Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.5-6 Pretoria Mai./Jun. 2019
http://dx.doi.org/10.17159/sajs.2019/5872
RESEARCH ARTICLES
Developmental stress in South African hominins: Comparison of recurrent enamel hypoplasias in Australopithecus africanus and Homo naledi
Mark F. Skinner
Department of Archaeology, University of York, York, United Kingdom
ABSTRACT
Discovery of a new hominin (Homo naledi) in the same geographical area as Australopithecus africanus creates the opportunity to compare developmental dental stress in higher latitude hominins with low that in latitude apes, among whom repetitive linear enamel hypoplasia (rLEH) recurs seasonally at about 6 or 12 months. In contrast to equatorial Africa, a single rainy/dry cycle occurs annually in non-coastal southern Africa. It is predicted that LEH will recur annually but not differ in duration between ancient and more recent hominins. Data were collected from epoxy casts of anterior teeth attributed to H. naledi (18 incisors, 13 canines) and A. africanus (29 incisors, 8 canines) using a digital microscope, surface scanner and scanning electron microscope. The location, number, width, depth and distance between defects (including perikymata counts and spatial measurements) of 136 LEH events were compared among crown moieties (deciles 4-6 and 7-9), tooth types and taxa. Enamel defects are concentrated in the cervical half of anterior crowns, and in similar numbers in each taxon. Contrary to expectations, H. naledi show bimodal LEH durations reconstructed at about 2 and 8 weeks compared to just 4 weeks in A. africanus. Both taxa show bimodally recurrent episodes of LEH centring on 2 and, more commonly and severely, 6 months. A combination of two independent annual stressor types, one disease and one seasonal, could explain the observations. These estimations of duration and recurrence of developmental stress require evaluation using actual perikymata periodicity for H. naledi and more refined understanding of palaeoenvironments for both taxa.
SIGNIFICANCE:
• Seasonal stress is a central concern in the biological and health sciences. Because of the innate way that enamel is deposited, the timing of stress in the childhood of apes, modern humans and their fossil ancestors can be measured with a precision of about 1 week.
• Application of this method to South African Pliocene Australopithecus africanus and Mid-Pleistocene Homo naledi reveals that, unexpectedly, both forms show semi-annual stress - a finding that is tentatively attributed to two independent annual stressors, possibly disease and malnutrition.
Keywords: dentition; perikymata; ontogeny; seasonality
Introduction
The great attraction of enamel hypoplasia studies in biological anthropology is that comparative studies of developmental well-being can include modern, ancient and even fossil assemblages.1-4 Previous studies of enamel hypoplasia in single teeth from large apes (gorillas, chimpanzees and orangutans) found that, generally speaking, several enamel furrows are often observable, especially on the canine teeth, representing a time span of 'felt stress' of 5 or more years5, and that the average interval between successive furrows is in the order of 6 months or multiples thereof; i.e. 12 or 18 months.6-9 Both western African apes (chimpanzees and lowland gorillas from Cameroon) and orangutans from Indonesia live in low latitude contexts, where commonly there are alternating twice yearly rainy and dry seasons driven by semi-annual passage of the inter-tropical convergence zone in Africa and twice yearly moisture-carrying monsoonal winds passing over the islands of Borneo and Sumatra.6,7 By contrast, chimpanzees from Senegal, who experience only one wet season alternating with one very long dry season, show a reconstructed average interval between repetitive episodes of enamel hypoplasia of just under a year.9,10
The assertion that repetitive linear enamel hypoplasia (rLEH) shows an average recurrence of 6 or 12 months, is not without challengers11,12, but see Smith et al.13 Furthermore, linking the recurrence with semi-annual moisture cycles, which are in turn linked to metabolic stressors such as seasonal food shortages, respiratory disease, malaria or intestinal worms8, remains speculative. With the discovery of Homo naledi, who lived in the same geographical area as Australopithecus africanus, it becomes possible to compare temporal ontogenetic patterns of enamel hypoplasia between low- and higher-latitude hominoids. Given the simple alternation, in central southern Africa, of a relatively warm, wet austral summer and cold, dry austral winter (i.e. only a single moisture cycle annually)14-16, it is predicted that South African hominins will show rLEH with an average recurrence of about 12 months (null hypothesis). Rejection of this hypothesis will weaken any direct connection between rLEH and seasonality.
The study of enamel hypoplasia among South African fossil hominins has a long history17; however, these previous studies have an emphasis only on prevalence and little attention has been given to ontogenetic patterning18. The concentration of transverse enamel defects in the cervical half of the A. africanus canine crown was interpreted as reflecting abrupt weaning stress.19 Recent studies of South African hominin enamel defects (137 P. robustus vs 200 A. africanus teeth) show increasing methodological sophistication and a concern for how intrinsic enamel formation (perikymata packing and crown formation span) could affect LEH expression and numbers of defects20,21 (perikymata are regularly deposited enamel increments). For the first time, a formal analysis of duration of stress from defect width was performed, finding that A. africanus showed a wide range of furrow widths confounding simple interpretation of duration of stress. There was no difference between assemblages in defect width or constituent perikymata counts. A geographically enlarged study found that east African Australopithecus (n=10) had a markedly larger number of 'within LEH' perikymata (mean=8.2, range 4-14) than A. africanus (mean=4.6, range 3-7).21 This latter study is noteworthy also for estimating defect duration in actual days from counts of perikymata in the occlusal wall of one Australopithecus from eastern Africa. Assuming a perikymata periodicity ranging from 8 to 10 days, stress lasted from 48 to 60 days. The author concluded, not unreasonably, that the number of perikymata within defects is a stronger predictor of defect width than is the spacing of perikymata adjacent to an LEH. In summary, studies of enamel hypoplasia in South African hominins show that, for comparative prevalence studies, the duration of crown formation span needs to be countenanced; and, secondly, that there are taxonomic and geographical differences in defect duration whose meaning needs further evaluation. There are no formal studies of defect depth or recurrence in South African hominins.
With the recent availability of surface-scanner microscopes and the discovery of a new hominin species (Homo naledi) that lived in the same higher-latitude geographical area as South African Australopithecus, we have the ability to conduct detailed studies of taxonomic and temporal variation in measures of enamel furrow defects and compare these to low-latitude apes with a view to furthering our understanding of aetiology. A major weakness in the reported research is that periodicity of perikymata in H. naledi remains unknown. Acknowledging this problem, reconstruction of temporal patterning of LEH is based on the range of Retzius periodicities reported for hominins in the literature.
Materials and methods
Linear enamel hypoplasia is described in two samples of South African fossil hominin teeth (Figure 1). They are drawn from greatly different time periods but lived in similar geographical areas and were likely subject to comparable annual cycles of insolation and moisture characteristic for the latitude.22,23 The climate today in the area is decidedly seasonal and quite arid generally. The A. africanus sample is drawn from Member 4 Sterkfontein, aged >2 million years24 and Makapansgat Member 3 (aged >2.6 my)25. Our picture of the climate for Pliocene Sterkfontein/Makapansgat can be glossed as probably wetter, more wooded and less seasonal, somewhat like Cameroon today26-28 (but see Avery29). Palaeoenvironmental reconstruction of the climate for the Sterkfontein Valley, based on micro-mammals, concluded that at the time of deposition the site was drier and more seasonal than today.29 However, there is evidence based on the presence of lianas in Member 4 that Sterkfontein exhibited dense, humid forest-type vegetation.26 Makapansgat to the north is thought to have been much wetter and wooded at Member 3 with reduced seasonality of rainfall.27,28 The drier austral winter months might have posed nutritional difficulties for the hominins.30
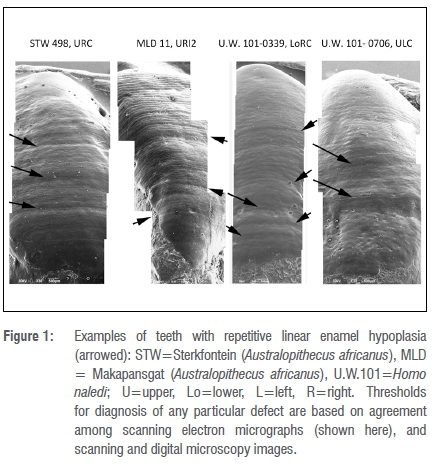
The H. naledi sample is derived from site U.W. 101, also known as the Dinaledi Chamber, within the Rising Star Cave system.31 These fossils are dated at 236 ka to 335 ka.32 The Middle Pleistocene of southern Africa has been described as semi-arid and, at intervals, more humid with an interval of greater cold.33
Cast preparation
Teeth from both sites were chosen specifically because they showed obvious enamel defect furrows and, often, countable perikymata in relevant crown parts. Here I report incisor and canine teeth only as they are thought to record most of any stress encountered during development (although not later childhood).34 While original teeth were examined for dental selection, this study is based on casts from moulds (Table 1). Moulds were taken by Mark Skinner and Debbie Guatelli-Steinberg in President Regular Jet impression material, so as to span from the incisal/occlusal edge to the cervical margin and a small portion of root. As described by Guatelli-Steinberg et al.35 casts were made by DGS and Mackie O'Hara in Struer's Epofix, a high-resolution epoxy. In addition, this study employs an earlier generation of casts for certain teeth whose labial surfaces were obscured in the later casts. Teeth were not assigned to individuals (the tooth being the unit of analysis) so there may be statistical redundancy; however, the advantage of this approach is that it avoids false associations of antimeres that can be so similar between individuals. The Dinaledi assemblage is commingled and there are numerous individuals of similar ontogenetic stage that are morphologically similar, making false associations a strong possibility. In order to have adequate sample sizes for tooth types (incisors/canines), no distinction is drawn between isomeres nor between central and lateral incisors.
Visualisation and instruments for recording developmental enamel defects
The term stress is defined here as a physically discernible effect of a stressor on enamel, i.e. a 'defect'. 'Stressor' is an inclusive term embracing nutritional, disease and Selyean-type physiological departures from homeostasis.36 LEH were imaged with three instruments: a Keyence digital microscope VHX-100, a 'µsurf Mobile Plus' optical scanner, and a JEOL JSM-6490LV scanning electron microscope. Besides employing these instruments for high magnifications of perikymata and enamel furrows, a comparison of image types from all three instruments at low power (ca 10-15X) increased confidence in identifying and numbering successive LEH. The task of matching LEH between low- and high-magnification pictures for perikymata counting was accomplished by noting small irregularities (e.g. scratches) or imperfections in the cast (e.g. bubbles) that could be located on images from all three instruments. LEH were measured with a 'µsurf Mobile Plus' optical scanner and analysed with µsoft Analysis Premium 6.2 software from NanoFocus® AG (Oberhausen, Germany). With this instrument, magnification was performed with a 10X lens that provides a square field of view 1600 μm on a side. Width and depth measurement outputs are averages, calculated by the instrument, from 516 measurements over this space. Scanner images were levelled, missing points filled in, and form removed. Form removal optimises measurement of defect depth by minimising the effect of object curvature. Because an object's surface is rarely level or completely flat, true depths are calculated trigonometrically from width and depth measures originally taken orthogonal to the instrument's plane.37 'Width' is defined as an orthogonal measure from the occlusal shoulder/high point, which visually demarcates the onset of an episode of LEH, to the deepest point of the defect, assessed with reference to the cervical high point of a defect. Typically, width defined this way is about half the overall width of an LEH.38 I also refer to this measure as 'onset width' to distinguish it from 'recovery width'.
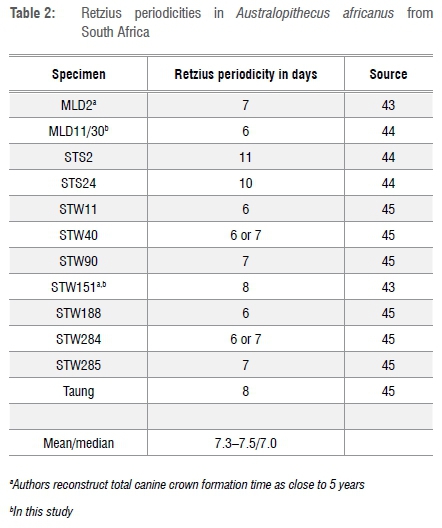
There are two options for estimating duration and recurrence of LEH: perikymata counting and spatial measurements. These measurements are not mutually exclusive and may be compared. Periodicities of perikymata are taken from the literature (see below). While periodicity of striae of Retzius, and their surface expression as perikymata, are thought to be invariant for the individual, it may be that some teeth, particularly deciduous teeth, show different periodicities than do permanent teeth from the same individual.39 Periodicity is reported to range in humans from 6 to 12 days in permanent teeth but, of these, 95% had periodicities between 7 and 10 days40 with a mode of 8 days41. As yet, Retzius periodicities for H. naledi are unknown. A northern African Homo sapiens (Djebel Ihroud, Morocco) of geological age somewhat less than that of the Dinaledi H. naledi remains has a stria periodicity of 10 days.42 Retzius periodicities in A. africanus from South Africa are shown in Table 2.
In that perikymata generally decrease in width and increase in number progressively from occlusal margin to cervix, especially in H. naledi35, it is necessary, for somewhat worn labial surfaces, to calculate the likely number of perikymata within an LEH in a particular crown location from the average width of perikymata within the relevant decile of crown height. Statistical analyses of perikymata number, LEH widths, depths and inter-defect distance use two-tailed parametric (Student's t-test) and non-parametric (Mann-Whitney) tests. Alpha is set at 0.05 for all statistical tests.
Results
Anatomical distribution and frequency of LEH defects
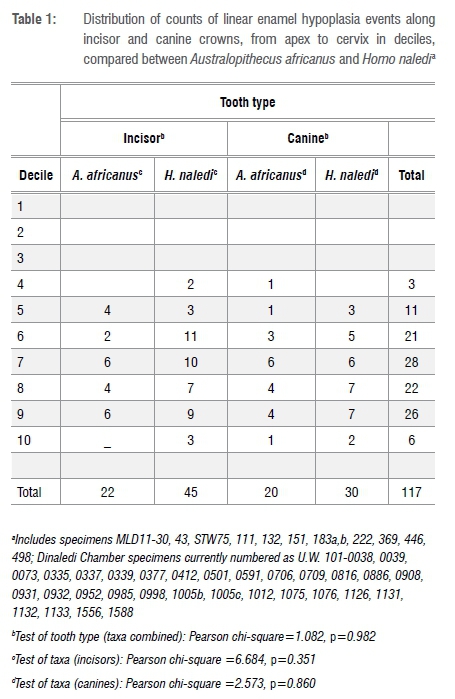
The objective of this study was to compare the duration, recurrence and severity of felt stress between H. naledi and A. africanus. Developmental stress, preserved as enamel hypoplasia in these ancient and more recent South African hominins, is concentrated in the cervical half of incisors and canines from both groups. There are no significant differences in the anatomical distribution of LEH along the tooth crown compared between tooth types or between taxa (Table 1). Similarly, the number of LEH furrows per tooth crown (medians range from two to three LEH) does not differ significantly between incisors and canines (H. naledi z=-1.305, p=0.192; A. africanus z=-0.942, p=0.346) nor between the ancient and more recent hominins for tooth types combined (z=-0.279, p=0.780). The lack of marked differences in the location and number of LEH between tooth crown types and taxa makes the following comparisons of LEH furrow measurements (size and perikymata number) more straightforward.
Furrows in enamel potentially vary not only as a result of ecological differences46 but also by location on the teeth (occlusal to cervical) and tooth types (incisor and canine)47. Until these variables are understood and controlled in some fashion, it is not possible to evaluate whether any apparent differences between sources are meaningful. Consequently, the first task is to evaluate the effect of location and tooth type on measures of LEH (width, depth, perikymata number).
Here I have chosen, in order to generate sufficient sample sizes for statistical analysis, to simplify crown location from one of ten deciles to two groupings: deciles 4, 5 and 6 versus deciles 7, 8 and 9. Deciles 1 through 3 were eliminated because there are no LEH in these deciles; decile 10 was not included because perikymata within this decile peter out. For similar reasons, data were combined by tooth type (e.g. combining data from maxillary and mandibular and lateral and central incisors and maxillary and mandibular canines). Variables of perikymata number within LEH, defect width and depth, in terms of anatomical factors, are evaluated in Table 3.
Comparison of the contrasting number of perikymata within the occlusal wall of an LEH defect in H. naledi incisor versus canine teeth suggests that the more occlusal decile moiety of the incisors is sampling an earlier phase of infancy than is the canine (given normal patterns of crown formation in hominoids48). Of 12 comparisons, in only 2 does crown location of an LEH matter (i.e. show statistical significance) (incisors from H. naledi, in which LEH in the occlusal moiety (deciles 4 to 6) contain few perikymata and are shallower) (Table 3). By contrast, in 5 of 12 instances, tooth type matters (interestingly most of these relate to the H. naledi LEH assemblage). In both taxa, canine defects are significantly deeper than are incisor defects, but only in the cervical decile moiety. Consequently, in order not to generate a confusing number of analytical groups and to maximise sample size for statistical analysis of potential differences in LEH expression between taxa, I have chosen, for most of the following analyses, to combine decile moieties but to keep tooth types separate.
Duration: Width of defects
Metrical
Dental crowns grow from cusp tip to cervix; consequently, there is a relationship between disturbances of enamel formation and time; that is, 'duration'. Acknowledging at the outset that enamel crowns may not form at a uniform rate, the first analysis is simply a comparison of defect width between tooth types and between taxa. In this work, width is technically 'onset width' and does not include recovery to normal enamel contour, i.e. 'recovery width'. Median widths of defects range from a high of 413 μm in H. naledi canines to a low of 263 μm in H. naledi incisors; with A. africanus widths falling within this range. The only difference of note relates to H. naledi, whose markedly narrow incisor defect widths occlusally (see above) lie behind the taxonomic differences (z=-1.959, p=0.05).
Number of perikymata within defects
Perikymata are the surface expression of depositional increments (Retzius lines) whose formation (number of days to form one perikyma/Retzius) is thought to be invariant within the permanent teeth of an individual, although somewhat variable within and between taxa (see above). Hence, even not knowing Retzius periodicity, it is reasonable, as a first step towards estimating LEH duration, to compare the number of perikymata within LEH (occlusal wall) between taxa.
Observed
There is no significant difference in perikymata number observed within an LEH between species for incisors (median counts=5.0 and 3.0 in H. naledi (n=29) and A. africanus (n=19), respectively) (z=-0.958, p=0.338) but there is for canines (median counts =7.0 and 4.0 in H. naledi (n=13) and A. africanus (n=9), respectively) (z=-2.501, p=0.012). This difference arises from the relatively large number of perikymata (mean=7.5) within LEH in H. naledi canines. Interestingly, it could be concluded that A. africanus incisors and canines are expressing the same kind of stressor but that H. naledi incisors and canines are recording stressors that differ in duration.
Predicted and observed
The previous analysis is based on rather a limited number of H. naledi canine defects; it is desirable to increase this sample if possible. All else being equal, there should be a strong correlation between LEH widths measured metrically and in terms of perikymata in the occlusal wall. In this study the correlation is 0.35 (n=67, p=0.003) and differs somewhat among tooth types and taxa. Apart from measurement error, the other major factor reducing the correlation is that LEH defects often contain more closely spaced perikymata because of reduced secretion (compression) affecting Retzius increment width.37,49,50
Given the noted correlation, it is reasonable to calculate, for those LEH defects without observable perikymata, the likely number of constituent perikymata given their widths. As discussed elsewhere35, the pattern of perikymata packing can differ between taxa and tooth types; specifically, in comparison to Australopithecus, H. naledi shows more widely spaced perikymata occlusally and narrower perikymata cervically, especially in the incisors. Consequently, in this study, all measured widths for defects without countable perikymata are divided by the mean perikymata width observed for a particular combination of decile, tooth type and species. As a check on the validity of this approach, one can examine the relationship between observed and predicted number of occlusal wall perikymata within an LEH (r=0.50, p<0.00003). The average number of observed versus predicted occlusal-wall perikymata (n=62) differs by only one perikyma (4 vs 3).
Assuming, then, that one can legitimately combine predicted with observed perikymata counts, we can now compare relative durations of LEH among taxa and tooth types in terms of perikymata count. The results are shown in Table 4a and Figure 2. Inclusion of LEH defects that did not include countable perikymata almost doubles the sample available for analysis of relative duration - from 70 to 120.
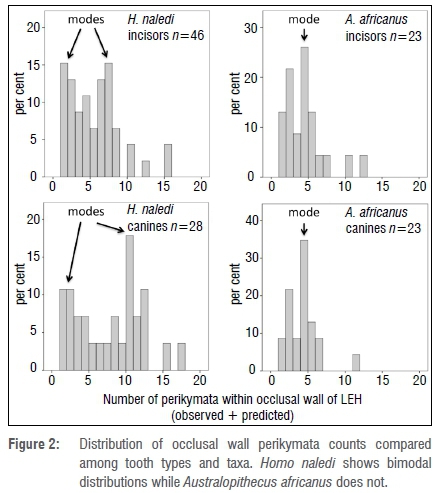
There is a striking difference between the two taxa in that both incisors and canines from H. naledi show a bimodal distribution of durations, which is not shown by A. africanus teeth (Figure 2).
Consequently, I have elected to divide durations into two groups: short duration with four or fewer perikymata in the occlusal wall versus long duration with five or more perikymata. The proportion of stressful episodes that are deemed long in duration (i.e. five or more perikymata) make up 57% in H. naledi compared to only 28% in A. africanus (Pearson chi-square = 9.278, p=0.003). In other words, H. naledi appears to have experienced stressful episodes of short and markedly long duration.
A comparison of defect durations (perikymata count) between tooth types and taxa is provided in Table 4b. It can be seen that it is the large number of (observed plus predicted) perikymata within defects in H. naledi canines that is creating significant differences between tooth types (z=-2.862, p=0.004) and between taxa (z=-2.524, p=0.009). The relatively wide defects in H. naledi canines are mirrored both in observed perikymata counts and in those perikymata counts predicted from measured widths.
Severity
Depth of defects
An assumption in this study is that, with caveats noted later, defect depth is a measure of 'felt stress'. Sustained stress, with no change in intensity, will produce deeper defects.20 A comparison of defect depth between those of short versus longer duration confirms this phenomenon ('short duration' median depth = 10.9 μm, 'long duration' median depth = 21.5 μm; z=-5.019, p<0.0001). Consequently, in an analysis of defect depth one has to separate the sample by defect duration. As shown in Table 5, after controlling for defect duration, LEH are significantly deeper in canines, compared to incisors, in H. naledi/long duration defects and in A. africanus/short and long duration defects. It may be concluded that defects in canine teeth are usually deeper than those in incisors; this difference is attributable to innate anatomical features of striae angle in canine teeth.20 In a comparison of sources, LEH are significantly deeper only in short duration defects in A. africanus canines. It may be concluded that there is not much difference between the sources in defect depth. Defects are significantly deeper in long duration defects for H. naledi incisors and both tooth types in A. africanus. In sum, the most important difference in a study of defect depth is that defects of long duration are more severe than are those of short duration.
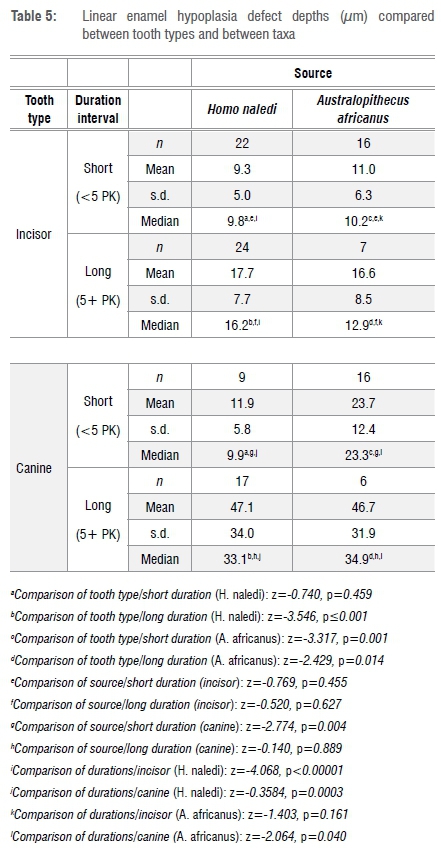
Angle of onset
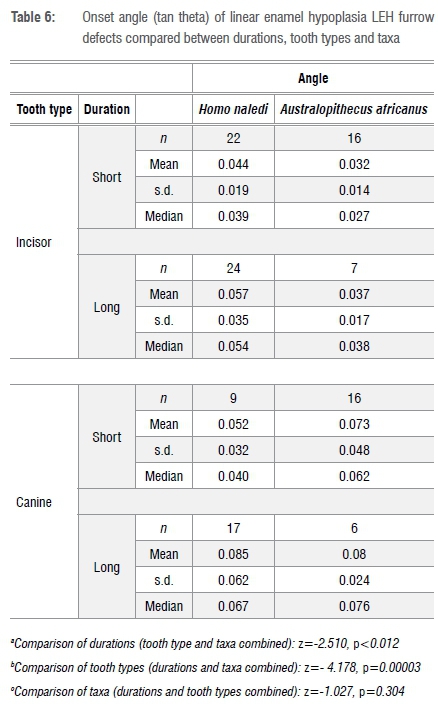
For this analysis, it is assumed that the steeper the angle of descent into an LEH furrow, the more intense is the felt stress.37 The angle is calculated from the relationship between width and depth of defect (Table 6). In the overall sample, the distribution of angle of onset is uni-modal and left skewed. Defects are significantly more steeply inclined (i.e. considered more intense) in defects of longer duration (as defined) and in canine teeth. There are no differences in defect angle between taxa. In a comparison of taxa, if one examines only the cervical moiety, there is a statistically significant difference for the incisor teeth (not the canines) apparently as a result of the more steeply inclined defects in H. naledi incisors (n=26 H. naledi, n=16 A. africanus; z=-3.212, p=0.001).
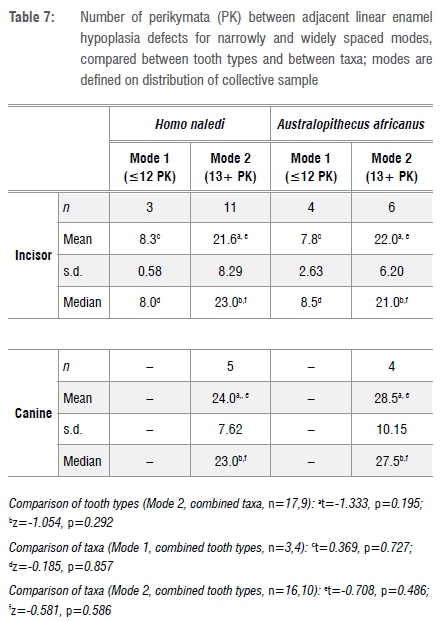
Recurrence: Number of perikymata between defects
Several of the teeth, belonging to both H. naledi and A. africanus, show fairly regularly spaced furrows that may be signalling a repetitive stressor (Figure 1). However, given the differences in perikymata packing among tooth types and taxa, this impression may well be illusory. The measures of central tendency (and tests of differences of means and medians) for spacing between adjacent LEH expressed as perikymata counts (Table 7) are misleading in that there is bimodality in the data. As may be seen in Figure 3, in the combined sample of tooth types and taxa (n=33) there are two spacing modes: one centred on about 8 perikymata and one on about 23 perikymata. Moreover, it is evident that while canine teeth from both taxa record only the wider spacing, the incisors from both taxa record the narrower and the wider spacing modes (Figure 3). I have elected to separate the two modes below and above 12 perikymata (see Figure 3) to recalculate the average number of perikymata (spacing) for each mode separately (Table 7). The sample sizes for each separate tooth type and taxon analytical unit are too small for statistical comparison. After lumping, with modes separated, there are no statistically significant differences in perikymata counts between LEH compared between tooth types and between taxa (Table 7). These results suggest that two spacings, centred on 8 and 23 perikymata, are common to both taxa (not forgetting that there are no canine teeth with narrowly spaced (as defined) LEH in the sample). It is important to note that the great majority (79%) of recurrences are of longer interval, not shorter.
Predicted timings of stress
Duration
The range of Retzius periodicities (RP) reported in the literature can be used to reconstruct probable durations and recurrences of LEH (Table 8). Median values are used in this exercise. A. africanus has reconstructed durations of LEH ranging from 24 to 40 days. The actual Retzius periodicity is known (see above) for two A. africanus individuals (STW151 RP=8 and MLD11/30 RP=6).43,44 For these two (ignoring possible redundancy from isomeres from single individuals), average duration =3.8 X 8=30.4 days (n=13 LEH in four teeth from STW151) and 3.3 X 6=19.8 days (n=6 LEH in two teeth from MLD11/30), respectively.
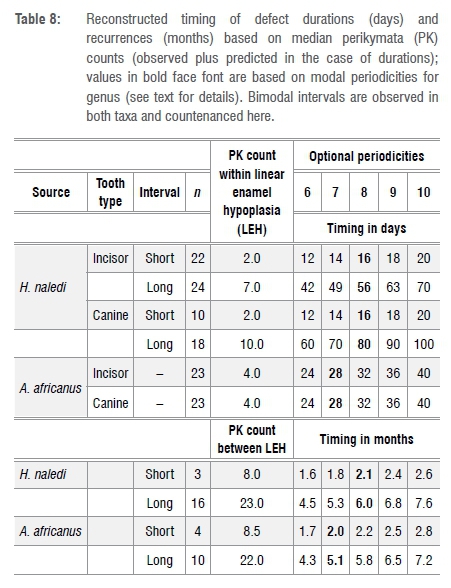
Earlier it was noted that H. naledi (but not A. africanus) shows two modal durations. Depending on the appropriate periodicity of Retzius formation, these range from 12 to 20 days and 42 to 100 days. Clearly, the Rising Star assemblage has a large proportion of stressful episodes (about 57%) that lasted a comparatively long time (ca 2+ months) compared to the A. africanus sample (ca 1 month) (see above).
Recurrence
Both taxa show bimodal recurrences of LEH: at 1.6−2.8 months and 4.3−7.6 months (range reflects optional periodicities (Table 8)). For A. africanus, where actual RP is known for two individuals (see above) recurrences occurred at an average of 6.1 months (MLD11/30, n=4 LEH) and 1.6 months (STW151, n=4 LEH) and 6.3 months (STW151, n=2 LEH). Recurrent values close to 6 months are reminiscent of LEH recurrences reported for low-latitude ape samples.6-8
This unexpected finding can be refined a bit if one allows the simplifying assumptions: (1) that reported modal stria periodicities (see above) prevail in the samples under study here and (2) that incisor teeth, which form relatively early in infancy, will record more shared stress in the mother/infant dyad than will the canine whose later crown formation is likely to record events impacting only on the older, more self-foraging, infant. For canines alone, using genus-specific modal stria periodicities, the predicted interval between onsets of LEH from perikymata counts in hominins from South Africa is 0.50 years in H. naledi and 0.53 years in A. africanus (0.51 years for both taxa (n=9 canines)). These tentative impressions, based on small samples, can be tested more fully when actual Retzius periodicities for H. naledi become known.
Summary of results
Anatomical features
•There are some significant differences between tooth types and decile moiety in the expression of LEH that have to be controlled when considering apparent differences between taxa.
•Defects of shorter duration (ca 2 perikymata) are naturally shallower.
General findings
•Defects of longer duration (ca 7 perikymata) are more intense (steeply angled).
• There is a bimodal distribution of defect recurrence intervals.
• Longer intervals between recurrences (ca 23 perikymata) are much more common than are short intervals (ca 8 perikymata) and are more intense than short intervals.
Comparison of taxa
• The concentrations of LEH in the cervical half of anterior teeth and the number of LEH per tooth crown are common to both taxa (H. naledi and A. africanus).
• H. naledi incisors have narrower LEH defects in occlusal moiety (deciles 4-6) and more severe defects in cervical moiety (deciles 7-9).
• H. naledi canines have defects that are twice as wide.
• In terms of defect duration, A. africanus show a unimodal tendency averaging around 1 month while H. naledi show a bimodal distribution averaging about 2 and 8 weeks.
• In terms of recurrence of LEH, both taxa show bimodal recurrences centring on 2 and, more commonly, 6 months.
Discussion
Incisors and the earlier forming parts of anterior tooth crowns, generally, tell a different story than does the later part of canine crown formation. Conceivably, the incisor ameloblasts are more sensitive to stress than are those which form the canine but I am unaware of observations to support such an inference. Rather, because incisors capture earlier commencing incidents in development, incisors will tend to record stress experienced through the mother as well as with its own development; also the younger infant has less developed immunity to disease.
The results for both taxa in this study are unexpected. Controlling for differences between crown moiety and tooth types, the two assemblages show both striking similarities and differences in the intensity, duration and recurrence of linear enamel hypoplasia. Firstly, bimodality of stress durations (at ca 2 and 8 weeks, evident in H. naledi) and bimodality of recurrence of stressful events (2 and 6 months, evident in both taxa) have not been reported previously. Secondly, the expectation that a single rainy/dry cycle, characterising the annual climate cycle in central South Africa, would produce annually recurrent episodes of LEH in hominins is not supported in this study. These findings will be discussed in turn.
Duration
An explanation for bimodality of durations, demonstrable in H. naledi but not in A. africanus, has to be sought. Just as a reminder, defects of shorter duration are less severe (low onset angle) while those of longer duration tend to be steeper. A single type of stressor would not be likely to produce such a pattern whereas two different types of stress might do so. For example, invocation of disease stress lasting about 2 weeks versus, for example, seasonal stress lasting about 8 weeks would seem reasonable but raises the question of why H. naledi would show this pattern while A. africanus does not. However, the reconstructed difference of about 12 days in duration (see Table 8) compared between A. africanus and the 'short duration' mode in H. naledi is probably trivial which, then, changes the question to simply why only H. naledi experienced sustained seasonal stress.
Recurrence
The median number of perikymata between 'longer interval' recurrences of LEH, combining tooth types to generate large enough samples for analysis, is 22 for H. naledi and 23 for A. africanus, a statistically insignificant difference. Employing modal periodicities for stria formation, the average time between defects among both South African hominins is very close to 6 months which does not fit well with the annual temperature and rainfall cycle of central South Africa. There are four possible explanations for the inference of semi-annual recurrence of stress among hominins from this latitude:
1.The finding is only a statistical artefact. However, I simply cannot think of how this result, reported to occur commonly in Indonesia7 and in central Africa5 and, now in this study, southern Africa, would be generated by chance.
2.Annual cycles of temperature and moisture, which increase and then decrease, may create two optimum thresholds for eclosion and oviposition/biting of disease-causing insects. For example, both dengue and malaria show twice-yearly occurrence in Vietnam with only a single annual rainy peak.51,52 Similarly, malaria recurs twice a year in southern China with only a single annual moisture cycle.53,54 Such an explanation fails to explain the difference in duration of stress between the two assemblages reported here.
3.There prevailed in this location in South Africa, phenological or meteorological phenomena that created physiological stress semi-annually of which we are unaware. Some Cape Peninsula baboons spend more time feeding and less time resting in the summer but are more reliant on energetically costly subterranean resources in the winter, suggesting that both summer and winter seasons are times of relative food scarcity.55 However, such an explanation for the pattern observed in this study seems unlikely given the similarity of the observed inter-LEH interval to that observed in most, but not all, low-latitude apes.
4.The value is real but is a result of the existence of two unrelated stressors, each of which has an annual cycle. For example the interaction of three environmental variables (rainfall, temperature and vegetation) in Burundi, each with an annual cycle, creates semi-annual incidence peaks in malaria.56 In such a scenario, one might assume that the stressors would be of different types and, as it would seem unlikely that two different stressors would occur for the same duration and with the same intensity, one would expect defect measurements to show bimodality. Indeed, as has been observed in this study, durations are bimodally distributed and 'longer durations' are more steeply angled (inferably, more intense). Support for the inference of independence can be found in the observation that exactly 50% of long interval recurrences (13/26) link with each of short and long duration LEH events.
Of these alternatives to explain semi-annual recurrence of stress, the last seems most likely; that is, invocation of two independent stressor types, each with an annual cycle. For example, a combination of independent annual cycles of short-term disease stress and longer-term seasonal stress could explain the pattern of LEH in H. naledi and A. africanus. In terms of climate, this could translate into winter and summer stressor types. It is not difficult to suggest candidates for seasonal stress. Winter stress would seem quite likely, e.g. at Sterkfontein, minimum cold temperature averages 3 °C in the austral winter months (June and July) and can go below freezing (www.meteoblue.com). At 3 °C, a wind of only 6 km/h creates a wind chill below freezing.57 Chacma baboons on the southern coast of South Africa, exposed to average minimum temperatures of 3 °C in the austral winter, show elevated cortisol levels, indicating physiological stress.58
Temperature regulation in the infant of a small-bodied hominin like H. naledi31 exposed to such conditions would indeed be a challenge and would predispose to respiratory disease. Respiratory disease is acknowledged to be the second-most common cause of mortality and morbidity in mountain gorillas59 and common in Tai Forest chimpanzees60,61. Human influenza season in South Africa is well defined and peaks in the austral winter months62 while most hospital admissions for paediatric diarrhoea occur in austral summer at the height of the rainy season63.
Conclusions
The discovery of H. naledi in the same geographical area as A. africanus has created an opportunity to evaluate the temporal patterning of developmental dental stress in higher latitude hominins with low latitude apes among whom LEH tends to recur on average at intervals of 6 months, or multiples thereof, linked it is thought to moisture cycles influencing the likelihood of disease and/or malnutrition. It was predicted, as a null hypothesis, that stress would tend to recur annually in the South African hominins and would be of similar duration in both taxa. Neither expectation is borne out. In terms of duration, H. naledi shows bimodal durations of stress centred on 2 and 8 weeks while A. africanus shows unimodal duration of stress centred on 4 weeks. Canine stress lasted significantly longer in H. naledi than in A. africanus. In terms of recurrence, stress tended to recur bimodally every 2 months (less common) and 6 months (more common) in both fossil assemblages. These results, while tantalising, await confirmation from studies of Retzius periodicity in the H. naledi specimens and refined understanding of comparative palaeoenvironments and climate for H. naledi and A. africanus.
Acknowledgements
I am grateful to Wenner-Gren, Matt Skinner, Luke Delezene, Lee Berger and the curators of the collections at the University of the Witwatersrand for the opportunity to work on these assemblages. Casts were created by Debbie Guatelli-Steinberg and Mackie O'Hara. I also acknowledge the generosity of Christophe Soligo at University College London for lending me his surface scanner and Meg Stark for training and access to the scanning electron microscope at the Biosciences Technology Facility, University of York.
References
1.Kelley J. Identification of a single birth cohort in Kenyapithecus kizili and the nature of sympatry betweeen K. kizili and Griphopithecus alpani at Pašalar. J Hum Evol. 2008;54:530-537. https://doi.org/10.1016/j.jhevol.2007.08.005 [ Links ]
2.Corruccini RS, Handler JS, Jacob KP. Chronologic distribution of enamel hypoplasias and weaning in a Caribbean slave population. Hum Biol. 1985;57:699-711. [ Links ]
3.Goodman AH, Armelagos GJ. Childhood stress and decreased longevity in a prehistoric population. Am Anthropol. 1988;90:936-944. https://doi.org/10.1525/aa.1988.90.4.02a00120 [ Links ]
4.Guatelli-Steinberg D. What can developmental defects of enamel reveal about physiological stress in nonhuman primates? Evol Anthropol. 2001;10:138-151. https://doi.org/10.1002/evan.1027 [ Links ]
5.Guatelli-Steinberg D, Skinner MF. Prevalence and etiology of linear enamel hypoplasia in monkeys and apes from Asia and Africa. Folia Primatol. 2000;71:115-132. https://doi.org/10.1159/000021740 [ Links ]
6.Skinner MF. Enamel hypoplasia in sympatric chimpanzee and gorilla. Human Evol. 1986;1:289-312. https://doi.org/10.1007/bf02436704 [ Links ]
7.Skinner MF. Variation in perikymata counts between repetitive episodes of linear enamel hypoplasia among orangutans from Sumatra and Borneo. Am J Phys Anthropol. 2014;154:125-139. https://doi.org/10.1002/ajpa.22485 [ Links ]
8.Skinner MF, Hopwood D. An hypothesis for the causes and periodicity of repetitive linear enamel hypoplasia (rLEH) in large, wild African (Pan troglodytes and Gorilla gorilla) and Asian (Pongo pygmaeus) apes. Am J Phys Anthropol. 2004;123:216-235. https://doi.org/10.1002/ajpa.10314 [ Links ]
9.Skinner MF, Pruetz JD. Reconstruction of periodicity of repetitive linear enamel hypoplasia (rLEH) from perikymata counts on imbricational enamel among dry-adapted chimpanzees (Pan troglodytes verus) from Fongoli, Senegal. Am J Phys Anthropol. 2012;149:468-482. https://doi.org/10.1002/ajpa.22145 [ Links ]
10.Kierdorf H, Witzel C, Kierdof U, Skinner MM, Skinner MF. "Missing perikymata" - Fact or fiction? A study on chimpanzee (Pan troglodytes verus) canines. Am J Phys Anthropol. 2015;157(2):276-283. https://doi.org/10.1002/ajpa.22720 [ Links ]
11.O'Hara MC. Investigating the regularity of linear enamel hypoplasia in Bornean and Sumatran orangutans and in a primate community from Sabah, Borneo. Columbus, OH: Ohio State University; 2016. [ Links ]
12.Smith TM, Boesch C. Developmental defects in the teeth of three wild chimpanzees from the Taï Forest. Am J Phys Anthropol. 2015;157:556-570. https://doi.org/10.1002/ajpa.22741 [ Links ]
13.Smith TM, Austin C, Hinde K, Vogel ER, Arora M. Cyclical nursing patterns in wild orangutans. Sci Adv. 2017;3, e1601517, 8 pages. https://doi.org/10.1126/sciadv.1601517 [ Links ]
14.Anonymous. Climate change knowledge portal for development practitioners and policy makers 2.0. Washington DC: The World Bank Group; 2013. [ Links ]
15.Harrison MSJ. A synoptic climatology of South African rainfall variations [doctoral thesis]. Johannesburg: University of the Witwatersrand; 1986. [ Links ]
16.Harrison MSJ. The annual rainfall cycle over the central interior of South Africa. S Afr Geogr J. 1984;66(1):47-64. https://doi.org/10.1080/03736245.1984.10559679 [ Links ]
17.White TD. Early hominid enamel hypoplasia. Am J Phys Anthropol. 1978;49(1):79-83. https://doi.org/10.1002/ajpa.1330490112 [ Links ]
18.Moggi-Cecchi J. Enamel hypoplasia in South African early hominids: A reappraisal. Am J Phys Anthropol. 2000;suppl30:230-231. [ Links ]
19.Bombin M. Transverse enamel hypoplasia on teeth of South African Plio-Pleistocene hominids. Naturwissenschaften. 1990;77:128-129. https://doi.org/10.1007/bf01134473 [ Links ]
20.Guatelli-Steinberg D. Macroscopic and microscopic analyses of linear enamel hypoplasia in Plio-Pleistocene South African hominins with respect to aspects of enamel development and morphology. Am J Phys Anthropol. 2003;120:309-322. https://doi.org/10.1002/ajpa.10148 [ Links ]
21.Guatelli-Steinberg D. Analysis and significance of linear enamel hypoplasia in Plio-Pleistocene hominins. Am J Phys Anthropol. 2004;123:199-215. https://doi.org/10.1002/ajpa.10324 [ Links ]
22.Peters CR, Maguire B. Wild plant foods of the Makapansgat area: A modern ecosystems analogue for Australopithecus africanus adapatations. J Hum Evol. 1981;10:565-583. https://doi.org/10.1016/s0047-2484(81)80048-6 [ Links ]
23.Chase BM, Faith JT, Mackay A, Chevalier M, Carr AS, Boom A, et al. Climatic controls on Later Stone Age human adaptation in Africa's southern Cape. J Hum Evol. 2018;114(suppl C):35-44. https://doi.org/10.1016/j.jhevol.2017.09.006 [ Links ]
24.Pickering R, Kramers JD. Re-appraisal of the stratigraphy and determination of new U-Pb dates for the Sterkfontein hominin site, South Africa. J Hum Evol. 2010;59(1):70-86. https://doi.org/10.1016/j.jhevol.2010.03.014 [ Links ]
25.Herries AIR, Pickering R, Adams JW, Curnoe D, Warr G, Latham AG, et al. A multi-disciplinary perspective on the age of Australopithecus in southern Africa. In: Reed K, Fleagle JG, Leakey RE, editors. The paleobiology of Australopithecus. Dordrecht: Springer Science+Business Media; 2013. p. 21-40. [ Links ]
26.Bamford M. Pliocene fossil woods from an early hominid cave deposit, Sterkfontein, South Africa. S Afr J Sci. 1999;95:231-237. [ Links ]
27.Hopley PJ, Latham AG, Marshall JD. Palaeoenvironments and palaeodiets of mid-Pliocene micromammals from Makapansgat Limeworks, South Africa: A stable isotope and dental microwear approach. Palaeogeogr Palaeoclimatol Palaeoecol. 2006;233:235-251. https://doi.org/10.1016/j.palaeo.2005.09.011 [ Links ]
28.Rayner RJ, Moon BP, Masters JC. The Makapansgat australopithecine environment. J Hum Evol. 1993;24:219-231. https://doi.org/10.1006/jhev.1993.1016 [ Links ]
29.Avery DM. The Plio-Pleistocene vegetation and climate of Sterkfontein and Swartkrans, South Africa, based on micromammals. J Hum Evol. 2001;41:113-132. https://doi.org/10.1006/jhev.2001.0483 [ Links ]
30.Maguire B. The potential vegetable dietary of Plio-Pleistocene hominids at Makapansgat. Palaeont Afr. 1980;23:69. [ Links ]
31.Berger LR, Hawks J, De Ruiter DJ, Churchill SE, Schmid P, Delezene LK, et al. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. eLife. 2015;4, e09560, 35 pages. https://doi.org/10.7554/eLife.09560 [ Links ]
32.Dirks PHGM, Roberts EM, Hilbert-Wolf H, Kramers JD, Hawks J, Dosseto A, et al. The age of Homo naledi and associated sediments in the Rising Star Cave, South Africa. eLife. 2017;6, e24231, 59 pages. http://doi.org/10.7554/eLife.24231 [ Links ]
33.Butzer KW, Stuckenrath R, Bruzewicz AJ, Helgren DM. Late Cenozoic paleoclimates of the Gaap Escarpment, Kalahari margin, South Africa. Quatern Res. 1978;10(3):310-339. https://doi.org/10.1016/0033-5894(78)90025-X [ Links ]
34.Skinner MF, Goodman AH. Anthropological uses of developmental defects of enamel. In: Saunders SR, Katzenberg A, editors. Skeletal biology of past peoples: Research methods. New York: Wiley-Liss; 1992. p. 153-174. [ Links ]
35.Guatelli-Steinberg D, O'Hara MC, Le Cabec A, Delezene LK, Reid DJ, Skinner MM, et al. Patterns of lateral enamel growth in Homo naledi as assessed through perikymata distribution and number. J Hum Evol. 2018;121:40-54. https://doi.org/10.1016/j.jhevol.2018.03.007 [ Links ]
36.Hillson S. Enamel hypoplasia=stress? Paper presented at: Stressed out: Debunking the stress myth in the study of archaeological human remains; 2017 May 19-20; London, UK. [ Links ]
37.Skinner MF, Skinner MM. Orangutans, enamel defects, and developmental health: A comparison of Borneo and Sumatra. Am J Primatol. 2017;79(8), e22668. https://doi.org/10.1002/ajp.22668 [ Links ]
38.Guatelli-Steinberg D, Reid DJ. What molars contribute to an emerging understanding of lateral enamel formation in Neandertals vs. modern humans. J Hum Evol. 2008;54:236-250. https://doi.org/10.1016/j.jhevol.2007.09.016 [ Links ]
39.Mahoney P, Miszkiewicz JJ, Pitfield R, Schlecht SH, Deter C, Guatelli-Steinberg D. Biorhythms, deciduous enamel thickness, and primary bone growth: A test of the Havers-Halberg Oscillation hypothesis. J Anat. 2016;228:919-928. https://doi.org/10.1111/joa.12450 [ Links ]
40.Hillson S. Tooth development in human evolution and bioarchaeology. Cambridge: Cambridge University Press; 2014. [ Links ]
41.Smith TM, Reid DJ, Dean MC, Olejniczak AJ, Ferrell RJ, Martin LB. New perspectives on chimpanzee and human molar crown development. In: Bailey SE, Hublin J-J, editors. Dental perspectives on human evolution. Dordrecht: Springer; 2007. p. 177-192. [ Links ]
42.Smith TM, Tafforeau P, Reid DJ, Grün R, Eggins S, Boutakiout M, et al. Earliest evidence of modern human life history in North African early Homo sapiens. Proc Natl Acad Sci USA. 2007;104(15):6128-6133. https://doi.org/10.1073/pnas.0700747104 [ Links ]
43.Le Cabec A, Tang N, Tafforeau P. Accessing developmental information of fossil hominin teeth using new synchrotron microtomography-based visualization techniques of dental surfaces and interfaces. PLoS ONE. 2015;10(4), e0123019, 23 pages. https://doi.org/10.1371/journal.pone.0123019 [ Links ]
44.Smith TM, Tafforeau P, Le Cabec A, Bonnin A, Houssaye A, Pouech J, et al. Dental ontogeny in Pliocene and Early Pleistocene hominins. PLoS ONE. 2015;10(2), e0118118, 20 pages. https://doi.org/10.1371/journal.pone.0118118 [ Links ]
45.Lacruz RS, Rozzi FR, Bromage TG. Variation in enamel development of South African fossil hominids. J Hum Evol. 2006;51:580-590. https://doi.org/10.1016/j.jhevol.2006.05.007 [ Links ]
46.Chollet MB, Teaford MF. Ecological stress and linear enamel hypoplasia in Cebus. Am J Phys Anthropol. 2010;142(1):1-6. https://doi.org/10.1002/ajpa.21182 [ Links ]
47.Guatelli-Steinberg D, Ferrell RJ, Spence J. Linear enamel hypoplasia as an indicator of physiological stress in great apes: Reviewing the evidence in light of enamel growth variation. Am J Phys Anthropol. 2012;148(2):191-204. https://doi.org/10.1002/ajpa.21619 [ Links ]
48.Kuykendall K. Dental development in chimpanzees (Pan troglodytes): The timing of tooth calcification stages. Am J Phys Anthropol. 1996;99:135-157. https://doi.org/10.1002/(SICI)1096-8644(199601)99:1<135::AID-AJPA8>3.0.CO;2-# [ Links ]
49.Gustafson A-G. A morphologic investigation of certain variations in the structure and mineralization of human dental enamel. Odont Tidskr 1959;67:361-472. [ Links ]
50.Witzel C, Kierdorf U, Dobney K, Ervynck A, Vanpoucke S, Kierdorf H. Reconstructing impairment of secretory ameloblast function in porcine teeth by analysis of morphological alterations in dental enamel. J Anat. 2006;209:93-110. https://doi.org/10.1111/j.1469-7580.2006.00581.x [ Links ]
51.Do TTT, Martens P, Luu NH, Wright P, Choisy M. Climatic-driven seasonality of emerging dengue fever in Hanoi, Vietnam. BMC Public Health. 2014;14:1078. https://doi.org/10.1186/1471-2458-14-1078 [ Links ]
52.Erhart A, Thang ND, Hung NQ, Toi L, Hung LX, Tuy TQ, et al. Forest malaria in Vietnam: A challenge for control. Am J Trop Med Hyg. 2004;70(2):110-118. https://doi.org/10.4269/ajtmh.2004.70.110 [ Links ]
53.Clements AC, Barnett AG, Cheng ZW, Snow RW, Zhou HN. Space-time variation of malaria incidence in Yunnan province, China. Malar J. 2009;8(1):180. https://doi.org/10.1186/1475-2875-8-180 [ Links ]
54.Bi P, Tong S, Donald K, Parton KA, Ni J. Climatic variables and transmission of malaria: A 12-year data analysis in Shuchen County, China. Public Health Rep. 2003;118:65-71. https://doi.org/10.1016/S0033-3549(04)50218-2 [ Links ]
55.Van Doorn AC, O'Riain MJ, Swedell L. The effects of extreme seasonality of climate and day length on the activity budget and diet of semi-commensal Chacma baboons (Papio ursinus) in the Cape Peninsula of South Africa. Am J Primatol. 2010;72:104-112. [ Links ]
56.Gomez-Elipe A, Otero A, Van Herp M, Aguirre-Jaime A. Forecasting malaria incidence based on monthly case reports and environmental factors in Karuzi, Burundi, 1997-2003. Malar J. 2007;6:129. https://doi.org/10.1186/1475-2875-6-129 [ Links ]
57.Steadman RG. Norms of apparent temperature in Australia. Australian Meteorological Magazine. 1994;43(1):1-16. [ Links ]
58.Weingrill T, Gray DA, Barrett L, Henzi SP. Fecal cortisol levels in free-ranging female chacma baboons: Relationship to dominance, reproductive state and environmental factors. Horm Behav. 2004;45:259-269. https://doi.org/10.1016/j.yhbeh.2003.12.004 [ Links ]
59.Spelmen LH, Gilardi KVK, Lukasik-Braum M, Kinani J-F, Elisabeth N, Lowenstine LJ, et al. Respiratory disease in Mountain gorillas (Gorilla beringei beringei) in Rwanda, 1990-2010: Outbreaks, clinical course, and medical management. J Zoo Wildl Med. 2013;44(4):1027-1035. https://doi.org/10.1638/2013-0014R.1 [ Links ]
60.Boesch C. Why do chimpanzees die in the forest? The challenges of understanding and controlling for wild ape health. Am J Primatol. 2008;70:722-726. https://doi.org/10.1002/ajp.20571 [ Links ]
61.Chi F, Leider M, Leendertz F, Bergmann C, Boesch C, Schenk S, et al. New Streptococcus pneumoniae clones in deceased wild chimpanzees. J Bacteriol. 2007;189(16):6085-6088. https://doi.org/10.1128/JB.00468-07 [ Links ]
62.Kyeyagalire R, Tempia S, Cohen AL, Smith AD, McAnemey JM, Dermaux-Msimang V, et al. Hospitalizations associated with influenza and respiratory syncytial virus among patients attending a network of private hospitals in South Africa, 2007-2012. BMC Infect Dis. 2014;14(694):1-10. https://doi.org/10.1186/s12879-014-0694-x [ Links ]
63.Robins-Browne RM, Still CS, Miliotis MD, Richardson NJ, Koornhof HJ, Freiman I, et al. Summer diarrhoea in African infants and children. Arch Dis Child. 1980;55:923-928. https://doi.org/10.1136/adc.55.12.923 [ Links ]
 Correspondence:
Correspondence:
Mark Skinner
Email: mskinner@sfu.ca
Received: 12 Dec. 2018
Revised: 20 Feb. 2019
Accepted: 21 Feb. 2019
Published: 29 May 2019
EDITOR: Maryna Steyn
FUNDING: Wenner-Gren Foundation; University of the Witwatersrand














