Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.5-6 Pretoria Mai./Jun. 2019
http://dx.doi.org/10.17159/sajs.2019/5534
INVITED REVIEW ARTICLE
Colourful chemistry of South African latrunculid sponges
Michael T. Davies-ColemanI; Edith M. AntunesI; Denzil R. BeukesII; Toufiek SamaaiIII, IV
IDepartment of Chemistry, University of the Western Cape, Cape Town, South Africa
IISchool of Pharmacy, University of the Western Cape, Cape Town, South Africa
IIIDepartment of Environmental Affairs (Oceans and Coasts), Cape Town, South Africa
IVDepartment of Biodiversity and Conservation Biology, University of the Western Cape, Cape Town, South Africa
ABSTRACT
Marine sponges - in common with many other sessile marine invertebrates seemingly devoid of obvious physical forms of defence against predators, e.g. spines or shells - are the sources of a diverse array of organic chemical compounds known as marine natural products or secondary metabolites. Recent research has indicated that the production of natural products via cellular secondary metabolic pathways in some sponge species may not occur within the sponge cells themselves, but rather in microbial endosymbionts which inhabit the surface and interstitial spaces within the sponge tissue. Regardless of their biosynthetic origin, the bioactivity, e.g. toxicity, of many of these marine natural products may be utilised by sponges as chemical feeding deterrents to discourage predation or to provide a chemical anti-fouling competitive edge in the intense competition for living space amongst filter-feeders on space-limited benthic reefs. Paradoxically, a small number of sponge natural products have serendipitously shown potential as new pharmaceuticals, e.g. novel anti-cancer drugs. Marine biodiscovery (or bioprospecting) is the search for new pharmaceuticals from marine organisms. Exploration of the taxonomy, natural products chemistry and biomedicinal potential of the rich diversity of South African latrunculid sponges (family Latrunculiidae), at Rhodes University, the South African Department of Environmental Affairs and the University of the Western Cape has continued unabated for over a quarter of a century as part of a collaborative marine biodiscovery programme. A short review of this multidisciplinary latrunculid sponge research is presented here.
SIGNIFICANCE:
• Research into the taxonomy, chemistry and microbiology of latrunculid sponges is the most comprehensive, multidisciplinary investigation of any group of African marine sponges.
• The potent cytotoxicity of the pyrroloiminoquinone alkaloid pigments isolated from latrunculid sponges may have biomedical applications.
• This review underlines the importance of conserving and protecting South Africa's unique marine invertebrate resources.
Keywords: marine sponges; Latrunculiidae; sponge taxonomy; marine alkaloids; bioactivity
Introduction
The first marine biodiscovery programme centred at a South African university was initiated by a SCUBA collection of approximately a dozen marine sponges by a team of Rhodes University ichthyologists, from a sub-tidal reef in the Tsitsikamma National Park, situated on the Southern Cape coast of South Africa during the spring of 1990. This small collection of marine sponges unearthed a single specimen of a dark brown coloured sponge (Figure 1). This sponge was identified by Samaai and Kelly as Tsitsikamma favus, the first species from a new genus in the family Latrunculiidae Topsent, 1922.1 Subsequent detailed studies of the taxonomy, marine natural products chemistry and marine microbiology of T. favus formed the basis of the first PhD studies at the University of the Western Cape in sponge taxonomy2, and in marine sponge natural products chemistry3-5 and marine sponge microbiology6 at Rhodes University.
Biodiversity of South African latrunculid sponges
In the southern hemisphere, sponges belonging to the family Latrunculiidae (Demospongia, Poecilosclerida; Figure 2) are commonly found on exposed coastal environments attached to hard rocky substrata (<50 m depth) in the cold, inshore, upwelled water environments around South Africa, New Zealand, southwestern Australia and Tasmania.7 The family Latrunculiidae was retained in the order Poecilosclerida, following a recent revised proposal for the classification of the Demospongiae (Figure 2).8 The family comprises seven genera7, of which four (Latrunculia, Cyclacanthia, Strongylodesma and Tsitsikamma) include species that are endemic to the Agulhas ecoregion of South Africa9. Tsitsikamma and Cyclacanthia, known from six species respectively7, are genera endemic to South Africa. The coastal regions of South Africa are recognised as a global hotspot of latrunculid sponge biodiversity.1,10
The pioneering, collaborative, marine invertebrate biodiscovery expeditions, coordinated from Rhodes University (1990-2004),10 revealed for the first time the significant latrunculid sponge diversity off South Africa. The continual discovery of new latrunculid species and genera from these expeditions fuelled increasing interest in these sponges and ultimately led to a taxonomic revision of the family Latrunculiidae in parallel with the natural products chemistry and bioactivity studies of the isolated natural products.1,2,11,12 Four genera - Latrunculia, Sceptrella, Strongylodesma and Tsitsikamma - were recognised in this revision (Figure 2). The genus Tsitsikamma is endemic to South Africa.13 The identification of sponges belonging to this family was predominantly based on a number of key morphological features, including, in most genera, the presence of the characteristic silica-based discorhabd microscleres which differ structurally between the different genera (Figure 3).
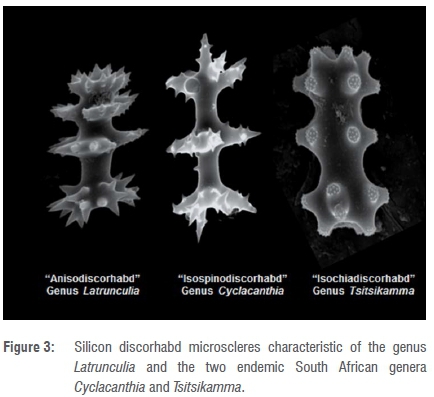
However, the genus Strongylodesma is an enigma within the family Latrunculiidae, because species within this genus do not contain these distinctive discorhabd microscleres.13 Shortly after this taxonomic revision was published, the family Latrunculiidae was further expanded to include another new endemic South African genus, Cyclacanthia, which incorporated three new species collected from the Agulhas ecoregion (C. bellae, C. cloverlyae and C. mzimayensis).14 The type specimen for this genus, C. bellae, described from Algoa Bay, was originally misidentified as L. bellae (Samaai and Kelly, 2003) and placed in the genus Latrunculia.13 Interestingly, Cyclacanthia differs from the genus Latrunculia, inter alia in ontogeny, morphology and presence of isospinodiscorhabd microscleres, in which there are three major whorls of projections, as opposed to the four characteristic of the anisodiscorhabds of the genus Latrunculia (Figure 3).15
The genus Latrunculia, comprising some 30 species of which over a half occur in the southern hemisphere, was further expanded to include another three new South African species: L. algoaensis, collected from Algoa Bay and L. gotzi and L. kerwathi collected from deep water reefs situated on the Agulhas continental shelf off South Africa.16 Latrunculid sponges from the genus Sceptrella are also deep water sponges; however, no representatives of this genus have yet been discovered off the South African coast and this genus appears confined to the northern and western Atlantic Ocean.1,17,18 The genera Bomba and Latrunclava have recently been added to the family Latrunculiidae, and representatives of these two genera are similarly not known to occur off southern Africa.7,18 A list of the new latrunculid sponges species collected off South Africa, their sites of collection, and references to their published taxonomic data are presented in Table 1.
Pyrroloiminoquinone alkaloids isolated from South African latrunculid sponges
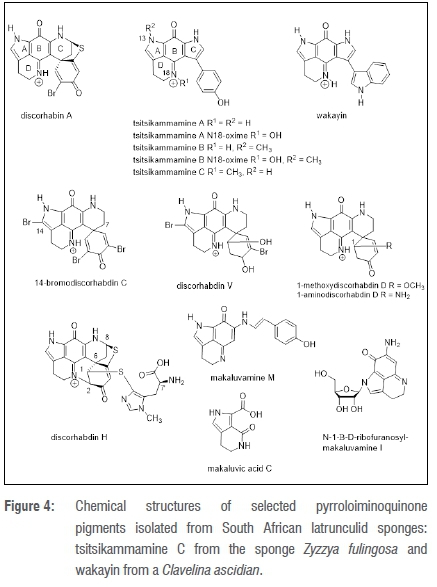
The general name pyrroloiminoquinone refers to the central chemical structural motif common to the group of alkaloid secondary metabolites isolated from sponges belonging to the families Latrunculiidae and Acarnidae (Figure 2).21 The pyrroloiminoquinone structure is characterised by a five-membered pyrrole ring (A) fused to a six-membered iminoquinone moiety (B), as typified by the chemical structure of the ubiquitous discorhabdin A (Figure 4).
Biosynthetically, the pyrroloiminoquinone motif is derived from tryptophan via decarboxylation and oxidative cyclisation. Subsequent addition of tyramine to a putative tricyclic pyrroloimininoquinone intermediate, affords a group of alkaloids known as the makaluvamines, which may be further elaborated via oxidative, intramolecular cyclisation to yield the discorhabdins and tsitsikammamines (Scheme 1).22
The name 'discorhabdin' originates from the name given to the discorhabd microscleres used by sponge taxonomists to identify latrunculid sponges, and was given to this group of marine natural products by Munro and colleagues, who isolated and identified the first discorhabdin compounds from a New Zealand Latrunculia sponge in 1986.23 Varying in colour from red and orange to dark green, pyrroloiminoquinone metabolites have been shown to be concentrated in the outer 0-2 mm of sponge tissue in the dark green or brown coloured sponges in which they are produced.24 Accordingly, pyrroloiminoquinone metabolites serve a dual purpose in latrunculid sponges, both as pigments and, because of their potent cytotoxicity and general bioactivity vide infra, as feeding deterrent compounds to deter predators.24
The isolation of the highly polar pyrroloiminoquinone compounds from methanolic extracts of marine sponges, and the subsequent determination of the chemical structures of these compounds, is not trivial. Nuclear magnetic resonance (NMR) spectroscopy is the pre-eminent spectroscopic technique used to elucidate the chemical structures of natural products. In the contemporary NMR spectroscopy toolbox, inverse detection, two-dimensional NMR spectroscopy experiments, e.g. heteronuclear single quantum coherence, heteronuclear multiple bond correlation are among the most useful to establish the chemical structure of an unknown organic compound.25 These experiments are designed to define the position in the chemical structure of magnetically insensitive nuclei with a low relative abundance and gyromagnetic ratio, e.g. carbon nuclei (13C), via a radio frequency pulse sequence that exploits the highly magnetically sensitive surrounding hydrogen protons (1H). 1H has an approximate four-fold greater gyromagnetic ratio compared to 13C and is normally more than twice as abundant as carbon atoms in organic compounds. Unfortunately, the relative paucity of hydrogen atoms in pyrroloiminoquinones in comparison with carbon atoms, as reflected, for example, by the molecular formula of discorhabdin A (C18H15BrN3O2S), often hampers the structure elucidation of these complex, highly conjugated, polycyclic compounds by NMR spectroscopy and thus provides an intriguing challenge for marine natural product chemists.
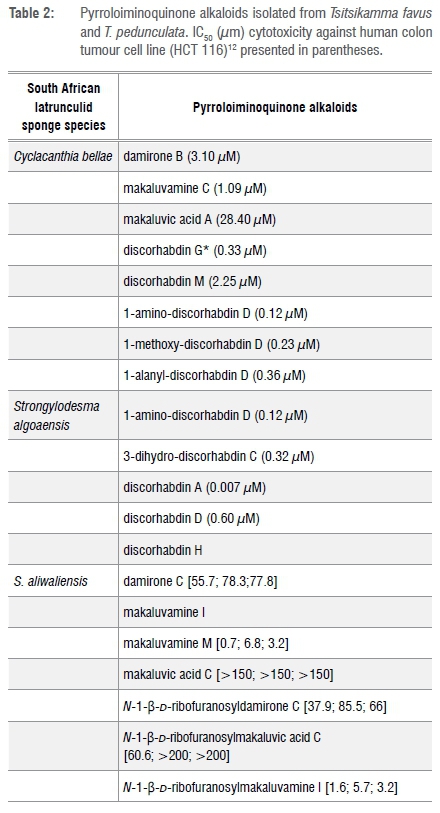
Over 100 marine pyrroloiminquinone natural products have been isolated from marine organisms collected from across the globe and the chemistry and bioactivities of this group of compounds have been regularly reviewed in the chemistry literature.21,26-28 Of the pyrroloiminoquinones isolated thus far from marine sponges, 30 (of which 16 were new compounds) have been isolated from five South African latrunculid sponge species: T. favus12,29, T. pedunculata12, C. bellae (formerly L. bellae)12, S. algoaensis12 and S. aliwalensis30,31 (Figures 1 and 5), and the names of these compounds are presented in Tables 2 and 3. Selected compounds from Tables 2 and 3, with novel chemical structural features observed for the first time in this group of compounds, are presented in Figure 4 and the significance of their discovery will be briefly discussed here.

Tsitsikammamine A and B were isolated from a 1:1 methanol:chloroform extract of T. favus (Figure 1) specimens collected from the Tsitsikamma National Park, along with the first discorhabdin analogues substituted at C-14 e.g. 14-bromodiscorhabdin C (Figure 4).29 The tsitsikammamines were the first bis-pyrroloiminoquinone (with two pyrrole rings A and C fused to either side of the central iminoquinone ring B) to be isolated from a sponge29, but were not the first compounds from this structural class to be isolated from a marine organism. Wakayin, which also possesses a bis-pyrroloiminoquinone structural scaffold (Figure 4), had previously been isolated from an ascidian, Clavelina sp.32 The biosynthesis of marine natural products, in common with the biosynthesis of terrestrial plant natural products, is not random and the chemical structures of the major natural products usually reflect family and genus structural generalities and species specificity. Therefore, the occurrence of natural products with almost identical chemical structures in two different marine phyla is unexpected and may be a possible indicator of a shared microbial endosymbiont primary producer of the natural product(s), as opposed to serendipitous convergent evolution of secondary metabolic pathways in phyletic disparate marine organisms. In an effort to unequivocally establish the biosynthetic origin of the tsitsikammamines, a detailed community structure analysis of the symbiotic bacteria found in T. favus sponges collected from Algoa Bay is currently underway at Rhodes University.9,33 The microbial community analyses are coupled to a metagenomic search for microbial biosynthetic gene clusters that might ultimately be responsible for the biosynthesis of the tsitsikammamines.
The structural novelty and bioactivity of the tsitsikammamines has attracted interest from synthetic chemists and tsitsikammamine A was first synthesised by Delfourne and co-workers.34 A follow-up natural products study of Tsitsikamma National Park specimens of T. favus afforded two further minor metabolite analogues of the tsitsikammamines, both of which contain an N18-oxime moiety (Figure 4).12 Marine natural products possessing either N-oxides or N-oximes moieties are rare and these two are the only examples of N-oximes amongst the known pyrroloiminoquinone metabolites. The closely related sponge, T. pedunculata (Figure 5a), collected from Algoa Bay yielded the same suite of discorhabdin compounds as that found in T. favus, in addition to four new minor discorhabdin metabolites also with C-14 substituents, e.g. discorhabdin V (Figure 4).12 Interestingly, the tsitsikammamines were not present in extracts of T. pedunculata, and are therefore not genus-specific chemotaxonomic markers as tentatively initially proposed.29
Natural product investigations of two other Algoa Bay latrunculid sponges, C. bellae (Figure 5b) and S. algoaensis (Figure 5c), yielded a further 11 pyrroloiminoquinone pigments, including the known compound discorhabdin A and makaluvamine C and two new compounds, namely 1-methoxydiscorhabdin D and 1-aminodiscorhabdin D from the specimens of the former species (Figure 4).12 Interestingly, discorhabdin H (Figure 4) isolated from S. algoaensis had also been previously identified from L. lunaviridis and L. microcanthoxea.4 The isolation of discorhabdin H provided an opportunity to unequivocally establish the absolute configuration of this compound. Degradative hydrolysis of the (-)-discorhabdin H, followed by comparative chiral gas chromatographic analysis of derivatised hydrolysis products with the equivalent derivative of L-histidine, confirmed the 7'S configuration of the C-1 thiomethylhistidine residue.12 The configuration of the remaining four chiral centres in discorhabdin were established, in collaboration with Copp in New Zealand, as 1R, 2R, 6R, 8S (Figure 4) by comparison of the electronic circular dichroism spectrum of (-)-discorhabdin H with that of a closely related model compound, discorhabin L, to which the application of time-dependent density functional theory calculations had been used to unequivocally establish the absolute configuration of this latter compound.35
A second Strongylodesma species, S. aliwaliensis (Figure 5d), collected from Aliwal Shoal, a sub-tropical reef system off Umkomaas in southern KwaZulu-Natal, South Africa, afforded three known pyrroliminoquinone compounds: e.g. makaluvamine M, a new pyrroloquinoline, makaluvic acid C, and three novel N-β-D-ribofuranosyl substituted pyrroloiminoquinone and pyrroloquinoline metabolites e.g. N-β-D-ribofuranosylmakaluvamine I (Figure 4).30,31 The sugar moiety in the latter three compounds was identified by chiral gas chromatography comparison of the peracetylated aldononitrile derivative of the acid hydrosylate derived from each compound with a similarly peracetylated aldononitrile derivative of D-ribose. The three ribofuranosyl-containing metabolites from S. alwaliensis remain the only known pyrroloiminoquinone glycosides isolated thus far from marine organisms.
Biomedicinal potential of pyrroloiminoquinone alkaloids
The impressive bioactivity of pyrroloiminoquinone metabolites, consistent with their ecological role as feeding deterrents in sponges vide supra, is continuously reported in the chemistry literature. Pyrroloiminoquinones have been shown to exhibit cytotoxicity in a variety of whole-cell biomedicinal screening programmes, including screens designed to discover new chemical entities with anti-cancer, anti-malaria and anti-microbial activity.28,36,37 In addition to whole cell screens, the bioactivity of marine pyrroloiminoquinones against medicinally relevant enzyme targets is also of interest.28,38,39 The isolation of a large cohort of pyrroloiminoquinone metabolites from four species of South African latrunculid sponges provided a unique opportunity to explore the cytotoxicity (Tables 2 and 3) and structure activity relationship of 20 of these compounds against a human colon tumour cell line (HCT-116).12 Discorhabdin A, with an IC50 of 7 nM, was the most active compound against this cancer cell line.12 The tetracyclic pyrroloiminoquinone core structure was found to be essential for activity, while a Δ7 olefin and substitution at C-1 in discorhabdins were found to enhance cytotoxicity.12 A subsequent screening of the S. aliwaliensis metabolites against oesophageal cancer cell lines (WHCO1, WHCO6 and KYSE30; Table 3) revealed that makaluvamine M (Figure 4) was the most cytotoxic compound amongst those isolated from S. aliwaliensis.40
Research into the biomedicinal potential and the possible mechanisms of cancer cell cytotoxicity of the tsitsikammamines has not abated. We initially established that tsitsikammamine A's inhibition of the topoisomerase I enzyme, responsible for catalysing the relaxation of super-coiled DNA, and this compound's DNA binding affinity were both comparable with that observed for the structurally homologous wakayin.12 In a subsequent comprehensive medicinal chemistry study, based around the chemical structure of tsitsikammamine A, Delfourne and co-workers synthesised both tsitsikammamine A34 and a series of 43 analogues of this South African latrunculid sponge natural product41,42. Two of these analogues, in which pyrrole ring C was exchanged for a pyrazole ring, exhibited improved topoisomerase I inhibitory activity cf tsitsikammamine A41, while a ring D-opened, bis-pyrroloquinone analogue of tsitsikammamine A exhibited low μM in vitro growth inhibitory (IC50) cytotoxicity against three different cancer cell lines42. The ensuing isolation of tsitsikammamine C43 (Figure 4) from an Australian sponge Zyzzya sp. (family Acarnidae; Figure 2) with potent low nanomolar IC50 in vitro anti-malarial activity against chloroquine-sensitive and chloroquine-resistant Plasmodium falciparum malaria parasites, has opened the door to a new era of tsitsikammamine anti-malarial drug research.44
Conclusion
The taxonomy, chemistry, symbiotic microbial community structure and biomedicinal potential of South African latrunculid sponges continues to attract interest a quarter of a century after the first investigations of the natural products chemistry and cytotoxicity of extracts of T. favus, a widely distributed, endemic subtidal sponge within the Agulhas ecoregion. South Africa's position as a global hotspot of latrunculid sponge biodiversity is well established and the search for new latrunculid sponge species continues. The generally non-specific, potent cytotoxicity of pyrroloiminoquinone metabolites continues to hamper their development as new pharmaceuticals. However, research into new targeted drug delivery systems, incorporating pyrroloiminoquinone compounds and currently underway at the University of the Western Cape, may open new opportunities for marine pyrroloiminoquinone metabolites as possible anti-cancer or anti-malarial drugs.
Acknowledgements
We acknowledge with gratitude the following institutions and research organisations for their financial and material support for the latrunculid sponge project over many years: Rhodes University, South African National Research Foundation, South African Department of Environmental Affairs (Oceans and Coasts), Tsitsikamma National Park, Scripps Institution of Oceanography, Glaxo SmithKline (formerly SmithKline Beecham), US National Cancer Institute, Coral Reef Research Foundation and the University of the Western Cape. We also gratefully acknowledge the numerous colleagues, postdoctoral research fellows and postgraduate students, cited in the references presented in this review, who contributed to the collection of South African latrunculid sponges, and the isolation, identification and bioactivity studies of pyrroloiminoquinone and related metabolites.
Authors' contributions
M.T.D-C. is the leader of the original research group working on South African latrunculid sponges and conceptualised the review and prepared the initial draft. E.M.A. and D.R.B. are key members of the original research group working on latrunculid sponge chemistry and bioactivity and provided critical review and commentary and assisted with the correction and editing of the original draft. T.S. is a key member of the original research group working on latrunculid sponge taxonomy and provided critical review and commentary and assisted with the correction and editing of the original draft.
References
1.Samaai T, Kelly M. Family Latrunculiidae Topsent, 1922. In: Hooper J, Van Soest R, editors. System Porifera: A guide to the classification of sponges. Boston, MA: Springer; 2002. p. 708-719. https://doi.org/10.1007/978-1-4615-0747-5_78 [ Links ]
2.Samaai T. Systematics of the Family Latrunculiidae Topsent (Porifera: Demospongiae) and consideration of the diversity and biogeography of shallow-water sponges of western South Africa [PhD thesis]. Cape Town: University of the Western Cape; 2002. [ Links ]
3.Hooper G. Biologically active natural products from South African marine invertebrates [PhD thesis]. Grahamstown: Rhodes University; 1996. [ Links ]
4.Beukes D. Structural and synthetic studies of South African marine natural products [PhD thesis]. Grahamstown: Rhodes University; 2000. [ Links ]
5.Antunes E. Pyrroloiminoquinone metabolites from South African latrunculid sponges [PhD thesis]. Grahamstown: Rhodes University; 2002. [ Links ]
6.Beckerling T. An investigation into the bacterial diversity associated with South African sponges that produce bioactive secondary metabolites [PhD thesis]. Grahamstown: Rhodes University; 2013. [ Links ]
7.Van Soest RWM, Boury-Esnault N, Hooper JNA, Rützler K, De Voogd NJ, Alvarez B, et al. World Porifera database. Latrunculiidae Topsent, 1922 [database on the Internet]. No date [cited 2019 Feb 26]. Available from: http://www.marinespecies.org/porifera/porifera.php?p=taxdetails&id=131671 [ Links ]
8.Morrow C, Cárdenas P. Proposal for a revised classification of the Demospongiae (Porifera). Front Zool. 2015;12(7):1-27. https://doi.org/10.1186/s12983-015-0099-8 [ Links ]
9.Matcher GF, Waterworth SC, Walmsley TA, Matsatsa T, Parker-Nance S, Davies-Coleman MT, et al. Keeping it in the family: Coevolution of latrunculid sponges and their dominant bacterial symbionts. Microbiol Open. 2017;6:1-13. https://doi.org/10.1002/mbo3.417 [ Links ]
10.Sunassee SN, Davies-Coleman MT. Marine bioprospecting in southern Africa. In: Chibale K, Masimbirembwe C, Davies-Coleman M, editors. Drug discovery in Africa. Heidelberg: Springer-Verlag; 2012. p. 193-210. https://doi.org/10.1007/978-3-642-28175-4_8 [ Links ]
11.Kelly M, Samaai T. Family Podospongiidae de Laubenfels, 1936. In: Hooper J, Van Soest R, editors. Systema Porifera: A guide to the classification of sponges. Boston, MA: Springer; 2002. p. 694-702. https://doi.org/10.1007/978-1-4615-0747-5_75 [ Links ]
12.Antunes EM, Beukes DR, Kelly M, Samaai T, Barrows LR, Marshall KM, et al. Cytotoxic pyrroloiminoquinones from four new species of South African latrunculid sponges. J Nat Prod. 2004;67:1268-1276. https://doi.org/10.1021/np034084b [ Links ]
13.Samaai T, Gibbons MJ, Kelly M. South African Latrunculiidae (Porifera: Demospongiae: Poecilo- sclerida): Descriptions of new species of Latrunculia du Bocage, Strongylodesma Lévi, and Tsitsikamma Samaai & Kelly. Zootaxa. 2003;371:1-26. https://doi.org/10.11646/zootaxa.371.1.1 [ Links ]
14.Samaai T, Govender V, Kelly M. Cyclacanthia n.g. (Demospongiae: Poecilosclerida: Latrunculiidae incertea sedis), a new genus of marine sponges from South African waters, and description of two new species. Zootaxa. 2004;725:1-18. [ Links ]
15.Samaai T, Keyzers R, Davies-Coleman M. A new species of Strongylodesma Lévi, 1969 (Porifera; Demospongiae; Poecilosclerida; Latrunculiidae) from Aliwal Shoal on the east coast of South Africa. Zootaxa. 2004;584:1-11. http://dx.doi.org/10.11646/zootaxa.584.1.1 [ Links ]
16.Samaai T, Janson L, Kelly M. New species of Latrunculia from the Agulhas shelf, South Africa, with designation of a type species for subgenus Biannulata (Demospongia, Poecilosclerida, Latrunculiidae). Zootaxa. 2012;3395:33-45. [ Links ]
17.Schmidt O. Grundzüge einer Spongien-Fauna des Atlantischen Gebietes [Basic features of a spongy fauna of the Atlantic area]. Leipzig: Wilhelm Engelmann; 1870. German. [ Links ]
18.Kelly M, Sim-Smith C, Stone R, Samaai T. New taxa and arrangements within the family Latrunculiidae (Demospongiae, Poecilosclerida). Zootaxa. 2016;4121:1-48. http://dx.doi.org/10.11646/zootaxa.4121.1.1 [ Links ]
19.Samaai T, Gibbons MJ, Kelly M. Revision of the genus Latrunculia du Bocage, 1869 (Porifera: Demospongia: Latrunculiidae) with descriptions of new species from New Caledonia and the Northeastern Pacific. Zootaxa. 2006;1127:1-71. [ Links ]
20.Samaai T, Gibbons MJ, Kelly M. A revision of the genus Strongylodesma Lévi (Porifera: Demospongiae: Latrunculiidae) with descriptions of four new species. J Mar Biol Assoc UK. 2009;89:1689-1702. https://doi.org/10.1017/s0025315409000101 [ Links ]
21.Antunes EM, Copp BR, Davies-Coleman MT, Samaai T. Pyrroloiminoquinone and related metabolites from marine sponges. Nat Prod Rep. 2005;22:62-72. https://doi.org/10.1039/b407299p [ Links ]
22.Lill RE, Major DA, Blunt JW, Munro MHG, Battershill CN, McLean MG, et al. Studies on the biosynthesis of discorhabdin B in the New Zealand sponge Latrunculia sp. B J Nat Prod. 1995;58:306-311. https://doi.org/10.1021/np50116a028 [ Links ]
23.Perry NB, Blunt JW, McCombs JD, Munro MHG. Discorhabdin C, a highly cytotoxic pigment of the genus Latrunculia. J Org Chem. 1986;51:5476-5478. https://doi.org/10.1021/jo00376a096 [ Links ]
24.Furrow FB, Amsler CB, McClintock JB, Baker BJ. Surface sequestration of chemical feeding deterrents in the Antarctic sponge Latrunculia apicalis as an optimal defense against sea star spongivory. Mar Biol. 2003;143:443-449. https://doi.org/10.1007/s00227-003-1109-5 [ Links ]
25.Milanowski DJ, Oku N, Cartner LK, Bokesch HR, Williamson RT, Saurí J, et al. Unequivocal determination of caulamidines A and B: Application and validation of new tools in the structure elucidation tool box. Chem Sci. 2018;9(2):307-314. https://doi.org/10.1039/c7sc01996c [ Links ]
26.Ding Q, Chichak K, Lown W. Pyrroloquinoline and pyridoacridine alkaloids from marine sources. Curr Med Chem. 1999;6:1-27. https://doi.org/10.1002/chin.199918299 [ Links ]
27.Urban S, Hickford S, Blunt JW, Munro MHG. Bioactive marine alkaloids. Curr Org Chem. 2000;4:765-807. https://doi.org/10.2174/1385272003376085 [ Links ]
28.Hu J-F, Fan H, Xiong J, Wu S-B. Discorhabdins and pyrroloiminoquinone-related alkaloids. Chem Rev. 2011;111:5465-5491. https://doi.org/10.1021/cr100435g [ Links ]
29.Hooper G, Davies-Coleman M, Kelly Borges M, Coetzee P. New alkaloids from a South African latrunculid sponge. Tetrahedron Lett. 1996;37:7135-7138. https://doi.org/10.1016/0040-4039(96)01560-2 [ Links ]
30.Keyzers RA, Samaai T, Davies-Coleman MT. Novel pyrroloquinoline ribosides from the South African latrunculid sponge Strongylodesma aliwaliensis. Tetrahedron Lett. 2004;45:9415-9418. https://doi.org/10.1016/j.tetlet.2004.10.106 [ Links ]
31.Keyzers RA, Arendse CE, Hendricks DT, Samaai T, Davies-Coleman MT. Makaluvic acids from the South African latrunculid sponge Strongylodesma aliwaliensis. J Nat Prod. 2005;68:506-510. https://doi.org/10.1021/np049589w [ Links ]
32.Copp B, Ireland C, Barrows L. Wakayin: A novel cytotoxic pyrroloiminoquinone alkaloid from the ascidian Clavelina species. J Org Chem. 1991;56:4596-4597. https://doi.org/10.1021/jo00015a005 [ Links ]
33.Walmsley TA, Matcher GF, Zhang F, Hill RT, Davies-Coleman MT, Dorrington RA. Diversity of bacterial communities associated with the Indian Ocean sponge Tsitsikamma favus that contains the bioactive pyrroloiminoquinones, tsitsikammamine A and B. Mar Biotechnol. 2012;14(6):681-691. https://doi.org/10.1007/s10126-012-9430-y [ Links ]
34.Rives A, Delaine T, Legentil L, Delfourne E. Total synthesis of the marine pyrroloiminoquinone alkaloid tsitsikammamine A. Tetrahedron Lett. 2009;50:1128-1130. https://doi.org/10.1016/j.tetlet.2008.12.078 [ Links ]
35.Grkovic T, Pearce AN, Munro MHG, Blunt JW, Davies-Coleman MT, Copp BR. Isolation and characterization of diastereomers of discorhabdins H and K and assignment of absolute configuration to discorhabdins D, N, Q, S, T, and U. J Nat Prod. 2010;73:1686-1693. https://doi.org/10.1021/np100443c [ Links ]
36.Nag S, Nadkarni D, Qin J-J, Vouganti S, Nguyen T, Xu S, et al. Anticancer activity and molecular mechanisms of action of makaluvamines and analogues. Mol Cell Pharmacol. 2012;4:69-81. [ Links ]
37.Wright C. Searching for new treatments for malaria. In: Heinrich M, Jager AK, editors. Ethnopharmacology. Chichester: Wiley-Blackwell; 2015. p. 123-124. https://doi.org/10.1002/9781118930717 [ Links ]
38.Goey A, Chau C, Sissung T, Cook K, Venzon D, Castro A, et al. Screening and biological effects of marine pyrroloiminoquinones: Potential inhibitors of the HIF1α/p 300 interaction. J Nat Prod. 2016;79:1267-1275. https://doi.org/10.1021/acs.jnatprod.5b00846 [ Links ]
39.Botic J, Defant A, Zanini P, Zuzek M, Frangez R, Janussen D, et al. Discorhabdin alkaloids from Antarctic Latrunculia sponges as a new class of cholinesterase inhibitors. Eur J Med Chem. 2017;136:294-304. https://doi.org/10.1016/j.ejmech.2017.05.019 [ Links ]
40.Whibley C, Keyzers R, Soper A, Davies-Coleman MT, Samaai T, Hendricks D. Antioesophageal cancer activity from southern African marine organisms. Ann N Y Acad Sci. 2005;1056:405-412. https://doi.org/10.1196/annals.1352.031 [ Links ]
41.Legentil L, Benel L, Bertrand V, Lesur B, Delfourne E. Synthesis and antitumour characterization of pyrazolic analogues of the marine pyrroloiminoquinone alkaloids: Wakayin and tsitsikammamines. J Med Chem. 2006;49:2979-2988. https://doi.org/10.1021/jm051247f [ Links ]
42.Rives A, Le Calve B, Delaine T, Legentil L, Kiss R, Delfourne E. Synthesis and antitumor evaluation of analogues of the marine pyrroloiminoquinone tsitsikammamines. Eur J Med Chem. 2010;45:343-351. https://doi.org/10.1016/j.ejmech.2009.10.019 [ Links ]
43.Davis R, Buchanan M, Duffy S, Avery V, Charman S, Charman W, et al. Antimalarial activity of pyrroloiminoquinones from the Australian marine sponge Zyzzya sp. J Med Chem. 2012;55:5851-5858. https://doi.org/10.1021/jm3002795 [ Links ]
44.Feng Y, Campitelli M, Davis R, Quin R. Chemoinformatic analysis as a tool for prioritization of trypanocidal marine derived lead compounds. Mar Drugs. 2014;12:1169-1184. https://doi.org/10.3390/md12031169 [ Links ]
 Correspondence:
Correspondence:
Michael Davies-Coleman
Email: mdavies-coleman@uwc.ac.za
Received: 28 Aug. 2018
Revised: 26 Feb. 2019
Accepted: 28 Feb. 2019
Published: 29 May 2019
EDITOR: John Butler-Adam
FUNDING: Rhodes University; National Research Foundation (South Africa); South African Department of Environmental Affairs: Oceans and Coasts; Tsitsikamma National Park, Scripps Institution of Oceanography; Glaxo SmithKline; US National Cancer Institute; Coral Reef Research Foundation; University of the Western Cape
















