Volume 112: Evaluation of five organophosphate insecticides and herbicides. Environ Heal. 2015;112(1):425-433. [ Links ]
18.European Food Safety Authority. Conclusion on the peer review of the pesticide risk assessment of the active substance glyphosate. EFSA J. 2015;13(11), Art. #4302, 107 pages. [ Links ]
19.Shaner DL, Lindenmeyer RB, Ostlie MH. What have the mechanisms of resistance to glyphosate taught us? Pest Manag Sci. 2012;68(1):3-9. https://doi.org/10.1002/ps.2261 [ Links ]
20.Chahal PS, Aulakh JS, Rosenbaum K, Jhala AJ. Growth stage affects dose response of selected glyphosate-resistant weeds to premix of 2,4-D choline and glyphosate (Enlist DuoTM Herbicide). J Agric Sci. 2015;7(11):1-10. https://doi.org/10.5539/jas.v7n11p1 [ Links ]
21.Landrigan PJ, Benbrook CM. GMOs, herbicides, and public health. N Engl J Med. 2015;373(8):693-695. https://doi.org/10.1056/NEJMp1505660 [ Links ]
22.Benbrook CM. Trends in glyphosate herbicide use in the United States and globally. Environ Sci Eur. 2016;28(3):1-15. https://doi.org/10.1186/s12302-016-0070-0 [ Links ]
23.Quinn L, De Vos BJ, Fernandes-Whaley M, Roos C, Bouwman H, Kylin H, et al. Pesticide use in South Africa: One of the largest importers of pesticides in Africa. In: Stoytcheva M, editor. Pesticides in the modern world - Pesticides use and management. Rijeka: InTech; 2011. p. 49. https://doi.org/10.5772/950 [ Links ]
24.Food & Agriculture Organization of the United Nations Statistics Division (FAOSTAT). Crops and livestock products [webpage on the Internet]. c2016 [cited 2019 Apr 10]. Available from: http://www.fao.org/faostat/en/#data/TP/visualize [ Links ]
25.Brown LR, Robinson DE, Nurse RE, Swanton CJ, Sikkema PH. Soybean response to simulated dicamba/diflufenzopyr drift followed by postemergence herbicides. Crop Prot. 2009;28(6):539-542. https://doi.org/10.1016/j.cropro.2009.02.004 [ Links ]
26.Department of Water Affairs and Forestry (DWAF). South African water quality guidelines. Volume 1: Domestic use. Pretoria: DWAF; 1996. Available from: http://www.dwa.gov.za/iwqs/wq_guide/Pol_saWQguideFRESH_vol1_Domesticuse.PDF 27. [ Links ]
27.South African Weather Service (SAWS). Historical rain maps [webpage on the Internet]. No date [cited 2019 Jan 24]. Available from: www.weathersa.co.za/climate/historical-rain-maps [ Links ]
28.Szekacs A, Mortl M, Darvas B. Monitoring pesticide residues in surface and ground water in Hungary: Surveys in 1990-2015. J Chem. 2015;2015:1-15. https://doi.org/10.1155/2015/717948 [ Links ]
29.Pusztai-Carey M, Carey PR, Lessard T, Yaguchi M. Isolation, quantitation and purification of insecticidal proteins from Bacillus thuringiensis. U.S. patent no. 5,356,788. 1994. October, 18, 1994. [ Links ]
30.Tank JL, Rosi-Marshall EJ, Royer TV, Whiles MR, Griffiths NA, Frauendorf TC, et al. Occurrence of maize detritus and a transgenic insecticidal protein (Cry1Ab) within the stream network of an agricultural landscape. Proc Natl Acad Sci USA. 2010;107(41):17645-17650. https://doi.org/10.1073/pnas.1006925107 [ Links ]
31.Strain KE, Whiting SA, Lydy MJ. Laboratory and field validation of a Cry1Ab protein quantitation method for water. Talanta. 2014;128:109-116. [ Links ]
32.Mörtl M, Németh G, Juracsek J, Darvas B, Kamp L, Rubio F, et al. Determination of glyphosate residues in Hungarian water samples by immunoassay. Microchem J. 2013;107:143-151. https://doi.org/10.1016/j.microc.2012.05.021 [ Links ]
33.Schoeman C, Mashiane M, Dlamini M, Okonkwo OJ. Quantification of selected antiretroviral drugs in a wastewater treatment works in South Africa using GC-TOFMS. J Chromatogr Sep Tech. 2015;6(4):1-7. [ Links ]
34.Madliger M, Gasser CA, Schwarzenbach P, Sander M. Adsorption of transgenic insecticidal Cry1Ab protein to silica particles. Effects on transport and bioactivity. Environ Sci Technol. 2011;45:4377-4384. https://doi.org/10.1021/es200022q [ Links ]
35.Tank JL, Rosi-Marshall EJ, Royer TV, Whiles MR, Griffiths NA, Frauendorf TC, et al. Occurrence of maize detritus and a transgenic insecticidal protein (Cry1Ab) within the stream network of an agricultural landscape. Proc Natl Acad Sci USA. 2010;107(41):17645-17650. https://doi.org/10.1073/pnas.1006925107 [ Links ]
36.Whiting SA, Strain KE, Campbell LA, Young BG, Lydy MJ. A multi-year field study to evaluate the environmental fate and agronomic effects of insecticide mixtures. Sci Total Environ. 2014;497-498:534-542. https://doi.org/10.1016/j.scitotenv.2014.07.115 [ Links ]
37.Strain KE, Lydy MJ. The fate and transport of the Cry1Ab protein in an agricultural field and laboratory aquatic microcosms. Chemosphere. 2015;132:94-100. https://doi.org/10.1016/j.chemosphere.2015.03.005 [ Links ]
38.Reddy KN, Rimando AM, Duke SO. Aminomethylphosphonic acid, a metabolite of glyphosate, causes injury in glyphosate-treated, glyphosate-resistant soybean. J Agric Food Chem. 2004;52(16):5139-5143. https://doi.org/10.1021/jf049605v [ Links ]
39.Battaglin WA, Meyer MT, Kuivila KM, Dietze JE. Glyphosate and its degradation product AMPA occur frequently and widely in U.S. soils, surface water, groundwater, and precipitation. J Am Water Resour Assoc. 2014;50(2):275-290. https://doi.org/10.1111/jawr.12159 [ Links ]
40.Myers JP, Antoniou MN, Blumberg B, Carroll L, Colborn T, Everett LG, et al. Concerns over use of glyphosate-based herbicides and risks associated with exposures: A consensus statement. Environ Health. 2016;15(1):1-13. https://doi.org/10.1186/s12940-016-0117-0 [ Links ]
41.Sanchís J, Kantiani L, Llorca M, Rubio F, Ginebreda A, Fraile J, et al. Determination of glyphosate in groundwater samples using an ultrasensitive immunoassay and confirmation by on-line solid-phase extraction followed by liquid chromatography coupled to tandem mass spectrometry. Anal Bioanal Chem. 2012;402(7):2335-2345. https://doi.org/10.1007/s00216-011-5541-y [ Links ]
42.Van Stempvoort DR, Spoelstra J, Senger ND, Brown SJ, Post R, Struger J. Glyphosate residues in rural groundwater, Nottawasaga river watershed, Ontario, Canada. Pest Manag Sci. 2016;72(10):1862-1872. https://doi.org/10.1002/ps.4218 [ Links ]
43.Smith NJ, Martin RC, St. Croix RG. Levels of the herbicide glyphosate in well water. Bull Environ Contam Toxicol. 1996;57(5):759-765. https://doi.org/10.1007/s001289900254 [ Links ]
44.Kolpin DW, Thurman EM, Lee EA, Meyer MT, Furlong ET, Glassmeyer ST. Urban contributions of glyphosate and its degradate AMPA to streams in the United States. Sci Total Environ. 2006;354(2-3):191-197. https://doi.org/10.1016/j.scitotenv.2005.01.028 [ Links ]
45.Wilson RD, Geronimo J, Armbruster JA. 2,4-D dissipation in field soils after applications of 2,4-D dimethylamine salt and 2,4-D 2-ethylhexyl ester. Environ Toxicol Chem. 1997;16(6):1239-1246. https://doi.org/10.1002/etc.5620160620 [ Links ]
46.Mountassif D, Kabine M, Mounchid K, Mounaji K, Latruffe N, El Kebbaj MS. Biochemical and histological alterations of cellular metabolism from jerboa (Jaculus orientalis) by 2,4-dichlorophenoxyacetic acid: Effects on d-3-hydroxybutyrate dehydrogenase. Pestic Biochem Physiol. 2008;90(2):87-96. https://doi.org/10.1016/j.pestbp.2007.08.001 [ Links ]
47.Hernandez AA, Silva MR, Moya CA. Compuestos organo-persistentes y daño genético en núcleos hepáticos de Goodea atripinnis del Lago de Chapala [Organo-persistent compounds and genetic damage in liver nuclei of Goodea atripinnis in Lake Chapala]. Scientia-CUCBA. 2011;13:1-8. Spanish. [ Links ]
48.Rodil R, Quintana JB, Concha-Graña E, López-Mahía P, Muniategui-Lorenzo S, Prada-Rodríguez D. Emerging pollutants in sewage, surface and drinking water in Galicia (NW Spain). Chemosphere. 2012;86(10):1040-1049. https://doi.org/10.1016/j.chemosphere.2011.11.053 [ Links ]
49.Tsaboula A, Papadakis EN, Vryzas Z, Kotopoulou A, Kintzikoglou K, Papadopoulou-Mourkidou E. Environmental and human risk hierarchy of pesticides: A prioritization method, based on monitoring, hazard assessment and environmental fate. Environ Int. 2016;91:78-93. https://doi.org/10.1016/j.chemosphere.2011.11.053 [ Links ]
50.Serrano L, DeLorenzo ME. Water quality and restoration in a coastal subdivision stormwater pond. J Environ Manage. 2008;88(1):43-52. https://doi.org/10.1016/j.jenvman.2007.01.025 [ Links ]
51.Ensminger MP, Budd R, Kelley KC, Goh KS. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008-2011. Environ Monit Assess. 2013;185(5):3697-3710. https://doi.org/10.1007/s10661-012-2821-8 [ Links ]
52.Wijnja H, Doherty JJ, Safie SA. Changes in pesticide occurrence in suburban surface waters in Massachusetts, USA, 1999-2010. Bull Environ Contam Toxicol. 2014;93(2):228-232. https://doi.org/10.1007/s00128-014-1251-4 [ Links ]
53.Atamaniuk TM, Kubrak OI, Storey KB, Lushchak VI. Oxidative stress as a mechanism for toxicity of 2,4-dichlorophenoxyacetic acid (2,4-D): Studies with goldfish gills. Ecotoxicology. 2013;22(10):1498-1508. https://doi.org/10.1007/s10646-013-1136-z [ Links ]
54.Rubio F, Veldhuis LJ, Clegg BS, Fleeker JR, Hall JC. Comparison of a direct ELISA and an HPLC method for glyphosate determinations in water. J Agric Food Chem. 2003;51(3):691-696. https://doi.org/10.1021/jf020761g [ Links ]
55.United States Environmental Protection Agency (USEPA). National primary drinking water regulations: Organic chemicals [webpage on the Internet]. c2018 [2019 Apr 09]. Available from: https://www.epa.gov/ground-water-and-drinking-water/national-primary-drinking-water-regulations [ Links ]
56.McDuffie HH, Pahwa P, McLaughlin JR, Spinelli JJ, Fincham S. Non-Hodgkin's lymphoma and specific pesticides exposures in men: Cross-Canada study of pesticides and health. Cancer Epidemiol Biomarkers Prev. 2001;10:1155. [ Links ]
57.Gervais JA, Luukinen B, Buhl K, Stone D. 2,4-D Technical fact sheet [document on the Internet]. c2008 [2018 Jul 15]. Available from: http://npic.orst.edu/factsheets/2,4-DTech.pdf [ Links ]
58.Marty MS, Neal BH, Zablotny CL, Yano BL, Andrus AK, Woolhiser MR, et al. An F1-extended one-generation reproductive toxicity study in Crl: CD(SD) rats with 2,4-dichlorophenoxyacetic acid. Toxicol Sci. 2013;136(2):527-547. https://doi.org/10.1093/toxsci/kft213 [ Links ]
59.Peterson MA, McMaster SA, Riechers DE, Skelton J, Stahlman PW. 2,4-D past, present, and future: A review. Weed Technol. 2016;30(02):303-345. https://doi.org/10.1614/WT-D-15-00131.1 [ Links ]
60.Green JM. The benefits of herbicide-resistant crops. Pest Manag Sci. 2012;68(10):1323-1331. https://doi.org/10.1002/ps.3374 [ Links ]
 Correspondence:
Correspondence:
Suranie Horn
suranie.prinsloo@nwu.ac.za
Received: 28 Jan. 2019
Revised: 10 Apr. 2019
Accepted: 14 May 2019
Published: 26 Sep. 2019
EDITORS: Priscilla Baker,Teresa Coutinho
FUNDING: National Research Foundation (South Africa)














