Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.115 no.3-4 Pretoria mar./abr. 2019
http://dx.doi.org/10.17159/sajs.2019/5461
WOMEN IN SCIENCE WITHOUT BORDERS: RESEARCH ARTICLES
Reclassification of early stage breast cancer into treatment groups by combining the use of immunohistochemistry and microarray analysis
Kathleen A. GrantI, II, III; Ettienne J. MyburghIV, V; Elizabeth MurrayVI, VII, VIII; Fredrieka M. PienaarIX; Martin KiddX; Colleen A. WrightI, XI; Maritha J. KotzeXII, XIII
IDivision of Anatomical Pathology, Department of Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIDepartment of Biomedical Sciences, Faculty of Health and Wellness, Cape Peninsula University of Technology, Cape Town, South Africa
IIIDST-NRF Centre of Excellence in Mathematical and Statistical Sciences (CoE-MaSS), South Africa
IVPrivate practice, Panorama Medi-Clinic, Cape Town, South Africa
VDivision of Surgery, Stellenbosch University, Cape Town, South Africa
VIRondebosch Medical Centre, Cape Town, South Africa
VIIMedi-Clinic Constantiaberg, Cape Town, South Africa
VIIIUniversity of Cape Town Private Academic Hospital, Cape Town, South Africa
IXCancerCare, Panorama Medi-Clinic, Cape Town, South Africa
XCentre for Statistical Consultation, Department of Statistics and Actuarial Sciences, Stellenbosch University, Stellenbosch, South Africa
XILancet Laboratories, Johannesburg, South Africa
XIIDivision of Chemical Pathology, Department of Pathology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
XIIISouth African National Health Laboratory Service, Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
Immunohistochemistry (IHC) is routinely used to approximate breast cancer intrinsic subtypes, which were initially discovered by microarray analysis. However, IHC assessment of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) status, is a poor surrogate of molecular subtype. Therefore, MammaPrint/BluePrint (MP/BP) microarray gene expression profiling is increasingly used to stratify breast cancer patients into different treatment groups. In this study, ER/PR status, as reported by standard IHC and single-gene mRNA analysis using TargetPrint, was compared with molecular subtyping to evaluate the combined use of MP/BP in South African breast cancer patients. Pathological information of 74 ER/PR positive, HER2 negative tumours from 73 patients who underwent microarray testing, were extracted from a central breast cancer genomics database. The IHC level was standardised by multiplying the intensity score (0-3) by the reported proportion of positively stained nuclei, giving a score of 0-300. Comparison between mRNA levels and IHC determination of ER/PR status demonstrated a significant correlation (p<0.001) for both receptors (ER: 0.34 and PR: 0.54). Concordance was shown in 61 (82%) cases and discordance in 13 (18%) of the 74 tumours tested. Further stratification by MP/BP identified 49 (66.2%) Luminal A, 21 (28.4%) Luminal B and 4 (5.4%) Basal-like tumours. Neither IHC nor TargetPrint could substitute BP subtyping, which measures the functional integrity of ER and can identify patients with false-positive tumours who are resistant to hormone therapy. These findings support the implementation of a pathology-supported genetic testing approach combining IHC and microarray gene profiling for definitive prognostic and predictive treatment decision-making in patients with early stage breast cancer.
Significance:
•Single-gene genomic oestrogen and progesterone receptor reporting adds limited additional information to the molecular stratification of breast cancer tumours and does not supersede the immunohistochemistry results.
•Neither single-gene genomic mRNA nor immunohistochemistry reporting of oestrogen and progesterone receptor status can replace the combined use of MammaPrint/BluePrint genomic molecular subtyping.
•Reliable distinction between Luminal A and B type tumours is not possible using immunohistochemistry or single-gene genomic mRNA assessment of oestrogen/progesterone and HER2 receptor status.
•Combining immunohistochemistry and microarray gene profiling enables the identification of endocrine treatment resistant hormone-positive tumours lacking ERα function (Basal-like), despite positive expression at the protein and single-gene RNA level
Keywords: oestrogen receptor; microarray; molecular subtype; BluePrint; MammaPrint
Introduction
Breast cancer defines a broad spectrum of histological lesions that are considered highly heterogeneous in presentation, morphological characteristics, prognosis and therapeutic outcome.1 Microarray-based gene expression profiling led to the discovery of intrinsic molecular subtypes underlying the variability in biological behaviour and response to treatment amongst breast cancer patients.2,3
Five distinct subtypes were described by Perou et al.2, although the normal-like subtype was subsequently considered to represent normal breast tissue within tumour. Luminal tumours expressed oestrogen receptor (ER) and responded to endocrine therapy. While Luminal A tumours show little benefit from the addition of chemotherapy, Luminal B tumours display some genetic similarities to Basal-like tumours in that they have a higher risk of being hormone resistant and show additional benefit from chemotherapy as demonstrated by the significant pathological complete response rate after neo-adjuvant chemotherapy. Human epidermal growth factor receptor-2 (HER2)-enriched and Basal-like subtypes are considered more aggressive with an unfavourable prognosis, although paradoxically exhibiting greater chemosensitivity compared to the Luminal subtype. Basal-like breast cancers are inherently resistant to endocrine therapy, and tumours subtyped as HER2 enriched respond to anti-HER2 therapy in addition to chemotherapy. Some tumours reported as HER2 positive are subtyped as Luminal B and retain some responsiveness to endocrine treatment in addition to chemotherapy and HER2 targeted treatments.4 Borley et al.5 demonstrated that the HER2 gene copy number provides additional information for stratifying breast cancer patients into different treatment groups, because HER2-positive patients with a low degree of HER amplification were shown to derive less benefit from trastuzumab (the chemotherapy agent more commonly known as Herceptin®).
Numerous studies using standard pathology have been performed to identify, with some accuracy, treatable molecular subtypes. Suggestions for incorporating markers such as the epidermal growth factor receptor (EGFR), proliferation marker Ki67, tumour suppressor gene protein p53, transmembrane tyrosine kinase receptor CD117(c-kit) and cytokeratin 5/6 into a standard immunohistochemistry (IHC) panel for breast cancer3,6 have not been adopted because of poor standardisation. High-quality assessed Ki67 is considered most useful when the indication for adding adjuvant chemotherapy to endocrine treatment is uncertain, but molecular classification can help to identify a larger group of early-stage breast cancer patients with low risk of recurrence.7 Some studies suggested that loss of progesterone receptor (PR) expression might be indicative of the Luminal B subtype8, but this association has not been universally reported. ER, PR and HER2 status have been incorporated into standard pathology reporting of breast cancers with reproducible prognostic and predictive value9,10. It is only with the advent of genetic tumour profiling that accurate molecular subtyping became part of daily clinical practice. A prospective study performed by Whitworth et al.11 proved that the combined use of the 70-gene MammaPrint (MP) and 80-gene BluePrint (BP) assays in microarray analysis of mRNA expression may be more accurate than standard IHC to guide treatment decisions. Notably, 22% of over 400 breast cancer patients studied were reclassified into a different subgroup compared with conventional assessment and showed an improved distribution of response rates in the relevant treatment groups. Similar findings were reported by Yao et al.12 Previous studies have shown that mRNA reporting of ER, PR and HER2 using microarrays is highly comparable to IHC testing.13-15 However, others have cautioned against the preferential use of hormone receptor reports using RNA-based reverse transcription polymerase chain reaction (RT-PCR) technology, highlighting discordance with the IHC results and the potential of denying patients who were ER or PR positive on IHC the benefit of endocrine therapy.16,17 Application of different methodologies for the same purpose therefore requires careful consideration.
Microarray-based tumour profiling using the 70-gene MP profile has been available in South Africa since 2007 and, from 2009, local referral criteria were introduced for reimbursement by medical aid providers.18 Initially, analysis was performed on fresh tissue only, but since 2012 the use of formalin fixed paraffin embedded (FFPE) tissue became available and has become the only method used. A central database was established by using an ethics approved protocol for comparative effectiveness studies on data of MP tests requested in southern Africa. In addition to ER/PR mRNA reporting by TargetPrint (TP) from 2009, BP - which determines the tumour molecular subtype19 - has also become part of the MP service from 2011. BP provides a comprehensive multigene expression analysis of the tumour molecular subtypes, which may not be sufficiently reflected by single-gene IHC or mRNA testing.20,21 Although our data on HER2 expression indicated a 100% correlation between fluorescence in-situ hybridisation (FISH) and microarray testing using TP22, discrepancies in ER/PR reporting between IHC and RNA-based RT-PCR techniques23,24 warranted evaluation in this study of the value and potential clinical impact of ER and PR status as reported by TP using RNA-based microarray analysis.
Our aim was to evaluate the combined use of MP/BP in clinical practice using a pathology-supported genetic testing approach incorporating ER, PR and HER2 status as part of the above-mentioned referral criteria, called the microarray pre-screen algorithm (MPA). ER and PR status as assessed by IHC was compared with that reported by TP in order to determine the correlation between the two techniques. We used the BP result to identify the molecular subtype and most probable response to therapy and correlated this result to the IHC and TP results. This study is the first to correlate IHC and mRNA hormone receptor status in South African breast cancer patients, using microarrays performed on FFPE specimens in the context of molecular breast cancer subtyping. As a consequence of the MPA employed in southern Africa, which generally excludes patients with ER/PR negative or HER2 positive tumours from testing, our series was limited to ER/PR positive, HER2 negative tumours only. South African patients reclassified as HER2 positive using TP and reflex FISH in a recent study22 were also excluded from the current analysis.
Methods
This study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki. Ethical approval was granted by the Health and Research Ethics Committee (HREC) of Stellenbosch University (reference number N09/06/166).
Study population
The records of 128 patients with 131 tumours were available in a central breast cancer genomic database, which comprised data of all patients referred for MP in southern Africa between 2007 and 2014. The database is maintained centrally under a rigorous quality control programme to ensure the integrity of the data. We extracted anonymised pathological data of 74 HER2 negative tumours from 73 patients who had TP and BP testing on FFPE tissue. The tumour pathology included tumour type, grade and size; ER, PR and HER2 status; MP risk status; TP results for ER, PR and HER2; and the molecular subtype as determined by BP.
Immunohistochemistry testing
Standard pathology reporting of hormone and HER2 receptor status using IHC to measure protein expression levels varies amongst different laboratories. To standardise the data for statistical analysis of hormone receptor status, estimation of the semi-quantitative expression of ER and PR was performed using the intensity score (0-3), multiplied by the reported proportion of positively stained nuclei, thereby calculating a final ER and PR score (0-300).
Microarray-based gene expression profiling
Microarray-based gene expression profiling (MP, TP and BP) was performed on 74 FFPE tissue samples obtained from 73 breast cancer patients, using a pathology-supported genetic testing strategy.18 An experienced pathologist evaluated tumour suitability for genomic analysis based on confirmation of a minimum tumour cell content of 30% in accordance with compliancy criteria laid out by the US Food and Drug Administration. These samples were transported under an export permit to the Netherlands where tumour assessment was performed at the centralised Agendia Laboratory in accordance with standard testing protocols.25 mRNA expression for ER and PR is reported on a continuous exponential scale from -1 to 1 and values of less than 0 are considered to be negative.
Comparative analysis
Quantitative analysis was performed by comparing the level of mRNA for ER and PR as reported by TP with the IHC score. ER and PR were considered to be positive when the IHC score was >10 or the mRNA score was >0. Qualitative analysis was performed to allow in-depth evaluation of the relationship between IHC and mRNA compared to tumour subtyping, with the aim of determining the clinical implications of individual versus combined assessment of pathology and microarray-based genetic testing.
Statistical analysis
Statistical analysis was performed using the Statistica v.13 software package. Observer agreement measures for IHC testing to determine hormone receptor status and microarray-based mRNA readout assessment were calculated from two-way contingency table analysis.26 The relationship between protein expression (IHC) and mRNA (TP) levels was assessed using Spearman rank correlation analysis. A possible association between loss of PR expression in ER-positive cases (ER+/PR-) and the high-risk Luminal B subtype as determined by microarray analysis was further assessed. Results corresponding to a p<0.05 were considered statistically significant.
Results
Description of tumour pathology in relation to molecular subtype
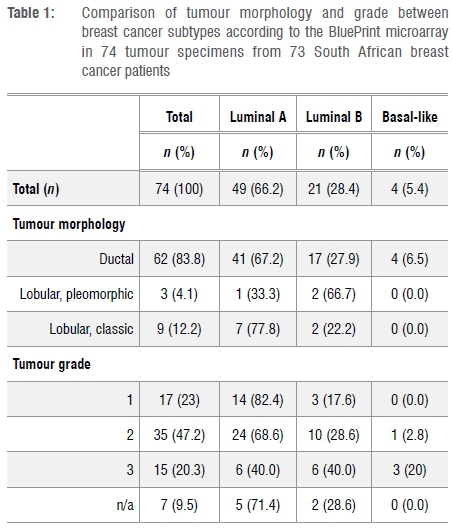
All tumours were ER and/or PR positive on IHC. Analysis of the pathological characteristics of the 74 HER2 negative tumours subjected to TP (ER, PR and HER2 status) and BP using FFPE specimens is presented in Table 1. Molecular subtyping using the BP microarray test stratified 49 (66.2%) tumours as Luminal A, 21 (28.4%) as Luminal B and 4 (5.4%) as Basal-like. In the patient with multi-focal pathology, one tumour was designated Luminal A and the other Luminal B.
Comparative analysis of ER and PR status
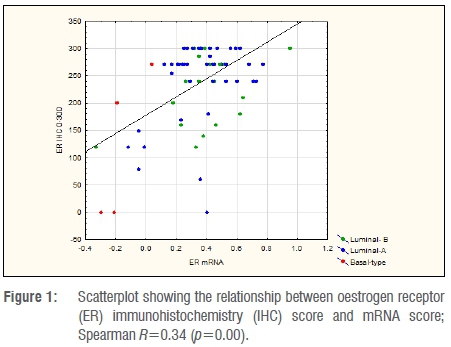
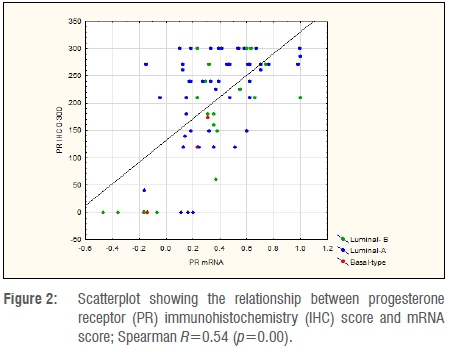
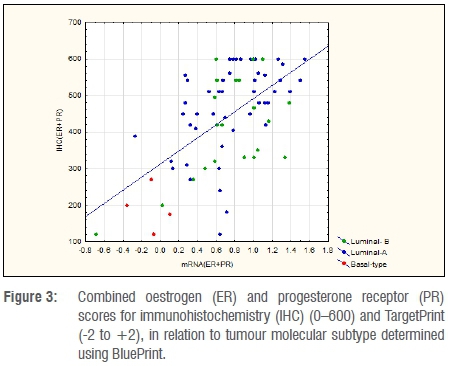
A quantitative comparison between mRNA levels and IHC determination of ER and PR is shown in Figures 1 and 2. Spearman R-values were 0.34 for ER and 0.54 for PR (p<0.001), indicating the correlation between mRNA and IHC. Table 2 indicates the mean values for IHC and mRNA scores for both ER and PR according to tumour subtype. Significant differences were observed amongst tumour subtypes for all variables except for mRNA expression of PR. Fisher's Least Significant Difference Test confirmed significant differences between Basal type and Luminal types with no difference seen between Luminal A and B subtypes. The sum of ER and PR scores, which resulted in maximum values of 600 and -2 to +2 for IHC and mRNA, respectively, indicated significant differences between Basal and Luminal tumours. Comparably, no differences were shown between Luminal A and B molecular subtypes. The combined score also amplified the differences between the groups, as illustrated in Figure 3.
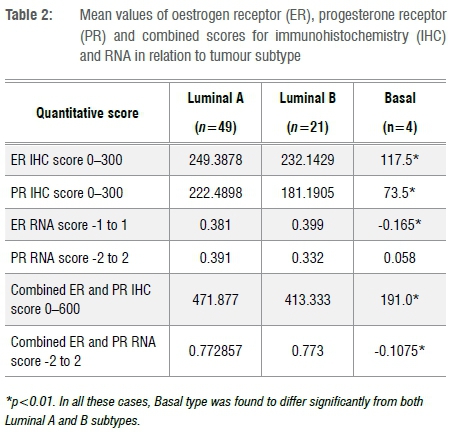
A qualitative analysis was performed to evaluate the relationship between IHC and mRNA in relation to tumour subtyping. Table 3 provides a summary of the data. Of the 74 tumours, ER and PR status was concordant in 61 (82%) and discordant in 13 (18%). In the concordant group, 39 (64%) were Luminal A, 17 (31%) were Luminal B and 3 (5%) were Basal breast cancer subtypes. In the discordant group (n=13), three tumours were ER and PR negative on mRNA, with one being Luminal A, one being Luminal B and the other being a Basal-subtype. Of these 13 discordant tumours, 10 (77%) were Luminal A, 2 (15%) were Luminal B and 1 (8%) was a Basal-type.
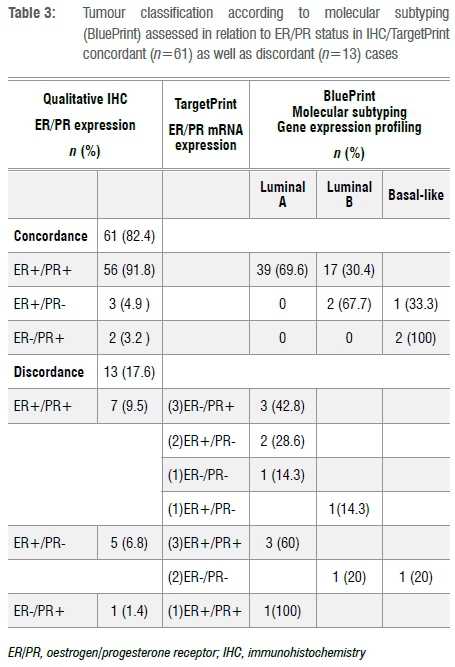
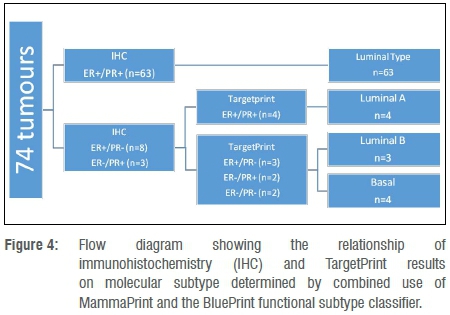
The relationship between IHC and mRNA status versus tumour subtype is indicated in Figure 4. Importantly, none of the ER+/PR+ tumours determined on IHC were reported as Basal subtype breast cancers. When either ER or PR expression was lost, on both IHC and mRNA, there was a 57% (4 out of 7) risk of a Basal subtype. No predictive pattern in hormone receptor expression determined by IHC could be identified to distinguish Luminal A from Luminal B tumours identified through combined use of MP/BP.
Discussion
Ongoing validation of emerging genomic technologies against current standards in breast cancer pathology is an important research focus. In South Africa, an increased level of confidence based on growing clinical experience supported the incorporation of MP/BP19,27 into routine clinical practice. Integration of these results into treatment decision may lead to a change in therapy in one of every two early-stage breast cancer patients treated in South Africa.28 In the present study, the molecular subtype based on BP was used as an indicator of the expected response to therapy. The Luminal type of breast cancer is expected to respond to endocrine therapy, whereas the Basal subtype is inherently resistant to endocrine therapy. IHC and TP results were evaluated for their ability to predict a non-Luminal subtype.
In contrast to previous results from our research group showing a 100% correlation in HER2 status between FISH and TP, irrespective of whether fresh or FFPE specimens were used22, our current qualitative results show 18% discordance between the IHC and TP determination of ER and PR status. More importantly, three (4%) of the ER+/PR+ tumours as determined by IHC/FISH were ER-/PR- on TP regardless of being a Luminal-type. If the TP results were interpreted without the IHC and/or BP results, the results could have a major implication on treatment decision-making for the patients. It is therefore important to realise that determination of mRNA status for ER and PR using single-gene mRNA analysis does not necessarily translate to protein expression or reflect the presence of a functioning receptor protein.29 Because molecular subtyping performed through BP is enriched for several genes involved in ER function, the combined score of 80 genes included in the BP microarray profile provides a better indication of an intact ER-mediated kinase pathway and subsequent response to endocrine therapy.20
Reasons for the apparent discrepancy between mRNA expression and receptor protein levels based on IHC can be found in our quantitative analyses which indicated that ER and PR protein expression levels varied substantially in relation to mRNA levels. In Figure 1, there were eight tumours with mRNA scores of <0, indicating ER mRNA negativity, but with a mean IHC score of 156 (0-300), and only two had an IHC score <10. As mRNA levels increased above 0, there was only one Luminal A tumour with an IHC score of 0. For PR there seemed to be better correlation of low mRNA levels to the IHC score, with 6 out of 9 tumours reported with a mRNA score <0 showing an IHC score of 0. Similar results were reported in a study using quantitative immunofluorescence for measuring mRNA in situ by Bordeaux et al.30 These authors found a nonlinear relationship between ERα protein expression and mRNA levels on tissue sections visualised using RNA scope probes. In a similar finding to this study, protein expressions varied considerably at very low levels of RNA. In their analysis, mRNA did not show any prognostic value but had some predictive value above and beyond that of the ERα protein expression.
The presence of a splice variant in the ER gene might also influence the apparent ER protein expression, as previously described by Groenendijk et al.31 Several ER splicing variants have been reported in the literature, resulting in one or more exons being omitted from the ER mRNA. Advanced techniques such as exome sequencing might be particularly effective in identifying these abnormalities.32 Antibodies used to detect ER protein during routine IHC assessment rely on epitope recognition encoded by the first exon of the ER gene; therefore, antibody binding at these sites will produce a positive ER result, despite the lack of normal ER functionality as a result of a splice variant. Similarly, RNA methods based on single-gene identification of ER status such as TP (microarray, previously provided as a separate readout with MP) and Oncotype DX (RT-PCR, included in 21-gene assay) will not routinely detect such variants. The ability of microarray analysis to detect functional ERα activity, could explain why a patient with the ERΔE7 splice variant as noted by Groenendijk et al.31 was classified as high risk by MP, but low risk by Oncotype DX (Recurrence Score of 8). Comprehensive genomic evaluation using multi-gene tests such as MP/BP is needed to identify these hormone-resistant tumours. This justifies recent discontinuation of TP and introduction of the European Conformity (CE)-marked next-generation sequencing based MP/BP® (Agendia, Inc.).
Numerous technical factors have an influence on the accuracy of standard IHC reporting, including the effect of cold ischaemic time on ER epitope availability, resulting in false low levels of protein expression.33,34 Different samples used for initial IHC versus subsequent receptor status determination using TP, could contribute to discordant results as some authors have reported high false positive and negative rates when testing was performed on tissue obtained from core needle biopsies as opposed to resected specimens.35 Sample differences might also play a role as some of the patients had the initial receptor status reported on core needle biopsies whereas TP was performed on the resection specimen. In most units, however, this is an accepted practice with good correlation, although it should be avoided in ER and PR negative tumours.36
Variations in individual ER and PR levels measured by IHC or mRNA levels did not discriminate between Luminal A and B tumours. Basal tumours had significantly lower scores for ER and PR on IHC as well as mRNA levels for ER. Utilising a summative ER/PR score for both IHC and mRNA allowed better discrimination between Basal tumours and Luminal tumours. When this combined score was employed, as illustrated in Figure 3, four out of six tumours with an IHC ≤ 270 and mRNA ≤0.1 were Basal while the remaining two were Luminal B breast cancer subtypes. This was also reflected in our qualitative analysis. All the tumours which were ER+/PR+ using IHC were Luminal A or B subtypes and the additional results provided by TP did not add any further information to assist in distinguishing Luminal A from Luminal B tumours identified by BP. In cases where ER or PR was negative on IHC, TP did add some additional value although the numbers in this group were limited (n=11). In this group, all four tumours for which TP showed an ER+/PR+ profile, were Luminal A and would potentially gain little or no benefit from additional chemotherapy. The remainder were either Luminal B or Basal subtypes, suggesting the addition of chemotherapy to the treatment plan.
Interpretation of the results is limited by a relatively small sample size as well as pre-selection of hormone-positive and HER2 negative patients in compliance with the MPA developed for reimbursement purposes in South Africa.18 This precluded evaluation of the effect TP might have on reclassification of IHC ER-/PR- tumours22, possibly classifying some of these as hormone receptor positive or Luminal type supported by BP results. The main strength of the study lies in the reclassification of a subset of patients into the Luminal B and Basal-like subtypes who require more aggressive treatment compared to patients with Luminal A type tumours. Because the 80-gene BP profile is enriched in ER-target genes and measures the functional integrity of ER, it has the potential to identify a subgroup of breast cancer patients who are ER-positive by IHC and/or single-gene mRNA expression analysis but would fail to respond to hormone treatment.31
In conclusion, our results show that in contrast to the added value of TP as a second opinion for HER2 status22, single-gene microarray readout of ER/PR status provided little additional information beyond that obtained from standard IHC results and performed poorly in predicting molecular tumour subtype. If genomic hormone receptor status alone would be used in clinical decision-making, it is possible that some patients might be erroneously denied endocrine therapy. However, in tumours for which ER or PR expression was lost on both IHC and TP, the molecular subtype determined by BP was less likely to be Luminal A, thus indicating the potential benefit of adjuvant chemotherapy. Basal tumours can potentially be identified by utilising the sum of the ER and PR results in both IHC and mRNA and selecting tumours for which IHC≤270 and mRNA≤0.1, or the flow diagram in Figure 4 can be employed. Accurate distinction between Luminal A and B molecular subtypes, and identification of the Basal-type despite apparent positive ER status on IHC and TP noted in at least one South African patient, were only possible by using the BP 80-gene profile. It was our consistent observation that loss of PR expression on IHC was not indicative of the Luminal B subtype. TP provided limited additional information compared to IHC, which justifies recent discontinuation of single-gene mRNA microarray testing of the ER, PR and HER2 genes now incorporated into the 80-gene BP profile.
The clinical relevance of a pathology-supported genetic testing approach to breast cancer management, combining microarray-based analysis as ancillary to existing clinico-pathological risk stratification and prognostication tools, is supported by the results presented in this study. The routine implementation of genomic profiling alongside standard pathology tests may increase clinician confidence in treatment decision-making and ultimately optimise individualised management of early-stage breast cancer patients by identifying molecular subgroups more accurately.
Acknowledgements
Research reported in this publication was supported by the Cancer Association of South Africa (CANSA) and the Strategic Health Innovation Partnerships (SHIP) Unit of the South African Medical Research Council (MRC) with funds received from the South African Department of Science and Technology (grant no. S003665). This work is also based on the research supported in part by the National Research Foundation of South Africa (grant no. 86417) and the Cape Peninsula University of Technology (CPUT) research fund. The Support Programme for Industrial Innovation provided financial support towards development of a laboratory interface for Agendia in the Netherlands to provide South African patients access to the MammaPrint test. The research database is maintained with funding received by the South African BioDesign Initiative of the Department of Science and Technology and the Technology Innovation Agency (grant no. 401/01). We acknowledge the DST-NRF Centre of Excellence in Mathematical and Statistical Sciences (CoE-MaSS), South Africa, for their support in the publication of this article. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the CoE-MaSS. Dr K. Brundyn and Dr G. Swart of PathCare are thanked for sample preparation and histopathology diagnoses.
Competing interests
M.J.K. is a director and shareholder of Gknowmix (Pty) Ltd. which developed a database tool for research translation under the auspices of the South African Medical Research Council. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Authors' contributions
K.A.G. was responsible for data collection, analysis and interpretation, and wrote the first draft of the manuscript. E.J.M. contributed to data acquisition, clinical interpretation and improvement of the database used in this study and revised the manuscript critically prior to submission for publication. E.M. contributed to data acquisition and improvement of the manuscript. R.P. contributed to acquisition of data and interpretation of the results. M.K. performed the final statistical analysis for the results presented. C.A.W. contributed to development of the study framework and takes responsibility for histopathology aspects. M.J.K. contributed substantially to conception and design of the study, revised the manuscript critically and finalised the paper together with K.A.G. All authors read and approved the final manuscript.
References
1.Polyak K. Heterogeneity in breast cancer. J Clin Invest. 2011;121(10):3786-3788. https://doi.org/10.1172/JCI60534 [ Links ]
2.Perou CM, Sørlie T, Eisen MB, Van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747-752. https://doi.org/10.1038/35021093 [ Links ]
3.Cheang MC, Voduc D, Bajdik C, Leung S, McKinney S, Chia SK, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14(5):1368-1376. https://doi.org/10.1158/1078-0432.CCR-07-1658 [ Links ]
4.Tran B, Bedard PL. Luminal-B breast cancer and novel therapeutic targets. Breast Cancer Res. 2011;13(6), Art. #221, 10 pages. https://doi.org/10.1186/bcr2904 [ Links ]
5.Borley A, Mercer T, Morgan M, Dutton P, Barrett-Lee P, Brunelli M, et al. Impact of HER2 copy number in IHC2+/FISH-amplified breast cancer on outcome of adjuvant trastuzumab treatment in a large UK cancer network. Br J Cancer. 2014;15;110(8):2139-2143. https://doi:10.1038/bjc.2014.147 [ Links ]
6.Nielsen TO, Hsu FD, Jensen K, Cheang M, Karaca G, Hu Z, et al. Immunohistochemical and clinical characterization of the Basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10(16):5367-5374. https://doi.org/10.1158/1078-0432.CCR-04-0220 [ Links ]
7.Viale G, De Snoo FA, Slaets L, Bogaerts J, Van 't Veer L, Rutgers EJ, et al. Immunohistochemical versus molecular (BluePrint and MammaPrint) subtyping of breast carcinoma. Outcome results from the EORTC 10041/BIG 3-04 MINDACT trial. Breast Cancer Res Treat. 2018;167(1):123-131. https://doi.org/10.1007/s10549-017-4509-9 [ Links ]
8.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, et al. Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann Oncol. 2013;24(3):661-668. https://doi.org/10.1093/annonc/mds430 [ Links ]
9.Viale G. The current state of breast cancer classification. Ann Oncol. 2012;10:207-210. https://doi.org/10.1093/annonc/mds326 [ Links ]
10.Phillips T, Murray G, Wakamiya K, Askaa J, Huang D, Welcher R, et al. Development of standard estrogen and progesterone receptor immunohistochemical assays for selection of patients for anti-hormonal therapy. Appl Immunohistochem Mol Morphol. 2007;15(3):325-331. https://doi.org/10.1097/01.pai.0000213135.16783.bc [ Links ]
11.Whitworth P, Stork-Sloots L, De Snoo FA, Richards P, Rotkis M, Beatty J, et al. Chemosensitivity predicted by BluePrint 80-gene functional subtype and MammaPrint in the Prospective Neoadjuvant Breast Registry Symphony Trial (NBRST). Ann Surg Oncol. 2014;21(10):3261-3267. https://doi.org/10.1245/s10434-014-3908-y [ Links ]
12.Yao K, Goldschmidt R, Turk M, Wesseling J, Stork-Sloots L, De Snoo F, et al. Molecular subtyping improves diagnostic stratification of patients with primary breast cancer into prognostically defined risk groups. Breast Cancer Res Treat. 2015;154:81-88. https://doi.org/10.1007/s10549-015-3587-9 [ Links ]
13.Roepman P, Horlings HM, Krijgsman O, Kok M, Bueno-de-Mesquita JM, Bender R, et al. Microarray-based determination of estrogen receptor, progesterone receptor, and HER2 receptor status in breast cancer. Clin Cancer Res. 2009;15(22):7003-7011. https://doi.org/10.1158/1078-0432.CCR-09-0449 [ Links ]
14.Gevensleben H, Göhring UJ, Büttner R, Heukamp LC, Kunz G, Dimpfl T, et al. Comparison of MammaPrint and TargetPrint results with clinical parameters in German patients with early stage breast cancer. Int J Mol Med. 2010;26(6):837-843. https://doi.org/10.3892/ijmm_00000532 [ Links ]
15.Viale G, Slaets L, Bogaerts J, Rutgers E, Van 't Veer L, Piccart-Gebhart MJ, et al. High concordance of protein (by IHC), gene (by FISH; HER2 only), and microarray readout (by TargetPrint) of ER, PgR, and HER2: Results from the EORTC 10041/BIG 03-04 MINDACT trial. Ann Oncol. 2014;25(4):816-823. https://doi.org/10.1093/annonc/mdu026 [ Links ]
16.Kraus JA, Dabbs DJ, Beriwal S, Bhargava R. Semi-quantitative immunohistochemical assay versus OncotypeDx® qRT-PCR assay for estrogen and progesterone receptors: An independent quality assurance study. Mod Pathol. 2012;25(6):869-876. https://doi.org/10.1038/modpathol.2011.219 [ Links ]
17.Park MM, Ebel JJ, Zhao W, Zynger DL. ER and PR immunohistochemistry and HER2 FISH versus Oncotype DX: Implications for breast cancer treatment. Breast J. 2014;20(1):37-45. https://doi.org/10.1111/tbj.12223 [ Links ]
18.Grant KA, Apffelstaedt JP, Wright C, Myburgh E, Pienaar R, De Klerk M, et al: MammaPrint Prescreen Algorithm (MPA) reduces chemotherapy in patients with early stage breast cancer. S Afr Med J. 2013;103(8):522-526. https://doi.org/10.7196/SAMJ.7223 [ Links ]
19.Krijgsman O, Roepman P, Zwart W, Carroll JS, Tian S, De Snoo FA, et al. A diagnostic gene profile for molecular subtyping of breast cancer associated with treatment response. Breast Cancer Res Treat. 2012;133(1):37-47. https://doi.org/10.1007/s10549-011-1683-z [ Links ]
20.Glück S, De Snoo F, Peeters J, Stork-Sloots L, Somlo G. Molecular subtyping of early-stage breast cancer identifies a group of patients who do not benefit from neoadjuvant chemotherapy. Breast Cancer Res Treat. 2013;139(3):759-767. http://doi:10.1007/s10549-013-2572-4 [ Links ]
21.Vieira A F, Schmitt F. An update on breast cancer multigene prognostic tests - emergent clinical biomarkers. Front Med. 2018;5, Art. #248, 12 pages. https://doi.org/10.3389/fmed.2018.00248 [ Links ]
22.Grant KA, Pienaar FM, Brundyn K, Swart G, Gericke GS, Myburgh EJ, et al. Incorporating microarray assessment of HER2 status in clinical practice supports individualised therapy in early-stage breast cancer. The Breast. 2015;24(2):137-142. https://doi.org/10.1016/j.breast.2014.12.006 [ Links ]
23.Khoury T, Yan L, Liu S, Bshara W. Oncotype DX RT-qPCR assay for ER and PR correlation with IHC: A study of 3 different clones. Appl Immunohistochem Mol Morphol. 2015;23(3):178-187. https://doi.org/10.1097/PAI.0000000000000078 [ Links ]
24.Hanna MG, Bleiweiss IJ, Nayak A, Jaffer S. Correlation of Oncotype DX Recurrence Score with histomorphology and immunohistochemistry in over 500 patients. Int J Breast Cancer. 2017;2017, Art. #1257078, 6 pages. https://doi.org/10.1155/2017/1257078 [ Links ]
25.Glas AM, Floore A, Delahaye LJ, Witteveen AT, Pover RC, Bakx N, et al. Converting a breast cancer microarray signature into a high-throughput diagnostic test. BMC Genomics. 2006,7, Art. #278, 10 pages. https://doi.org/10.1186/1471-2164-7-278 [ Links ]
26.Landis JR, Koch GG: The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159-174. [ Links ]
27.Myburgh EJ, Langenhoven L, Grant KA, Van der Merwe L, Kotze MJ. Clinical overestimation of HER2 positivity in early estrogen and progesterone receptor-positive breast cancer and the value of molecular subtyping using BluePrint. J Glob Oncol. 2016;3(4):314-322. https://doi.org/10.1200/JGO.2016.006072 [ Links ]
28.Pohl H, Kotze MJ, Grant KA, Van der Merwe L, Pienaar FM, Apffelstaedt JP, et al. Impact of MammaPrint on clinical decision-making in South African patients with early-stage breast cancer. Breast J. 2016;16,22(4):442-446. https://doi.org/10.1111/tbj.12605 [ Links ]
29.Itoh M, Iwamoto T, Matsuoka J, Nogami T, Motoki T, Shien T, et al. Estrogen receptor (ER) mRNA expression and molecular subtype distribution in ER-negative/progesterone receptor-positive breast cancers. Breast Cancer Res Treat. 2014;143(2):403-409. https://doi.org/10.1007/s10549-013-2763-z [ Links ]
30.Bordeaux JM, Cheng H, Welsh AW, Haffty BG, Lannin DR, Wu X, et al. Quantitative in situ measurement of estrogen receptor mRNA predicts response to tamoxifen. PLoS ONE. 2012;7(5), e36559, 8 pages. https://doi.org/10.1371/journal.pone.0036559 [ Links ]
31.Groenendijk FH, Zwart W, Floore A, Akbari S, Bernards R. Estrogen receptor splice variants as a potential source of false-positive estrogen receptor status in breast cancer diagnostics. Breast Cancer Res Treat. 2013;140(3):475-484. https://doi.org/10.1007/s10549-013-2648-1 [ Links ]
32.Taneri B, Asilmaz E, Gaasterland T. Biomedical impact of splicing mutations revealed through exome sequencing. Mol Med. 2012;30(18):314-319. [ Links ]
33.Welsh AW, Moeder CB, Kumar S, Gershkovich P, Alarid ET, Harigopal M, et al. Standardization of estrogen receptor measurement in breast cancer suggests false-negative results are a function of threshold intensity rather than percentage of positive cells. J Clin Oncol. 2011;29(22):2978-2984. https://doi.org/10.1200/JCO.2010.32.9706 [ Links ]
34.Gown AM. Current issues in ER and HER2 testing by IHC in breast cancer. Mod Pathol. 2008;21(Suppl 2):S8-S15. https://doi.org/10.1038/modpathol.2008.34 [ Links ]
35.Seferina SC, Nap M, Van den Berkmortel F, Wals J, Voogd AC, Tjan-Heijnen VC. Reliability of receptor assessment on core needle biopsy in breast cancer patients. Tumour Biol. 2013;34(2):987-994. https://doi.org/10.1007/s13277-012-0635-5 [ Links ]
36.Dekker TJ, Smit VT, Hooijer GK; Reliability of core needle biopsy for determining ER and HER2 status in breast cancer. Ann Oncol. 2013;24(4):931-937. https://doi.org/10.1093/annonc/mds599 [ Links ]
 Correspondence:
Correspondence:
Kathleen Grant
grantk@cput.ac.za
Received: 17 Aug. 2018
Revised: 22 Feb. 2019
Accepted: 27 Feb. 2019
Published: 27 Mar. 2019














