Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.5-6 Pretoria Mai./Jun. 2019
http://dx.doi.org/10.17159/sajs.2019/4662
RESEARCH ARTICLES
Metatarsophalangeal proportions of Homo naledi
Sarah TraynorI; Mark BanghartII; Zachary ThrockmortonIII
IMedical Education Office, Academic Affairs, University of Wisconsin School of Medicine and Public Health, Madison, Wisconsin, USA
IISocial Science Computing Cooperative, University of Wisconsin-Madison, Madison, Wisconsin, USA
IIIDepartment of Anatomy, Arkansas College of Osteopathic Medicine, Fort Smith, Arkansas, USA
ABSTRACT
Post-cranial differences between extant apes and humans include differences in the length, shape and size of bone elements relative to each other; i.e. differences in proportions. Foot proportions are influenced by the different functional requirements of climbing and bipedal locomotion. Phalangeal length is generally correlated with locomotor behaviour in primates and there is variation in hominins in relative phalangeal lengths - the functional and evolutionary significance of which is unclear and currently debated. Homo naledi has a largely modern rearfoot (i.e. tarsal skeleton) and midfoot (i.e. metatarsal skeleton). The proximal pedal phalanges of H. naledi are curved, but the relative lengths are unknown, because the phalanges cannot reliably be associated with metatarsals, or in many cases even with ray number. Here, we assess the lengths of the proximal pedal phalanges relative to the metatarsals in H. naledi with resampling from modern human and chimpanzee (Pan troglodytes) samples. We use a novel resampling method that employs two boundary conditions, assuming at one extreme that elements in the sample are associated, and at the other extreme that no elements are associated. The associated metatarsophalangeal proportions from digits 1 and 2 are within the 95% confidence interval of the modern human distribution. However, the associated and unassociated proportions from digits 3-5 fall above the 95% confidence interval of the human distribution, but below and outside of the chimpanzee distribution. While these results may indicate fossil preservation bias or other sample-derived statistical limitations, they potentially raise the intriguing possibility of unique medial versus lateral pedal column functional evolution in H. naledi. Additionally, the relevant associated proportions of H. naledi are compared to and are different from those of H. floresiensis. Both species suggest deep phylogenetic placement so the ancestral condition of the pedal phalanges in the genus Homo remains unclear.
SIGNIFICANCE:
• Modern humans demonstrate straight and relatively short pedal phalanges, whereas H. naledi demonstrates curved phalanges of an unknown relative length. This research analyses the relative length of the proximal phalanges to the metatarsals to determine if H. naledi has relatively short phalanges similar to modern humans or is distinct from modern humans in both its phalangeal length and curvature.
• This analysis further develops a statistical resampling method that was previously applied to large fossil assemblages with little association between bones.
• A more comprehensive understanding of pedal morphology of H. naledi could provide insight into the ancestral pedal form of the genus Homo as the overall morphology of H. naledi appears to be deeply rooted in the genus.
Keywords: pedal proportions; resampling; Homo; phalanges; metatarsals
Introduction
Evidence of hominin bipedality is obtained from multiple sources: hominin limb proportions from fragmentary post-cranial fossil evidence1; basicranial position of foramen magnum2; preserved fossil partial foot skeletons3-6; and footprints preserved in volcanic ash or lakeshore sediment in eastern Africa at the Laetoli and Ileret sites7-9. Modern humans have a robust and long hallucal ray, aligned with the lateral digits, which is morphologically and functionally distinct from those in the living great apes. The lateral digits in humans are markedly short compared to those of living great apes, and the lateral toes are much shorter in humans relative to the lengths of the metatarsals.10-13 These traits functionally support human bipedal walking and running, including the distinctive 'toe-off' phase of the gait cycle.14,15 Short toes eliminate some of the mechanical costs of walking16, while a stiff and elongated midfoot (i.e. metatarsal skeleton) is thought to promote the posterior-anterior transfer of weight through the foot's medial column and from heel-strike to toe-off for a more efficient bipedal gait11.
In addition to digit length and midfoot stability, the relative lengths of the proximal pedal phalanges are potentially informative for assessing bipedal gait efficiency. We focused on proximal phalanges because they are more readily identifiable and are thus more numerous than intermediate or distal phalanges in fossil and comparative collections. In this study, we assess proximal phalangeal lengths relative to metatarsal lengths, or the metatarsophalangeal proportions, in the fossil sample of Homo naledi.
Although pedal traits can be inferred for several species of hominins based on partial feet or isolated foot bones, relatively complete hominin feet in the fossil record are rare. Consequently, the pedal proportions that are characterised by the relations of multiple foot bones, such as the direct proportions of the lengths of the metatarsals and phalanges, are unknown for most hominin species. In the later Pleistocene fossil record, the Neanderthals exhibit proximal phalangeal and metatarsal lengths that are largely indistinguishable from those of modern humans.17 Like Neandertals and modern humans, Homo erectus also demonstrates clear post-cranial adaptations for obligate bipedalism, meaning a commitment to terrestrial bipedalism and loss of all unambiguously climbing adaptations.18 The metatarsal ratios of H. erectus material from Dmanisi are human-like in their proportions to one another; however, the lengths of the pedal phalanges are unknown for this species.19 Evidence of H. erectus foot morphology has been largely obtained from the Ileret footprints in Kenya, which date to 1.5 million years ago (mya), and appear to have been produced by a more modern-appearing foot architecture than the more ancient Laetoli footprints.8 The Late Pleistocene Homo floresiensis is represented by a partial foot. This foot has curved phalanges that are longer relative to the metatarsals than the range presented by modern humans. Additionally, H. floresiensis is thought to have an elongated foot - partially because of its longer phalangeal length.5
Prior to the origin of Homo, the only hominin specimen that has preserved pedal phalanges in association with metatarsals is ARA-VP-6/500, the partial skeleton of Ardipithecus ramidus.20 Because of damage to the distal ends of the metatarsals of ARA-VP-6/500, researchers normalised the complete phalanges from ray 4 by body size and concluded that the phalangeal lengths were closer to a mean length for Gorilla. Long lateral digits in addition to the evidence of an opposable hallux led researchers to conclude that the species likely had an ability to grasp large branches and support itself arboreally.20 The preserved base of the third metatarsal suggests that Ar. ramidus had a more stable midfoot and this bone has a relatively slight curvature compared to third metatarsals of living orangutans and chimpanzees. The proximal phalanges of Ar. ramidus exhibit substantial curvature, as does the single proximal pedal phalanx, AME-VP-1/71, from the Amba East locality in the Middle Awash, Ethiopia.21
Some aspects of pedal morphology can be assessed in other species of hominins. However, these do not contribute to the discussion of relative proximal phalangeal length, because of the lack of pedal material preserved at many of the well-known early hominin sites. This leaves a substantial gap in our understanding of pedal phalanx proportions in Australopithecus and Homo habilis, as the OH 8 foot, representing H. habilis at Olduvai Gorge, Tanzania, does not retain complete metatarsals or proximal phalanges.22
The AL 333 locality at Hadar, Ethiopia, presents a commingled assemblage of bones representing at least 18 individuals of Australopithecus afarensis.23 There is substantial debate about the morphology of Au. afarensis in terms of its locomotor adaptations; the pedal morphology is no exception.24 No metatarsal lengths are known from this assemblage; however, it does preserve complete, strongly curved phalanges. Stern and Susman16 noted that the proximal phalanges of Au. afarensis are likely long relative to the metatarsals, because they are longer relative to the diameter of the femoral head than expected for modern humans. Longer toes would require more work during the swing phase of the bipedal gait. With the assumption that a costly trait would not persist without some countervailing functional utility, they suggested that longer and curved toes probably indicate arboreal behaviour in this species.
Phalangeal curvature has taken on a substantial weight in arguments about the function of fossil feet because the curvature is thought to be a reliable indicator not only of adaptation but of use during an individual's lifetime. Phalangeal curvature is considered epigenetically sensitive, such that the repeated use of the fingers and toes in grasping is believed to influence the development of curvature.25-29 If this is true, then the curvature exhibited by certain species is not merely a retention from arboreal ancestors but an indication of the way the foot was used.30 Increasing the curvature of the phalanges decreases the amount of stress from the fibres of the flexor sheath, and forceful gripping/strong flexion becomes safer (i.e. mitigates the likelihood of avulsion fractures and other joint failures) at higher curvatures.31 A similar functional response would occur in the pedal phalanges if gripping with the toes occurred throughout a lifetime.
White and Suwa32 argued that the relevant functional parameter is not toe length, as was suggested by Stern and Susman16, but instead overall foot length, that is, the toes contribute little to locomotor costs relative to the overall length of the foot. White and Suwa demonstrated that the relative length of the Au. afarensis foot to the femur was within modern human variation, ergo the length of the foot would not negatively affect the bipedal gait. Later, additional fossil remains from Hadar and from the Woranso-Mille study area of Ethiopia were recovered, demonstrating that some individuals of Au. afarensis had tall statures well within the range of modern humans.33 This evidence further emphasises that the relative length of a single pedal phalanx is unlikely to indicate accurately the proportion of phalanges within a fossil species.
In South Africa, at the site of Sterkfontein, STW 573 or 'Little Foot', a specimen representing Australopithecus africanus, is missing the distal-most aspects of the foot and does not present pedal phalangeal lengths.34 However, from other morphological indicators in the rearfoot and midfoot, Au. afarensis and Au. africanus demonstrate unique morphologies, which are also unique from H. habilis, indicating variation in pedal form in early hominins.28
As a consequence of variable pedal forms of hominin species in the Pliocene, the ancestral condition of the pedal phalanges in the genus Homo is unknown. H. floresiensis is a later Homo species, yet is primitive in its overall morphology. It is not clear whether H. floresiensis is phylogenetically linked to H. erectus or if it is more distantly related and rooted deeper in the genus Homo like H. naledi. H. floresiensis exhibits curved and elongated phalanges relative to associated metatarsals, which is unique to that of contemporaneous H. sapiens.35 Because of the phylogenetic ambiguity, it is unclear if the H. floresiensis foot represents the primitive condition of the basal Homo foot.
The recently described species, H. naledi, occupies a phylogenetic position deeply rooted in genus Homo, yet the depositional age of the fossil bones and associated sediments within the Rising Star Cave system in South Africa is 236 kya to 335 kya36, which implies that H. naledi was sharing the landscape with more derived Middle Pleistocene hominin species. H. naledi is presently represented by over 1700 fossil fragments in the Dinaledi Chamber, of which more than 100 are pedal elements.37 Note that no fossils relevant to this study have been recovered from the Lesedi Chamber.38 The Dinaledi Chamber assemblage contains the remains of at least 15 commingled individuals, including 7 adults, which appear to represent a limited time of deposition.39 Although most of the material discovered in the Dinaledi Chamber was unassociated and commingled, a remarkably complete hand and at least one nearly complete foot were preserved in situ.27,40 However, while this foot has an associated hallux, it lacks any clear associations of the phalanges for digits 2-5 and the lengths of the phalanges relative to the metatarsals are unknown for H. naledi.
The morphology of the H. naledi foot is similar to the hand in that it is mosaic, with modern features such as an adducted hallux and an elongated talus, while retaining ancestral features such as a minimally developed medial longitudinal arch and curved pedal phalanges.27 With clear adaptations to bipedality in the pelvis and lower limb37,41, and features of the upper limb that appear to reflect an enhanced ability to climb relative to that found in modern humans, Neanderthals or H. erectus27,42, the H. naledi hominins engaged in a combination of locomotor grasping43 and bipedal locomotion. The foot of H. naledi provides valuable insight into these activity patterns, in that it primarily presents derived bipedal morphology, yet retains curvature of the phalanges that reflects some degree of climbing behaviour.
The lengths of the phalanges relative to the metatarsals may provide key evidence about the timing and sequence by which the early hominin foot, with its relatively long toes, evolved toward more human-like proportions. H. naledi has a more human-like midfoot and rearfoot, placing its anatomical configuration much closer to modern humans and Neanderthals than to H. habilis, H. floresiensis or Australopithecus. This might suggest that H. naledi would also have shorter lateral digits, more similar to modern humans and Neanderthals.44 However, the metatarsophalangeal proportions of the associated H. naledi Foot 1 (F1) cannot be directly assessed, because it is missing the phalanges of digits 2-5, and although the hallucal phalanges are present, the proximal hallucal phalanx is incomplete and its length cannot be estimated with certainty.40 Yet, the H. naledi pedal sample contains 21 unassociated proximal phalanges and metatarsals (Table 1). Because of the unknown association among the elements, we applied two assumptions about the possible associations in the pedal sample in order to assess the metatarsophalangeal proportions of H. naledi. We compared the fossil proportions to the associated and resampled45 distributions of modern humans and chimpanzees (Pan troglodytes), and to the associated proportions of H. floresiensis.
Materials and methods
Our procedure was similar to the procedure used by Rolian and Gordon46 to study the manual proportions of the Hadar Au. afarensis material. Analogous to the H. naledi context, the Hadar AL 333 locality is a commingled assemblage with limited bony associations.
The modern human comparative sample was derived from plain film pedal radiographs taken during routine medical care. All radiographs were de-identified prior to measurement in compliance with the Health Insurance Portability and Accountability Act (HIPPA) and Institutional Review Board (IRB) regulations; acquiring such radiographs does not require interaction with patients on the part of the researchers, and because the radiographs are anonymised, they are not considered human subjects and are exempted from IRB oversight. Radiographs of skeletally immature or pathological individuals were not included in this study. Measurements of pedal phalangeal and metatarsal lengths (mm) were taken in the dorsal-plantar view using standard equipment (lightbox and calipers). The sample of 110 adults included a variety of ancestries, was mixed-sex (48 male and 62 female), and was from a habitually shod US population. Agoada47 demonstrated that linear measurements collected from pedal radiographs are accurate depictions of pedal skeletal element dimensions in humans, therefore this study considered the radiographic measurements equivalent to an osteological pedal sample. These radiographic linear measurements were compared to fossil bone linear measurements.
The chimpanzee (Pan troglodytes) comparative sample included 39 individuals (17 female, 18 male, 4 indeterminate) from the skeletal collections of the American Museum of Natural History, Cleveland Museum of Natural History, and the Smithsonian Natural History Museum. Chimpanzees demonstrate more ancestral metatarsophalangeal proportions (i.e. longer proximal phalanges relative to metatarsals) than modern humans11 so the ancestral condition can be considered in contrast to the derived modern human sample. The maximum lengths of the proximal phalanges and metatarsals were measured with calipers held flush on proximal and distal ends.
The H. naledi fossil sample included the maximum lengths of 21 adult proximal phalanges and metatarsals (Table 1). These phalanges and metatarsals are described further in the Supplementary Information of Harcourt-Smith and colleagues.40 The pedal elements represent a minimum of four adult individuals, although at least seven adult individuals are known from dental remains, and there is no reason to assume the pedal material samples fewer individuals than the dentition.37 When resampling, many researchers have emphasised the importance for modern comparative samples to match the fossil sample in the minimum number of individuals (MNI) represented by the site.48-51 Of the two fossil MNI, we chose to resample from the larger MNI of seven because it reduced the probability that multiple comparisons in each resampled set would come from the same individual.
Distinct from the Rolian and Gordon analysis46, the present analysis considered the relationship among the bones of the commingled sample. While assessing proportions within a commingled assemblage, one cannot assume the fossil sample is a random, independent sample of a fossil population. There is a true state among two bones in a commingled assemblage. Either these two bones belong to the same individual, or they belong to different individuals. Hence, looking at a sample of bones with unknown associations, these two possible states constitute two boundary conditions. While bones may belong to a single individual, they may alternatively all belong to different individuals. These two states provide the boundaries within which all other partial associations must fall, including when some bones belong to one individual, but other bones belong to other individuals.
In this study, we probed the two boundary conditions by carrying out two separate tests. For each digit, two different analyses were performed. In the first analysis, the procedure assumed that an association was present between two bones in the sample, meaning they belong to the same individual. The assumed associated pair of bones was compared to a distribution generated from paired bones that were each from the same individual. In the other analysis, the procedure assumed that all bones were unassociated, which means that they were all from different individuals. This unassociated sample of bones was compared to a resampled distribution generated from samples of bones that were all from different individuals.
Usually, the commingled context of H. naledi would prevent the comparison of the indirect proportions of H. naledi to the direct proportions of H. floresiensis. However, because this novel approach to studying commingled assemblages addresses the associations among the elements, the associated proportion of H. naledi and direct proportion of H. floresiensis can be compared. The H. floresiensis pedal material contains the maximum lengths of five proximal phalanges, excluding the hallucal proximal phalanx, and three metatarsals.5,35 Jungers and colleagues35 assigned the longest and shortest phalanges to the second and fifth metatarsals, respectively. Therefore, the proportions of second and fifth digits of H. naledi and H. floresiensis were compared to better understand the pedal morphology of two species, both thought to be primitive in their morphology.
Digit 1
The first proximal phalanx (PP1) and first metatarsal (MT1) fossils were morphologically distinguishable from digit 1. There were two PP1 and four MT1 elements in the fossil sample (Table 1). If the sample of six elements included a minimum of one associated pair of PP1 and MT1 elements, the shortest proximal phalanx and the longest metatarsal in the sample create the most conservative pairing as they generate the smallest proportion. The human data set was composed of known individuals, or associated elements; thus, all PP1/MT1 proportions were calculated for the human sample. The minimum associated proportion for H. naledi was then compared to the distributions of associated human proportions.
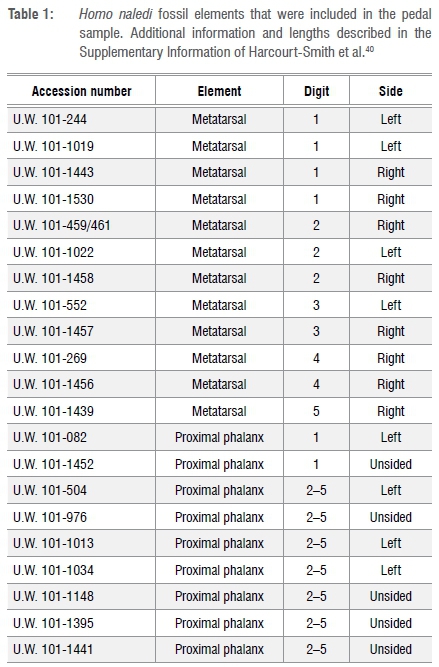
If the PP1 and MT1 elements were unassociated, a resampling procedure was required to analyse the indirect proportions. From the initial data set of 110 modern humans, 7 individuals were randomly sampled without replacement, to equal the MNI of H. naledi (Figure 1, step 1). Two of the seven individuals were randomly sampled without replacement and their PP1 lengths were collected (Figure 1, step 2). To ensure no association, of the remaining five individuals (Figure 1, step 3), four were randomly sampled without replacement and their MT1 lengths collected (Figure 1, step 4). Six elements were sampled - two PP1 and four MT1, ensuring the modern sample was equivalent to the H. naledi sample for digit 1. The arithmetic mean proportion, or the mean length of the phalanges divided by the mean length of the metatarsals, was calculated for these six elements (Figure 1, step 5). The resampling procedure was run 100 000 times (Figure 1, step 6) then the H. naledi mean proportion was compared to the resampled human distribution of unassociated mean proportions.
Digits 2-5
Regarding digits 2-5, the metatarsals were distinguishable from the digit, but the proximal phalanges were not. Resampling does not require complete fossils or complete data sets to perform an analysis; this provides the opportunity to study incomplete data sets and compare them to more complete extant samples. Resampling designs a scenario in which the largest possible range of ratios is generated from the available fossil material and is then compared to the resampled distributions of ratios from an equivalent number of elements representing extant taxa. Hence, digit attribution is not required for the relative length of the proximal phalanges to the metatarsals to be studied. Because the proximal phalanges of digits 2-4 were not distinguishable from each other, all proximal phalanges not assigned to digit 1 were pooled. This method of phalangeal pooling was previously performed by Rolian and Gordon46 to assess the manual proportions of Au. afarensis. It was reasonable to use the approach here to assess pedal proportions because of the similar morphological ambiguity of both the manual and pedal proximal phalanges of the lateral digits. The resampling procedure will be demonstrated with digit 2, but was also applied to digits 3-5.
The digit 2 sample comprised three metatarsals (MT2) and seven pooled proximal phalanges (PP2-5; Table 1). If the sample included a minimum of one associated pair of elements, the identical digit 1 procedure was performed for digit 2. The H. naledi minimum proportion for digit 2 was generated from the shortest pooled phalanx and the longest MT2, with the assumption that if a phalanx from PP2-5 was associated with the MT2, it was a second proximal phalanx (PP2). This minimum fossil proportion was compared to the distribution of modern human proportions for digit 2 (PP2/MT2).
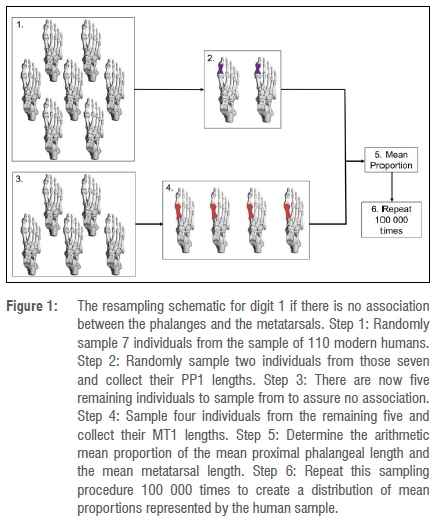
If the digit 2 PP2-5 and MT2 elements were unassociated, the modern human phalanges were pooled to mimic the fossil sample composition and a similar resampling procedure to that of digit 1 was performed (Figure 2). From the modern human sample of 110 individuals, 7 individuals were randomly sampled without replacement (Figure 2, step 1). Of those seven, three individuals were randomly sampled and their MT2 lengths were collected (Figure 2, step 2). The proximal phalanges of digits 2-5 from the remaining four individuals were pooled (16 phalanges), and the third, fourth and fifth proximal phalanges of the three individuals from whom MT2 lengths were collected (nine phalanges), for a total of 25 pooled phalanges (Figure 2, step 3). From the pooled phalangeal sample, seven phalanges were randomly sampled without replacement (Figure 2, step 4). In total, three MT2 elements and seven PP2-5 elements were sampled, equivalent to the composition of the fossil sample. The arithmetic mean proportion was calculated from the arithmetic mean of PP2-5 lengths and the arithmetic mean of the MT2 lengths (Figure 2, step 5). The resampling procedure was run 100 000 times (Figure 2, step 6) and the mean fossil proportion was compared to the resampled distribution of mean proportions. Both associated and unassociated procedures were repeated for digits 3-5.
Analysis
The analysis was performed with R software version 3.1.2.52 Each H. naledi proportion was compared to its corresponding cumulative distribution function (CDF), which represented human variation for a given proportion. If a fossil value falls outside of the human distribution, it is considered significantly different. There is no associated p-value for the comparison. We tested the null hypothesis that H. naledi is not significantly different from modern humans in its metatarsophalangeal proportions. Because the assemblage is commingled, the true state of the bones is unknown, therefore both associated and unassociated states must be considered for each digit. The null hypothesis was not rejected if both assumptions failed to reject the null, meaning if both H. naledi proportions fell within the 95% confidence interval of their respective modern human CDF. Likewise, the null hypothesis was rejected if both assumptions rejected the null, or if both H. naledi proportions fell outside the 95% confidence interval of their respective CDF. Finally, the null hypothesis was not rejected if only one assumption failed to reject the null. Both assumptions were considered equally plausible, therefore if one H. naledi proportion fell within the 95% confidence interval of its respective CDF, it could represent the true state of the bones so the null hypothesis cannot be rejected.
If the fossil value fell within the upper 97.5% of the human distribution, meaning that the fossil proportion was larger than modern humans, the fossil proportion was compared to a chimpanzee distribution to test if the fossil proportion was more similar to the ancestral condition of longer phalanges in relation to metatarsal length. If the null hypothesis was rejected for a given digit, the associated and unassociated fossil proportions for that digit were compared to the corresponding chimpanzee CDFs. The chimpanzee distributions were generated using the same methods described above.
Results
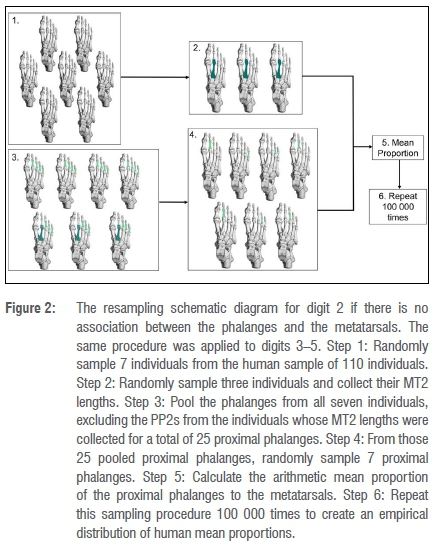
If at least one association between the elements was assumed to be present in the fossil sample, the minimum direct proportion of digit 1 fell at the 80th percentile of the modern human CDF (Figure 3). Similarly, the minimum direct proportion of digit 2 fell within the 95% confidence interval of its respective CDF (Figure 3). Both digit 1 and digit 2 unassociated mean proportions fell outside of 95% confidence intervals. However, because both states were equally plausible, if only one assumption failed to reject the null, the null hypothesis could not be rejected. If there was at least one pair of associated elements in the H. naledi pedal material, we failed to reject the null that H. naledi resembles modern humans in its metatarsophalangeal proportions, particularly those in the medial pedal column. With the present pedal data of H. naledi, we conclude that the proportions of first and second digits could be similar to those of modern humans.
In contrast to digits 1 and 2, all minimum associated and unassociated proportions of the more lateral digits 3-5 fell above the 95% confidence interval of their respective modern human CDFs (Figure 3) and so we rejected the null hypothesis that the metatarsophalangeal proportion values in the lateral column of H. naledi are similar to those of modern humans. This could be a result of preservation bias, in which the larger proximal phalanges were more likely to be preserved than the smaller phalanges from the more lateral digits. If the smaller phalanges of lateral digits are not represented in the H. naledi sample, it would result in a higher metatarsophalangeal proportion value for the lateral digits compared to modern humans. This could also be a result of a biological difference between the lateral and medial pedal columns in the H. naledi foot. The more lateral phalanges could be longer relative to the metatarsals than in modern humans, which would generate the higher proportions seen in this study. Alternatively, the metatarsals could be shorter. Either way, the proportions of the lateral digits are different from those of modern humans and could represent different medial versus lateral pedal column development in this species.
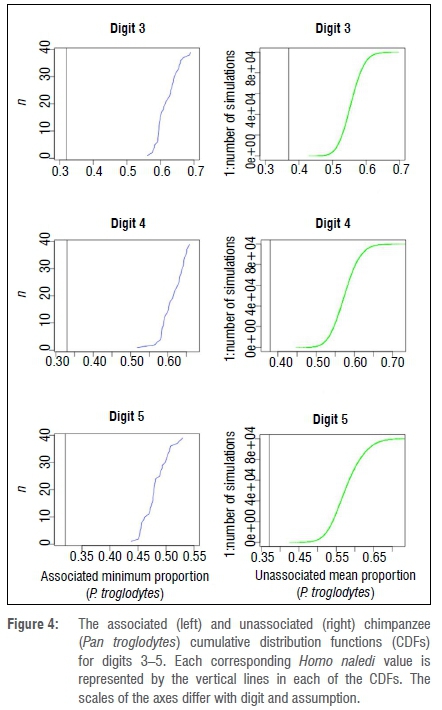
Because the fossil proportions of the lateral digits were different from those of modern humans, we compared digits 3-5 to corresponding chimpanzee CDFs. The unassociated minimum and associated mean proportions of digits 3-5 of H. naledi fell below all respective chimpanzee CDFs (Figure 4). This demonstrates that although the values of metatarsophalangeal proportions are higher in H. naledi than they are in humans, they are not within the range of the more ancestral chimpanzee values.
Regarding the H. floresiensis pedal elements, both H. floresiensis digit 2 (0.43) and digit 5 (0.39) proportions fell outside the modern human confidence intervals provided by this study (Figure 3) and both were larger than the H. naledi minimum associated proportions (0.29, 0.32). This analysis demonstrates that H. floresiensis has different proportions from those of modern humans, which confirms the results of Jungers and colleagues35, but also demonstrates that H. naledi is distinct from H. floresiensis in its pedal proportions.
Discussion
Homo naledi has a human-like hindfoot and midfoot, but it has curvature of the proximal pedal phalanges like some extant primate species and Au. afarensis.39 It was unclear if its primitive phalangeal morphology was accompanied by primitive phalangeal proportions (i.e. longer phalanges relative to metatarsals), as direct proportions are not possible in this unassociated sample. In the present study, we analysed the length of the proximal phalanges relative to the metatarsals in H. naledi and compared these proportions to samples of modern humans, chimpanzees and H. floresiensis. on these comparisons, H. naledi could have medial column proportions similar to those of modern humans, but different lateral proportions from those of modern humans and chimpanzees. Additionally, H. naledi has proportions different from those of H. floresiensis.
Given the lack of associated proximal phalanges and metatarsals, the resampling method generates distributions of likely proportions in modern humans, considering the sample size and composition of H. naledi fossils, and permits us to study the pedal proportions in the largest pedal sample in the African hominin fossil record to date. Consequently, H. naledi provides insight into the evolution of this mosaic morphology in hominins, as this species demonstrates manual27 and medial pedal phalangeal lengths similar to those of modern humans, but exhibits manual27 and pedal curvature40 dissimilar to modern humans.
Although palaeoanthropologists assess the length and curvature of the manual and pedal phalanges to identify certain locomotor behaviours in hominin fossils, the evolutionary mechanism through which length is modified is less clear. The human-like proportions of the manual and pedal phalanges of H. naledi could indicate serial homology53,54, or the continued modularity55 and shared developmental trajectories of these two structures56,57. However, developmental genetics13 have demonstrated the existence of regulatory elements that are expressed in one limb but not the other, suggesting manual and pedal skeletal element covariation is not constant. Additionally, cortical neural mapping suggests that the hand in human and nonhuman primates developed more independently from the foot than previously assumed.58
If covariation of the hand and feet are inconsistent, the shorter phalanges of H. naledi may indicate a locomotor adaptation unique to H. floresiensis. Shorter toes have been demonstrated to minimally decrease mechanical work of the digital flexor muscles while walking16, and drastically decrease the mechanical work while running15. In addition to shorter medial phalanges, H. naledi also exhibits an elongated tibia59, which has been demonstrated to significantly positively correlate with optimal walking speeds60. At the same time, the curvature of the pedal phalanges, in addition to other primitive features of the upper limb, suggest that H. naledi was likely engaging in locomotor grasping with a human-proportioned medial pedal column. An implication of the results is that the lateral side of the foot might have been more effective for pedal grasping rather than the medial side. Lateral forefoot grasping could represent a hominin strategy for limited climbing given the loss of an opposable, grasping hallux. Future directions of this research include comparing these H. naledi pedal proportions to those of additional primate samples to better understand the lateral pedal morphology of H. naledi.
A foot with a combination of traits like that of H. naledi has not previously been observed in the fossil record. Because of the paucity of pedal material in early hominins, the ancestral foot of Homo is unknown. The foot of H. floresiensis has been hypothesised to represent the primitive condition of the genus Homo with curved and elongated proximal phalanges. Both H. floresiensis digit proportions are larger than inferred for H. naledi and are additionally outside the modern human distribution. H. naledi toe proportions are different from those of H. floresiensis, while both species suggest deep phylogenetic placement in the genus Homo. Without knowing the proportions in H. erectus, it is unclear as to which pedal form, if either, represents the ancestral form to H. erectus and later Homo.
Acknowledgements
We thank John Hawks, Lee Berger, Bernhard Zipfel, Trenton Holliday, Karen Strier, Travis Pickering, Richard McFarland, Karen Steudel and Aaron Sams for their comments regarding this analysis and suggestions to improve this manuscript. We thank Thomas Cody Prang for providing the chimpanzee comparative data and the anonymous reviewers whose input improved the manuscript. We also thank the senior graduate students in the biological section of the Department of Anthropology at UW-Madison, the Social Science Computing Center at UW-Madison, Sara Throckmorton, the Arkansas College of Osteopathic Medicine, and the Evolutionary Studies Institute at the University of the Witwatersrand.
Authors' contributions
S.T.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - initial draft; writing - revisions. M.B.: Methodology; data analysis; validation. Z.T.: Conceptualisation; data collection; sample analysis; writing - revisions.
References
1.Ruff CB, Burgess ML, Ketcham RA, Kappelman J. Limb bone structural proportions and locomotor behavior in A.L. 288-1 ('Lucy'). PLoS ONE. 2016;11(11), e0166095, 26 pages. http://dx.doi.org/10.1371/journal.pone.0166095 [ Links ]
2.Russo GA, Kirk EC. Foramen magnum position in bipedal mammals. J Hum Evol. 2013;65(5):656-670. http://dx.doi.org/10.1016/j.jhevol.2013.07.007 [ Links ]
3.Haile-Selassie Y, Saylor BZ, Deino A, Levin NE, Alene M, Latimer BM. A new hominin foot from Ethiopia shows multiple Pliocene bipedal adaptations. Nature. 2012;483(7391):565-569. http://dx.doi.org/10.1038/nature10922 [ Links ]
4.Gebo DL, Schwartz GT. Foot bones from Omo: Implications for hominid evolution. Am J Phys Anthropol. 2006;129(4):499-511. http://dx.doi.org/10.1002/ajpa.20320 [ Links ]
5.Jungers WL, Harcourt-Smith WE, Wunderlich RE, Tocheri MW, Larson SG, Sutikna T, et al. The foot of Homo floresiensis. Nature. 2009;459(7243):81-84. http://dx.doi.org/10.1038/nature07989 [ Links ]
6.Wood BA. Olduvai Bed I post-cranial fossils: A reassessment. J Hum Evol. 1974;3(5):373-378. http://dx.doi.org/10.1016/0047-2484(74)90199-7 [ Links ]
7.Leakey MD, Hay RL. Pliocene footprints in the Laetoli Beds at Laetoli, northern Tanzania. Nature. 1979;278(5702):317-323. http://dx.doi.org/10.1038/278317a0 [ Links ]
8.Bennett MR, Harris JW, Richmond BG, Braun DR, Mbua E, Kiura P, et al. Early hominin foot morphology based on 1.5-million-year-old footprints from Ileret, Kenya. Science. 2009;323(5918):1197-1201. http://dx.doi.org/10.1126/science.1168132 [ Links ]
9.Masao FT, Ichumbaki EB, Cherin M, Barili A, Boschian G, Iurino DA, et al. New footprints from Laetoli (Tanzania) provide evidence for marked body size variation in early hominins. eLife. 2016;5, e19568, 29 pages. http://dx.doi.org/10.7554/eLife.19568 [ Links ]
10.Schultz AH. Relations between the lengths of the main parts of the foot skeleton in primates. Folia Primatol. 1963;1(3-4):150-171. http://dx.doi.org/10.1159/000165791 [ Links ]
11.Aiello L, Dean C. An introduction to human evolutionary anatomy. London: Academic Press; 1990. [ Links ]
12.Midlo C. Form of hand and foot in primates. Am J Phys Anthropol. 1934;19(3):337-389. http://dx.doi.org/10.1002/ajpa.1330190314 [ Links ]
13.Indjeian VB, Kingman GA, Jones FC, Guenther CA, Grimwood J, Schmutz J, et al. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell. 2016;164(1):45-56. http://dx.doi.org/10.1016/j.cell.2015.12.007 [ Links ]
14.Susman RL, Stern Jr JT, Jungers WL. Arboreality and bipedality in the Hadar hominids. Folia Primatol. 1984;43(2-3):113-156. http://dx.doi.org/10.1159/000156176 [ Links ]
15.Rolian C, Lieberman DE, Hamill J, Scott JW, Werbel W. Walking, running and the evolution of short toes in humans. J Exp Biol. 2009;212(5):713-721. http://dx.doi.org/10.1242/jeb.019885 [ Links ]
16.Stern Jr JT, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am J Phys Anthropol. 1983;60(3):279-317. http://dx.doi.org/10.1002/ajpa.1330600302 [ Links ]
17.Trinkaus E, Hilton CE. Neandertal pedal proximal phalanges: Diaphyseal loading patterns. J Hum Evol. 1996;30(5):399-425. http://dx.doi.org/10.1006/jhev.1996.0035 [ Links ]
18.Harcourt-Smith WEH. The origins of bipedal locomotion. In: Henke WHC, Tattersall I, editor. Handbook of paleoanthropology. New York: Springer; 2007. p. 1483-1518. http://dx.doi.org/10.1007/978-3-642-39979-4_48 [ Links ]
19.Pontzer H, Rolian C, Rightmire GP, Jashashvili T, De León MS, Lordkipanidze D, et al. Locomotor anatomy and biomechanics of the Dmanisi hominins. J Hum Evol. 2010;6:492-504. http://dx.doi.org/10.1016/j.jhevol.2010.03.006 [ Links ]
20.Lovejoy CO, Suwa G, Spurlock L, Asfaw B, White TD. The pelvis and femur of Ardipithecus ramidus: The emergence of upright walking. Science. 2009;326(5949):71-71e6. http://dx.doi.org/10.1126/science.1175831 [ Links ]
21.Haile-Selassie Y. Late Miocene hominids from the middle Awash, Ethiopia. Nature. 2001;412(6843):178-181. http://dx.doi.org/10.1038/35084063 [ Links ]
22.Day MH, Napier JR. Fossil foot bones. Curr Anthropol. 1965;6(4):419-420. http://dx.doi.org/10.1086/200626 [ Links ]
23.Latimer BM, Lovejoy CO, Johanson DC, Coppens Y. Hominid tarsal, metatarsal, and phalangeal bones recovered from the Hadar Formation: 1974-1977 collections. Am J Phys Anthropol. 1982;57(4):701-719. http://dx.doi.org/10.1002/ajpa.1330570412 [ Links ]
24.Stern JT. Climbing to the top: A personal memoir of Australopithecus afarensis. Evol Anthropol. 2000;9(3):113-133. http://dx.doi.org/10.1002/1520-6505(2000)9:3%3C113::AID-EVAN2%3E3.3.CO;2-N [ Links ]
25.Preuschoft H. Functional anatomy of the lower extremity. In: Bourne G, editor. The chimpanzee. Vol. 3. Basel: Karger; 1970. p. 221-294. [ Links ]
26.Congdon KA. Interspecific and ontogenetic variation in proximal pedal phalangeal curvature of great apes (Gorilla gorilla, Pan troglodytes, and Pongo pygmaeus). Int J Primatol. 2012;33(2):418-427. http://dx.doi.org/10.1007/s10764-012-9590-7 [ Links ]
27.Kivell TL, Deane AS, Tocheri MW, Orr CM, Schmid P, Hawks J, et al. The hand of Homo naledi. Nat Commun. 2015;6, Art. #8431, 9 pages. http://dx.doi.org/10.1038/ncomms9431 [ Links ]
28.Harcourt‐Smith WEH, Aiello LC. Fossils, feet and the evolution of human bipedal locomotion. J Anat. 2004;204(5):403-416. http://dx.doi.org/10.1111/j.0021-8782.2004.00296.x [ Links ]
29.Ward CV. Interpreting the posture and locomotion of Australopithecus afarensis: Where do we stand? Am J Phys Anthropol. 2002;119(S35):185-215. http://dx.doi.org/10.1002/ajpa.10185 [ Links ]
30.Tuttle RH, Webb DM, Baksh M. Laetoli toes and Australopithecus afarensis. J Hum Evol. 1991;6(3):193-200. http://dx.doi.org/10.1007/BF02438142 [ Links ]
31.Fuss FK, Tan MA, Niegl G. Arboreality is reflected in the phalangeal curvature: Biomechanical and mathematical evidence. J Comp Hum Biol. 2009;60(3):269. http://dx.doi.org/10.1016/j.jchb.2009.02.047 [ Links ]
32.White TD, Suwa G. Hominid footprints at Laetoli: Facts and interpretations. Am J Phys Anthropol. 1987;72(4):485-514. http://dx.doi.org/10.1002/ajpa.1330720409 [ Links ]
33.Haile-Selassie Y, Latimer BM, Alene M, Deino AL, Gibert L, Melillo SM, et al. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci USA. 2010;107(27)12121-12126. https://doi.org/10.1073/pnas.1004527107 [ Links ]
34.Clarke RJ, Tobias PV. Sterkfontein Member 2 foot bones of the oldest South African hominid. Science. 1995;269(5223):521. http://dx.doi.org/10.1126/science.7624772 [ Links ]
35.Jungers WL, Larson SG, Harcourt-Smith W, Morwood MJ, Sutikna T, Awe RD, et al. Descriptions of the lower limb skeleton of Homo floresiensis. J Hum Evol. 2009;57(5):538-554. http://dx.doi.org/10.1016/j.jhevol.2008.08.014 [ Links ]
36.Dirks PH, Roberts EM, Hilbert-Wolf H, Kramers JD, Hawks J, Dosseto A, et al. The age of Homo naledi and associated sediments in the Rising Star Cave, South Africa. eLife. 2017;6, e24231, 59 pages. http://dx.doi.org/10.7554/eLife.24231 [ Links ]
37.Berger LR, Hawks J, De Ruiter DJ, Churchill SE, Schmid P, Delezene LK, et al. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. eLife. 2015;4, e09560, 35 pages. http://dx.doi.org/10.7554/eLife.09560 [ Links ]
38.Hawks J, Elliott M, Schmid P, Churchill SE, De Ruiter DJ, Roberts EM, et al. New fossil remains of Homo naledi from the Lesedi Chamber, South Africa. eLife. 2017;4, e24232, 63 pages. http://dx.doi.org/10.7554/eLife.24232 [ Links ]
39.Dirks PH, Berger LR, Roberts EM, Kramers JD, Hawks J, Randolph-Quinney PS, et al. Geological and taphonomic context for the new hominin species Homo naledi from the Dinaledi Chamber, South Africa. eLife. 2015;4, e09561, 37 pages. http://dx.doi.org/10.7554/eLife.09561 [ Links ]
40.Harcourt-Smith WE, Throckmorton Z, Congdon KA, Zipfel B, Deane AS, Drapeau MSM, et al. The foot of Homo naledi. Nat Commun. 2015;6, Art. #8432, 8 pages. http://dx.doi.org/10.1038/ncomms9432 [ Links ]
41.Marchi D, Walker CS, Wei P, Holliday TW, Churchill SE, Berger LR, et al. The thigh and leg of Homo naledi. J Hum Evol. 2017;104:174-204. http://dx.doi.org/10.1016/j.jhevol.2016.09.005 [ Links ]
42.Feuerriegel EM, Green DJ, Walker CS, Schmid P, Hawks J, Berger LR, et al. The upper limb of Homo naledi. J Hum Evol. 2017;104:155-173. http://dx.doi.org/10.1016/j.jhevol.2016.09.013 [ Links ]
43.Kivell TL. Evidence in hand: recent discoveries and the early evolution of human manual manipulation. Phil Trans R Soc B. 2015;370(1682), Art. #20150105, 11 pages. http://dx.doi.org/10.1098/rstb.2015.0105 [ Links ]
44.Susman RL. Evolution of the human foot: Evidence from Plio-Pleistocene hominids. Foot Ankle Int. 1983;3(6):365-376. http://dx.doi.org/10.1177/107110078300300605 [ Links ]
45.Manly BF. Randomization, bootstrap and Monte Carlo methods in biology. New York: Chapman & Hall/CRC; 2006. [ Links ]
46.Rolian C, Gordon AD. Reassessing manual proportions in Australopithecus afarensis. Am J Phys Anthropol. 2013;152(3):393-406. http://dx.doi.org/10.1002/ajpa.22365 [ Links ]
47.Agoada D. The relationship between linear osteological and radiographic measurements of the human calcaneus and talus. 2018;301(1):21-33. http://dx.doi.org/10.1002/ar.23697 [ Links ]
48.Johanson DC, Taieb M, Gray BT, Coppens Y. Geological framework of the Pliocene Hadar Formation (Afar, Ethiopia) with notes on paleontology including hominids. Geological Society, London, Special Publications. 1978;6(1):549-564. http://dx.doi.org/10.1144/GSL.SP.1978.006.01.37 [ Links ]
49.Venkataraman VV, Rolian C, Gordon AD, Patel BA. A resampling approach and implications for estimating the phalangeal index from unassociated hand bones in fossil primates. Am J Phys Anthropol. 2013;151(2):280-289. http://dx.doi.org/10.1002/ajpa.22278 [ Links ]
50.Lockwood CA, Richmond BG, Jungers WL, Kimbel WH. Randomization procedures and sexual dimorphism in Australopithecus afarensis. J Hum Evol. 1996;31(6):537-548. http://dx.doi.org/10.1006/jhev.1996.0078 [ Links ]
51.Green DJ, Gordon AD. Metacarpal proportions in Australopithecus africanus. J Hum Evol. 2008;54(5):705-719. http://dx.doi.org/10.1016/j.jhevol.2007.10.007 [ Links ]
52.Team RC. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2013. [ Links ]
53.Hall BK. Homology and embryonic development. In: Hecht MH, Macintyre RJ, Clegg MT, editor. Evolutionary biology. New York: Springer; 1995. p. 1-37. http://dx.doi.org/10.1007/978-1-4615-1847-1_1 [ Links ]
54.Young NM, HallgrÍmsson B. Serial homology and the evolution of mammalian limb covariation structure. Evolution. 2005;59(12):2691-2704. http://dx.doi.org/10.1554/05-233.1 [ Links ]
55.Von Dassow G, Munro E. Modularity in animal development and evolution: Elements of a conceptual framework for EvoDevo. J Exp Zool. 1999;285(4):307-325. http://dx.doi.org/10.1002/(SICI)1097-010X(19991215)285:4%3C307::AID-JEZ2%3E3.0.CO;2-V [ Links ]
56.Rolian C, Lieberman DE, Hallgrímsson B. The coevolution of human hands and feet. Evolution. 2010;64(6):1558-1568. http://dx.doi.org/10.1111/j.1558-5646.2009.00944.x [ Links ]
57.Rolian C. The role of genes and development in the evolution of the primate hand. In: Kivell TL, Lemlin P, Richmond BG, Schmitt D, editors. The evolution of the primate hand. New York: Springer; 2016. p. 101-130. http://dx.doi.org/10.1007/978-1-4939-3646-5_5 [ Links ]
58.Hashimoto T, Ueno K, Ogawa A, Asamizuya T, Suzuki C, Cheng K, et al. Hand before foot? Cortical somatotopy suggests manual dexterity is primitive and evolved independently of bipedalism. Phil Trans R Soc B. 2013;368(1630), Art. #20120417, 12 pages. http://dx.doi.org/10.1098/rstb.2012.0417 [ Links ]
59.Walker CS, Desilva JM, Holliday TW, Marchi D, Garvin HM, Zachary C, et al. Relative length of the immature Homo naledi tibia UW 101-1070: Evidence for elongation of the leg. Paper presented at: 85th Annual Meeting of the American Association of Physical Anthropologists; 2016 April 15; Atlanta, GA, USA. Am J Phys Anthropol; 2016:326. [ Links ]
60.Wall-Scheffler CM. Energetics, locomotion, and female reproduction: Implications for human evolution. Annu Rev Anthropol. 2012;41:71-85. http://dx.doi.org/10.1146/annurev-anthro-092611-145739 [ Links ]
 Correspondence:
Correspondence:
Sarah Traynor
Email: setraynor@wisc.edu
Received: 09 Mar. 2018
Revised: 12 Oct. 2018
Accepted: 29 Jan. 2019
Published: 29 May 2019
EDITOR: Maryna Steyn
FUNDING: Arkansas College of Osteopathic Medicine (USA)














