Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.3-4 Pretoria mar./abr. 2019
http://dx.doi.org/10.17159/sajs.2019/5589
SCIENTIFIC CORRESPONDENCE
Parental care or opportunism in South African Triassic cynodonts?
Julien Benoit
Evolutionary Studies Institute, School of Geosciences, University of the Witwatersrand, Johannesburg, South Africa
Keywords: Cynodontia; shelter-sharing; lactation; juvenile mortality; parental care
In a paper published in Nature in 2018, Hoffman and Rowe1 describe the discovery of an adult tritylodontid cynodont, Kayentatherium, from the Jurassic of the Kayenta Formation (Arizona, USA), accompanied by at least 38 perinatal juveniles, all at the same very early stage of development. Such a high number of juveniles in one clutch is found only in a handful of oviparous reptiles, and never in viviparous or ovoviviparous species1, suggesting that these cynodonts laid eggs. As tritylodontids are amongst the closest relatives to Mammaliaformes, and sometimes even reconstructed as their sister clade2, this textbook changing discovery implies that all non-mammaliaform synapsids had an essentially reptilian-like reproductive biology1.
Hoffman and Rowe's1 discovery forces a reappraisal of the interpretation of the rich fossil record of parental care in South African non-mammaliaform cynodonts3.
First, the implications of Hoffman and Rowe's1 discovery for the evolution of parental care and lactation need to be discussed. With at least 38 hatchlings to feed, it can be safely assumed that lactation was not the main source of nutrients in Kayentatherium neonates (nor, by extension, in all more basal non-mammaliaform synapsids). Instead, perinatal juveniles of Kayentatherium already had functional teeth despite their early age, which (1) impedes lactation4 and (2) strongly suggests that they were self-sufficient at birth and capable of foraging nearby vegetation5. Extensive parental care was thus most likely not essential to newborn survival in Kayentatherium. In contrast, early Mammaliaformes are characterised by (1) a diphyodont dentition, which indicates lactation4, and (2) a remarkably smaller body mass compared to their non-mammaliaform cynodont ancestors, which imposes a physical limitation to the size of the eggs and, incidentally, the yolk they contain, thus enforcing parental care and lactation6.
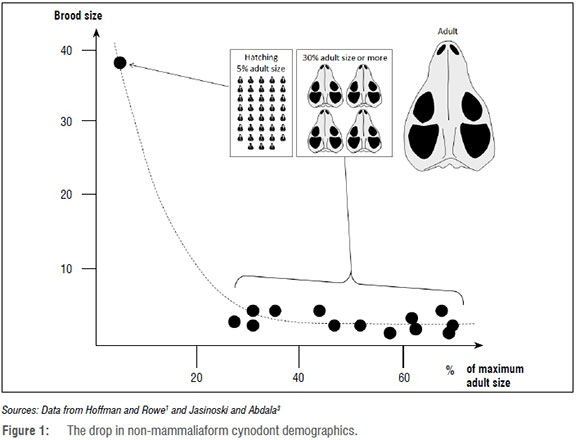
The presence of an adult Kayentatherium next to perinatal juveniles indeed suggests that the eggs were incubated. This observation makes this specimen a landmark for any discussion about the origin of endothermy in mammalian ancestry, but more importantly for this discussion, it also suggests that a mammary gland could have produced a milk-like secretion as in modern monotremes that use their mammary glands to moisturise their leathery eggs to prevent desiccation.7 Therefore, despite the fact that Kayentatherium likely did not suckle its young, the possibility that this taxon possessed an ancestral mammary gland that secreted a moisturising milk-like fluid cannot be excluded, thus not completely invalidating hypotheses that similar structures were already present in tritylodontids.8
The high number of juveniles accompanying the Kayentatherium described by Hoffman and Rowe1 sharply contrasts with the rest of the fossil record of non-mammaliaform cynodont adult-juvenile associations, primarily documented in the South African Karoo genera Galesaurus and Thrinaxodon3. Before Hoffman and Rowe's1 discovery, the maximum number of juvenile non-mammaliaform cynodonts that were found associated with an adult was four3. Similarly, an adult-juvenile association is documented for Heleosaurus, a more basal pelycosaur synapsid, for which four juveniles were found together alongside an adult.9 The Kayentatherium juveniles are substantially smaller, and most likely less mature, than any other juvenile non-mammaliaform cynodonts previously recovered in a parent and offspring association. The Kayentatherium juvenile skull size is about 5% of the maximum adult size for the taxon1, whereas the skull size of the otherwise youngest non-mammaliaform cynodont recorded alongside an adult is 31% of the maximum adult size3. A burrow cast found with three juvenile Langbergia (but no adult) contains an individual with a skull 27% of the maximum adult size for this taxon.3 Juvenile Heleosaurus are 39-54% the size of the associated adult.9 All of these specimens are in a very advanced growth stage compared to the Kayentatherium perinates described by Hoffman and Rowe1. If these demographics are representative of the general condition in non-mammaliaform cynodonts, it means that from the time interval during which juveniles were growing from 5% to about 30% adult size, some 90% of the litter was lost. This high level of early juvenile mortality would further support the absence of lactation and parental care in non-mammaliaform cynodonts. In this case, far from evidencing parental care, the South African fossil record would actually support that non-mammaliaform cynodonts were not extensively taking care of their young beyond incubation.
A revised interpretation
Given that Mammaliaformes is a clade deeply nested within Cynodontia, so far the null hypothesis has historically been to consider the association of juveniles and adults in non-mammaliaform cynodonts as evidence for parental care. However, amongst the 12 specimens that show aggregation of many individuals (one in Kayentatherium, one in Langbergia, eight in Thrinaxodon and two in Galesaurus), only 4 actually include an adult associated with juveniles, whereas 3 show interspecific associations.3,10 Thus, there is nearly as much evidence for parental care in non-mammaliaform cynodonts as there is evidence for interspecific shelter-sharing in the Triassic, advocating a case for the frequent co-occurrence of adults and juveniles actually being the result of opportunistic shelter-sharing (as some mammals do today11) as opposed to parental care. For the smallest individuals of any age or species, aggregation might have been advantageous as it provides protection against predators and extreme temperature changes in the dry and highly seasonal conditions of the Triassic.3,10,12,13 The smallest individuals would be tolerated by the largest ones as they emit more body heat as a result of their high surface to volume ratio, thereby aiding in heat conservation during periods of hibernation or aestivation. Following this hypothesis, these fossils could instead suggest simple mutualistic behaviour rather than elaborate parental care.
Considering the facts presented above, a new interpretation of the aggregation of non-mammaliaform cynodonts is proposed here. Instead of parental care, these associations could instead actually offer evidence that opportunistic inter- and intraspecific shelter-sharing was tolerated as it provided a selective advantage to all participating parties.
Acknowledgements
I thank Viktor Radermacher and Sophie Vrard for proofreading and the NRF African Origin Platform and the DST-NRF Centre of Excellence in Palaeosciences for funding.
References
1.Hoffman EA, Rowe TB. Jurassic stem-mammal perinates and the origin of mammalian reproduction and growth. Nature. 2018;561:104-108. https://doi.org/10.1038/s41586-018-0441-3 [ Links ]
2.Ruta M, Botha-Brink J, Mitchell SA, Benton MJ. The radiation of cynodonts and the ground plan of mammalian morphological diversity. Proc Biol Sci. 2013;280(1769), Art. #20131865, 10 pages. https://doi.org/10.1098/rspb.2013.1865 [ Links ]
3.Jasinoski SC, Abdala F. Aggregations and parental care in the Early Triassic basal cynodonts Galesaurus planiceps and Thrinaxodon liorhinus. PeerJ. 2017;5, e2875, 35 pages. https://doi.org/10.7717/peerj.2875 [ Links ]
4.Luo Z-X, Kielan-Jaworowska Z, Cifelli RL. Evolution of dental replacement in mammals. Bull Carnegie Mus Nat Hist. 2004;36:159-175. [ Links ]
5.Koteja P. Energy assimilation, parental care and the evolution of endothermy. Proc R Soc Lond B. 2000;267:479-484. https://doi.org/10.1098/rspb.2000.1025 [ Links ]
6.Hopson JA. Endothermy, small size, and the origin of mammalian reproduction. Am Nat. 1973;446-452. https://doi.org/10.1086/282846 [ Links ]
7.Oftedal OT. The origin of lactation as a water source for parchment-shelled eggs. J Mammary Gland Biol Neoplasia. 2002;7:253-266. https://doi.org/10.1023/A:1022848632125 [ Links ]
8.Benoit J, Manger PR, Rubidge BS. Palaeoneurological clues to the evolution of defining mammalian soft tissue traits. Sci Rep. 2016;6, Art. #25604, 10 pages. https://doi.org/10.1038/srep25604 [ Links ]
9.Botha-Brink J, Modesto SP. A mixed-age classed 'pelycosaur' aggregation from South Africa: Earliest evidence of parental care in amniotes? Proc Biol Sci. 2007;274(1627):2829-2834. https://doi.org/10.1098/rspb.2007.0803 [ Links ]
10.Fernandez V, Abdala F, Carlson KJ, Cook DC, Rubidge BS, Yates A, et al. Synchrotron reveals early Triassic odd couple: Injured amphibian and aestivating therapsid share burrow. PLoS ONE. 2013;8(6), e64978, 7 pages. https://doi.org/10.1371/journal.pone.0064978 [ Links ]
11.Reichman O, Smith SC. Burrows and burrowing behavior by mammals. Curr Mammal. 1990;2:197-244. [ Links ]
12.Botha-Brink J. Burrowing in Lystrosaurus: Preadaptation to a postextinction environment? J Vert Paleontol. 2017;37(5), e1365080. https://doi.org/10.1080/02724634.2017.1365080 [ Links ]
13.Botha-Brink J, Huttenlocker A, Angielczyk KD, Codron D, Ruta M. Breeding young as a survival strategy during earth's greatest mass extinction. Sci Rep. 2016;6, Art. #24053, 9 pages. https://doi.org/10.1038/srep24053 [ Links ]
 Correspondence:
Correspondence:
Julien Benoit
julien.benoit@wits.ac.za
Published: 27 March 2019














