Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.115 n.3-4 Pretoria Mar./Apr. 2019
http://dx.doi.org/10.17159/sajs.2019/5516
WOMEN IN SCIENCE WITHOUT BORDERS: RESEARCH ARTICLES
Assessing the potential effects of nevirapine in South African surface water on fish growth: A chronic exposure of Oreochromis mossambicus
Marie Clémentine U. NibamurekeI; Irene E.J. BarnhoornII; Gesina M. WagenaarI
IDepartment of Zoology, University of Johannesburg, Johannesburg, South Africa
IIDepartment of Zoology, University of Venda, Thohoyandou, South Africa
ABSTRACT
Aquatic environments around the world have become mixtures of different types of pollutants, including pharmaceuticals. The presence of pharmaceuticals in aquatic environments has raised concerns regarding the possibility of unintended effects on aquatic animals. South Africa is currently the largest consumer of HIV antiretroviral drugs (ARVs) worldwide. Nevirapine (NVP), a first-line ARV, has been associated with serious liver toxicity in humans and has been repeatedly detected in South African surface water. We investigated the potential effect of NVP on the growth of larvae and juveniles of the Mozambique tilapia (Oreochromis mossambicus) through a chronic laboratory exposure. Larval and early juvenile stages were exposed to the highest reported environmental relevant concentration of NVP in South African surface water (1.48 µg/L) for 60 days in a static renewal system. Body mass and total length measurements were recorded and analysed for individuals aged 1, 5, 30 and 60 days. In total, 455 fish were assessed. The growth parameters of larvae exposed to NVP were not statistically significantly different (p>0.05) from those of control larvae. However, the juveniles exposed to NVP showed a slightly lower mean growth rate between the 30th and 60th day compared with the control fish. These results suggest that the concentration of NVP in South African surface water has no significant detrimental effects on fish growth during the first 2 months of their life. Further studies to investigate the effects on all life stages of fish are needed as it is evident that the growth rate of exposed fish could be affected after this stage.
Significance:
•This study was the first to investigate the effect of an antiretroviral drug in surface water on fish growth.
•Chronic exposure to the highest environmentally relevant concentration of nevirapine in South African waters did not affect the growth of early life stages of Mozambique tilapia.
•The levels of antiretrovirals in aquatic systems should be monitored closely as their consumption is likely to increase in the future
Keywords: pharmaceuticals; HIV antiretrovirals; aquatic toxicology; fish health; early life stages
Introduction
The presence of human pharmaceuticals in aquatic environments around the world is considered to be one of the pollution problems of emerging concern as the effect of these pharmaceuticals on aquatic animals is still largely unknown.1,2 There are growing concerns that pharmaceuticals in water may have unintended effects on fish.3,4 Case studies include the collapse of a fish population exposed to a synthetic oestrogen in Canada5; reduced hatching success of zebrafish (Danio rerio) embryos exposed to sulfonamides6; histological changes in the organs of rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus caprio) exposed to diclofenac7; and liver damage and altered health in Mozambique tilapia (Oreochromis mossambicus) exposed to efavirenz, an antiretroviral (ARV)8. It is evident that pharmaceutical pollution in aquatic environments needs to be investigated thoroughly as pharmaceuticals are important in our everyday life to prevent and treat diseases in humans, animals and crops, leading to increased economic income of households and countries.1
Consumption of pharmaceuticals around the world is diversified, and, therefore, the types and concentrations of pharmaceuticals in surface water differ from one region to another.9 South Africa is amongst the countries with the highest HIV prevalence in the world, with approximately 7.9 million people infected.10 More than 60% (4.4 million) of people living with HIV are receiving ARVs - making South Africa the biggest ARV treatment programme in the world.10,11 Studies in different countries have showed that the current technologies used to treat waste water in wastewater treatment plants do not remove, or only partially remove, some of the pharmaceuticals, including ARVs.12-14 In addition, during the disinfection processes, some pharmaceuticals form compounds which can be more active than their parent compound.15 It is therefore not a surprise that South African surface water contains all types of ARVs used in the country, and that these ARVs have also been detected in tissues of fish.16,17 Currently, little information is available on the effects ARVs may have on fish health.
Nevirapine (NVP), a first-line ARV prescribed in South Africa, has been repeatedly detected in surface water around the country16, because it is resistant to biodegradation18 and because the chlorination process used in wastewater treatment plants in the country results in the formation of various NVP degradation products, some of which have similar antiviral activity to the parent compound15,19. NVP is known to cause serious undesirable side effects in humans including liver toxicity and skin rash.20 Being continuously added to surface water as a result of daily consumption, NVP as well as all other ARVs and pharmaceuticals in general, are constantly present in surface water and available to fish living in the water.21 This situation raises concerns about the possible effects ARVs and NVP in particular may have on fish health.
Human pharmaceuticals are highly active chemical compounds designed to have specific physiological effects on target tissues.1 Therefore, serious concerns arise when non-target organisms such as fish come into contact with such highly active compounds which were not intended for them. Nobody can predict with certainty what effects these compounds may have on fish. Recent laboratory exposure studies have shown that some of those pharmaceuticals can disturb fish physiology and metabolism.22-24 Some studies have also emphasised that early life stages of fish may be more vulnerable to pollutants in water which can impede their growth and development and these pollutants could, therefore, negatively affect fish communities and populations.25
Fish growth is determined amongst others by the type and quantity of food, the physicochemical water quality and the competition among individuals.26 When conditions become unfavourable, for example, in the case of pollution or food scarcity, fish will show impaired growth.27 In fisheries, mass and length measurements are important growth parameters which are recorded frequently to calculate mass-length relationships to estimate the well-being and age of the fish.28,29 The mass-length relationship of a healthy fish should show a strong positive correlation as the fish is growing.29
One of the most used mass-length relationships in fisheries is the condition factor (CF) which is expressed as the ratio between fish mass and length.28-30 Researchers in fisheries using different fish species have shown that the CF of a healthy adult fish is close to 1.28-30 Pharmaceuticals which may affect fish growth by disturbing metabolism, may lead to reduced growth which may be noticed through changes in growth parameters.30 Potentially detrimental effects of pharmaceuticals of concern on aquatic organisms should be determined, and effects of individual pharmaceuticals should be assessed on individual species to determine specific effects.31
We report on the potential effects of NVP on the growth of larvae and juveniles of the southern African indigenous freshwater fish species Oreochromis mossambicus through a laboratory chronic exposure.
Methods
Ethical clearance
This study was approved by the University of Johannesburg's Ethical Committee on 20 November 2015 (ref no. 201242617).
Breeding of fish
Sexually mature O. mossambicus were chosen from the University of Johannesburg's aquarium stock and organised into two breeding sets (comprising one male and two female individuals) in two 700-L glass tanks. The experiment was conducted in an environment-controlled room: the temperature of the water was kept at 27±1 °C, a photoperiod cycle of 14 h light:10 h dark was maintained, and an oxygen line was connected to each tank (providing ≥80% dissolved oxygen). The fish were inspected every morning for possible eggs, and once eggs were available, they were collected from the mouth of the female fish approximately 24 h after fertilisation and placed in 1-L glass bottles fitted with small hatching jars.32
Exposure conditions and procedures
The experiment was conducted four times and included three groups: control group with dilution water only; the solvent control group (<0.01% v/v dimethylsulfoxide or DMSO in water) and the NVP group (1.48 µg/L16). The exposure started with eggs in 1-L glass jars containing the exposure media. The start of the larval stage was recorded as the first day after hatching.33 On the fifth day after hatching, the larvae were transferred to 20-L glass tanks where the exposure continued until they were 30 days old. Then they were transferred to 90-L tanks and the exposure continued until they were 60 days old. The transfer to bigger tanks was done in order to give the fish more space for adequate growth and development.33 As the exposure lasted 60 days, exogenic feeding started when the yolk sac was almost depleted, which was approximately 12 days post-fertilisation. The start of exogenic feeding also marked the end of the larval stage and the start of the juvenile life stage.33,34 The fish were fed three times per day with complete tilapia fry crumble #1 (500-750 µm) (Tilapia Fry Crumble, AVI Products (Pty) LTD 2001/015923/07, Durban, South Africa).
The experiment was conducted in the same climate-controlled room as the breeding to avoid exposure of the embryos to different temperatures; the temperature in the different exposure containers was kept at 27±1°C and a 14:10 h light:dark photoperiod was maintained. Oxygen was supplied and kept at ≥80%. Each exposure container was checked twice daily to remove organic waste including dead embryos/larvae if any, food remains and excretion. The pH in each container was monitored daily and maintained between 7.6 and 8.1.
At least 10 individuals aged 1, 5, 30 and 60 days old were sampled per group by placing them on ice for 3-5 min, which served as an anaesthesia. Then the total length and wet mass of each individual were measured using a small graduated board (in mm) under a dissecting microscope and an electronic scale accurate to 0.001 g. The sampling of specimens was done randomly using a plastic pipette with the tip cut to make a ±3-mm opening for 1- and 5-day-old larvae, and a small net for 30- and 60-day-old juveniles. After taking the measurements, the fish were killed and fixed in 10% neutral buffered formalin for further analyses. The CF of each fish was calculated from the recorded mass and length measurements following Carlander35. The age of the fish was recorded and expressed in days post-hatching, with Day 1 being the first day after hatching.
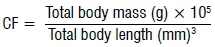
The mean specific growth rate (SGR) which expresses the mean daily mass gain was also calculated in % for each group for 30- and 60-day-old juveniles using the following formula36:

where Ln represents the natural logarithm while T1 and T2 represent the days of initial and final mass recordings, respectively.
Water physical and chemical parameters
The physical parameters of the water were monitored daily. Every 96 h the exposure medium was renewed (to a half) to maintain NVP concentration and water quality as per recommended guidelines.37
The exposure media were sampled for instrumental analysis to determine the concentration of NVP. The analyses were done by an ISO 17025 accredited laboratory using ultra-high-pressure liquid chromatography (UPLC) coupled to quadrupole time-of-flight mass spectrometry (QTOF/MS).38
Statistical analysis
The IBM SPSS Statistics software (version 24) was used for statistical data analysis with a significance level of p<0.05. Data were checked for normality of distribution using the Shapiro-Wilk test; the Spearman correlation coefficient moment (rho) was used to test the strength and direction of association between length and mass measurements; and non-parametric Kruskal-Wallis and Mann-Whitney U tests were used to test the significance of differences between the NVP-exposed fish and control fish.
Results
The analysis of test media samples showed that NVP concentrations never fell below 80% of the nominal concentration. NVP was below the detection limit in both the control and solvent control media.
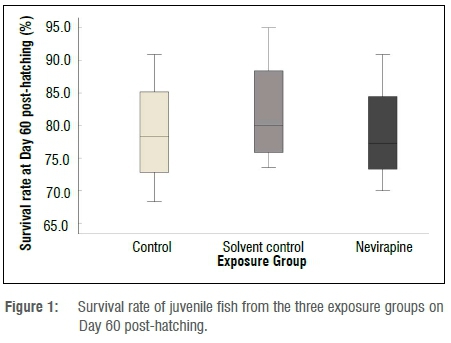
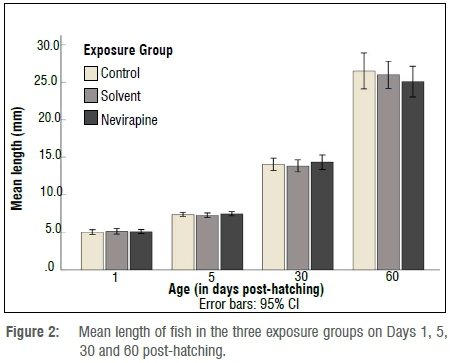
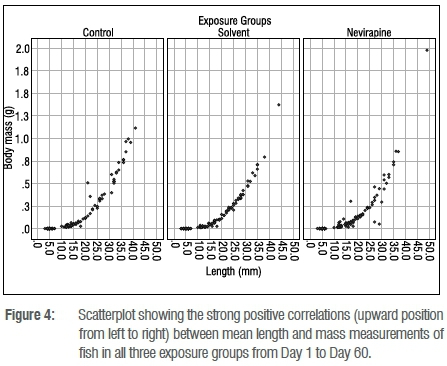
At the end of the exposure, the mean survival rate of 60-day-old fish was above 78% in all the groups (Figure 1). No statistically significant difference in survival rate was found between the NVP-exposed fish and the control fish (p=0.683). The length and mass measurements of larvae in all exposure containers and groups were very similar for 1- and 5-day-old fish, but 30- and 60-day-old fish showed variations in growth rate in the same container and in all groups. The mean length and mass, respectively, of 60-day-old juveniles were 26.5±8 mm and 0.410±0.30 g for the control group; 26±6.1 mm and 0.336±0.24 g for the solvent control fish; and 25.1±7 mm and 0.319±0.33 g for the NVP-exposed fish (Figures 2 and 3). The mean length and mass from Day 1 to Day 60 are presented in Table 1. The largest and heaviest 60-day-old juvenile fish was from the NVP-exposed fish with a total length of 49 mm and mass of 1.976 g; and the smallest fish was from the control group with a total length of 13 mm and mass of 0.032 g. Statistical comparison of length and mass measurements of larvae and juveniles in the NVP-exposed group with those from the control and solvent groups showed no statistically significant difference (p=0.995 and p=0.808, respectively). As the fish aged, they were also growing in length and gaining mass. To assess the strength of the relationship between length and mass measurements in all three groups, the Spearman correlation coefficient rho was used. A strong positive and significant (p=0.0001) correlation was found between length and mass measurements from Day 1 to Day 60 post-hatching in all the groups: control group rho=0.958, solvent control group rho=0.955 and nevirapine group rho=0.949 (Figure 4). The relationships length-age and mass-age were also strong, positive and significant (p=0.0001) in all groups, respectively: 0.883 and 0.691 for the control fish; 0.917 and 0.696 for the solvent control fish; and 0.890 and 0.574 for the NVP-exposed fish. The length-age relationship was stronger than the mass-age relationship in all groups. A standard multiple regression showed that in all groups, although both length and mass could be used to predict the age of the fish, length was a better predictor than mass, with adjusted R2=0.862 for the control group (beta=1.54, p=0.0001), R2=0.918 for the solvent control group (beta=1.447, p=0.0001) and R2=0.872 for the NVP group (beta=1.303, p=0.0001). The calculated mean CF for the 60-day-old fish was 1.8±0.6, 1.7±0.1 and 1.7±0.8 for the control, the solvent control and NVP-exposed fish, respectively (Figure 5).
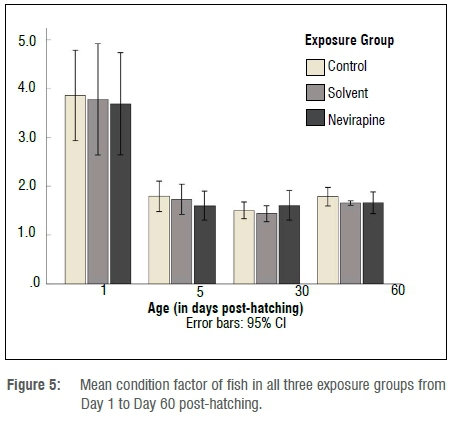
Statistical analysis showed no significant differences between the NVP-exposed fish and both control groups (p=0.427). The mean CF values for the fish in each group are presented in Table 1.The mean daily specific growth rate (SGR) of the fish was calculated as a percentage using mass measurements from Day 1 to Day 30 and from Day 30 to Day 60 post-hatching. SGR shows the mean percentage increase in body mass per day. The SGRs for the first month were 8.4±1.6%, 8.4±1.5% and 8.9±2.3% for the control, solvent and NVP-exposed fish, respectively, while for the second month they were 7.5±0.7%, 7.2±0.8% and 6.4±0.7% (Table 2).
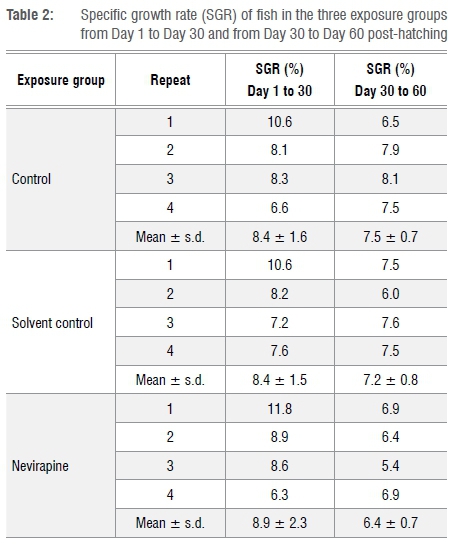
The SGR for NVP-exposed fish between the 30th and 60th day was noticeably lower than that of the control fish, but statistical analyses showed no significant differences in SGR between the three groups (p=0.138).
Discussion
We used a laboratory chronic exposure to examine the possible effects of the highest environmental concentration of NVP previously reported in South African surface water on the growth of early life stages of O. mossambicus. Although our results showed no significant differences in length and mass of larvae and juveniles between those exposed to NVP and control fish, it was noted that 30- and 60-day-old juveniles showed variations in growth rate in the same container and in all groups. However, this finding is not abnormal in the Oreochromis genus; previous studies on various developmental stages (embryos, larvae and juveniles) of O. niloticus from several broods observed variations in development and growth rates among fish from the same brood - the authors of those studies suggested that the variations were probably caused by the density of fish and the inadequate quality of food.33,34
For the present study, the exposure started with the same number of embryos in each group for each repeat, and all larvae and early juveniles received the same treatment in terms of feeding and care. However, after hatching, there were mortality cases in all groups as shown by the survival rate presented in Figure 1. Even though the mortality rate was low with no significant differences in survival rate between groups, it may have caused a certain disturbance in the density of fish in the tanks concerned, leading to observed slight variations in growth parameters within groups. In addition, the length and mass measurements recorded in this study as well as the growth rate are comparable to those from a previous study on O. mossambicus from a natural environment in which 1-day-old larvae had a mean standard length of 4 mm39 and O. niloticus reared in captivity had lengths of 4.9±0.1 mm for 1-day-old larvae and 17±0.8 mm for 30-day-old juveniles.33
The strong positive correlation between length and mass measurements, and between fish age and both length and mass measurements, in all groups, confirmed that as the larval and juvenile stages of O. mossambicus were aging, they were also increasing both in length and in mass, as is the case for healthy fish.28,40 If NVP in water had a negative impact on fish growth, the relationship between length and mass would have been affected, and the correlation would not have been strong and positive. In fisheries, healthy fish are characterised by strong positive mass-length, age-length and age-mass relationships; and length measurements are the most used in regressive models to predict either fish age or fish mass.29 For the present study, it was also found that length measurements in all groups were good predictors of the age of fish.
The calculated CF showed that larvae and juvenile fish from all three groups had a high mean CF of about 4 for 1-day-old larvae; however, as the fish were growing, the CF decreased and approached 1 - the normal CF for healthy adult fish.12 Froese28 previously reported that fish early life stages have a normal CF above 1 because they increase more rapidly in length than they do in other dimensions. The mean CF of the fish in all the groups was within normal range for early life stages of fish; therefore, NVP had no significant negative impact on the CF.
The fish in all three groups had a high and rapid mean SGR from Day 1 to Day 30; from Day 30 to Day 60, the mean SGR decreased in all groups but was still high compared to the SGR recorded for O. niloticus in their natural environment.40O. mossambicus early life stages are known to have a fast and high initial growth rate to allow them to reach large sizes quickly in order to avoid predation.41 According to Sparre and Venema29, in normal conditions, the growth rate of a healthy fish decreases as the fish gets older, becoming zero when the fish gets to its maximal growth. Thus, even though the NVP-exposed juveniles showed a reduced mean SGR between the 30th and the 60th day of their life, statistical analysis showed no significant differences between the three groups. It is therefore evident that, despite the observed variations in the rate of development, the fish in all the three groups showed comparable growth rates from the first day of their life. Therefore, NVP did not significantly affect the growth rate of the fish. The variations observed in growth rate of the fish in the same group have also been observed in previous studies on Oreochromis and are believed to be mainly caused by fish density, food quality and variation in temperature of the water.33,34,40,41 As this study was conducted in a controlled-environment room, and the fish in all groups were fed at the same time and with the same amount of food, the only factor that could have caused the variations in growth rate would be the slight variations in fish density, as explained above.
Conclusion
This study was the first in which the effects of NVP on larval and juvenile stages of O. mossambicus were assessed. It can be concluded that the highest concentration (1.48 µg/L) of NVP detected in South African waters at the start of this study, did not have significant detrimental effects on O. mossambicus early juvenile growth in terms of length and body mass during the 2 first months of life. However, a slightly reduced growth rate was noted between the 30th and the 60th day in the NVP-exposed fish compared to the control fish. Future studies should further investigate this finding by extending the exposure to adult stages.
Acknowledgements
We thank the University of Johannesburg Aquarium staff for their help, especially during the initial phase of the study, with the setting up of the environmental room in which the experiments were conducted and their guidance and assistance in fish breeding and keeping. We also acknowledge Mr C. Sihoka for his assistance with statistical analysis. This research was funded by the National Research Foundation of South Africa (Innovative Funding for Rated Researchers [UID: 86056] and DST Innovation Doctoral Scholarships [UID: 98719]); the Global Excellence and Stature of the University of Johannesburg, South Africa (to student No. 201242617); and the University of Venda (RPC project SMNS/17/ZOO/03). We acknowledge the DST-NRF Centre of Excellence in Mathematical and Statistical Sciences (CoE-MaSS), South Africa, for their support in the publication of this article. Opinions expressed and conclusions arrived at are those of the authors and are not necessarily to be attributed to the CoE-MaSS.
Authors' contributions
U.M.C.N.: Conceptualisation; methodology; data collection; sample analysis; data analysis; validation; data curation; writing - the initial draft. G.M.W.: Conceptualisation; project leadership; project management; funding acquisition; writing - revisions; student supervision. I.E.J.B.: Writing - revisions; student supervision; funding acquisition.
References
1.Boxall ABA. The environmental side effects of medication. EMBO Rep. 2004;5(12):1110-1116. https://doi.org/10.1038/sj.embor.7400307 [ Links ]
2.Corcoran J, Winter MJ, Tyler CR. Pharmaceuticals in the aquatic environment: A critical review of the evidence for health effects in fish. Crit Rev Toxicol. 2010;40(4):287-304. https://doi.org/10.3109/10408440903373590 [ Links ]
3.World Health Organization (WHO). Pharmaceuticals in drinking water. WHO/HSE/WSH/11.05. Geneva: World Health Organization; 2011. Available from: http://www.who.int/water_sanitation_health/publications/2011/pharmaceuticals_20110601.pdf [ Links ]
4.Kolpin DW, Furlong ET, Meyer MT, Thurman EM, Zaugg SD, Barber LB, et al. Pharmaceuticals, hormones, and other organic wastewater contaminants in U.S. streams 1999-2000: A national reconnaissance. Environ Sci Technol. 2002;36(6):1202-1211. https://doi.org/10.1021/es011055j [ Links ]
5.Kidd AK, Blanchfield JP, Mills HK, Palace PV, Evans ER, Lazorchak MJ, et al. Collapse of a fish population after exposure to a synthetic estrogen. Proc Natl Acad Sci USA. 2007;104(21):8897-8901. https://doi.org/10.1073/pnas.0609568104 [ Links ]
6.Lin T, YanQiu C, Chen W. Impact of toxicological properties of sulphonamides on the growth of zebrafish embryos in the water. Environ Toxicol Pharmacol. 2013;36:1068-1076. https://doi.org/10.1016/j.etap.2013.09.009 [ Links ]
7.Triebskorn R, Casper H, Scheil V, Schwaiger J. Ultrastructural effects of pharmaceuticals (carbamazepine, clofibric acid, metoprolol, diclofenac) in rainbow trout (Oncorhynchus mykiss) and common carp (Cyprinus carpio). Anal Bioanal Chem. 2007;387:1405-1416. https://doi.org/10.1007/s00216-006-1033-x [ Links ]
8.Robson L, Barnhoorn IEJ, Wagenaar I. The potential effects of efavirenz on Oreochromis mossambicus after acute exposure. Environ Toxicol Pharmacol. 2017;56:225-232. https://doi.org/10.1016/j.etap.2017.09.017 [ Links ]
9.Aus der Beek T, Weber FA, Bergmann A, Hickmann S, Ebert I, Hein A, et al. Pharmaceuticals in the environment - Global occurrences and perspectives. Environ Toxicol Chem. 2016;35(4):823-835. https://doi.org/10.1002/etc.3339 [ Links ]
10.Human Sciences Research Council (HSRC). The fifth South African national HIV prevalence, incidence, behaviour and communication survey, 2017: HIV impact assessment summary report. Cape Town: HSRC Press; 2018. Available from: http://www.hsrc.ac.za/uploads/pageContent/9225/SABSSMV_Impact_Assessment_Summary_ZA_ADS_cleared1%20(002).pdf [ Links ]
11.World Health Organization (WHO). Global update on HIV treatment 2013: Results, impact and opportunities: WHO report in partnership with UNICEF and UNAIDS. Geneva: World Health Organization; 2013. Available from: http://www.who.int/hiv/pub/progressreports/update2013/en/ [ Links ]
12.Prasse C, Schlusener MP, Schulz R, Ternes TA. Antiviral drugs in wastewater and surface waters: A new pharmaceutical class of environmental relevance? Environ Sci Technol. 2010;44(5):1728-1735. https://doi.org/10.1021/es903216p [ Links ]
13.Swati J, Raj VK, Prabhat P, Sangeeta V. A review on fate of antiviral drugs in environment and detection techniques. Int J Environ Sci. 2011;1(7):1526-1541. https://doi.org/10.6088/ijessi.00107020012 [ Links ]
14.Madikizela LM, Tavengwa NT, Chimuka L. Review: Status of pharmaceuticals in African water bodies: Occurrence, removal and analytical methods. J Environ Manage. 2017;193:211-220. https://doi.org/10.1016/j.jenvman.2017.02.022 [ Links ]
15.Wood TP, Basson AE, Duvenage C. The chlorination behaviour and environmental fate of the antiretroviral nevirapine in South African surface water. Water Res. 2016;104:349-360. https://doi.org/10.1016/j.watres.2016.08.038 [ Links ]
16.Wood TP, Duvenage CSJ, Rohwer E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface waters. Environ Pollut. 2015;199:235-243. https://doi.org/10.1016/j.envpol.2015.01.030 [ Links ]
17.Swanepoel C, Bouwman H, Pieters R, Bezuidenhout C. Presence, concentrations and potential implications of HIV-anti-retrovirals in selected water resources in South Africa. WRC report no. 2144/1/14. Pretoria: Water Research Commission; 2015. https://doi.org/10.13140/RG.2.2.20637.51688 [ Links ]
18.Vankova M. Biodegradability analysis of pharmaceuticals used in developing countries; screening with OxiTop®-C 110 [thesis]. Tampere, Finland: Tampere University of Technology; 2011. [ Links ]
19.Antunes MMA, Sidarus M, Novais AD, Harjivan GS, Santos PP, Da Silva JLF, et al. Oxidation of 2-hydroxynevirapine, a phenolic metabolite of the anti-HIV drug nevirapine: Evidence for an unusual pyridine ring contraction. Molecules. 2012;17:2616-2627. https://doi.org/10.3390/molecules17032616 [ Links ]
20.Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother. 2007;59:342-346. https://doi.org/10.1093/jac/dkl524 [ Links ]
21.Brausch JM, Connors KA, Brooks BW, Rand GM. Human pharmaceuticals in the aquatic environment: A review of recent toxicological studies and considerations for toxicity testing. Rev Environ Contam Toxicol. 2012;218:1-99. https://doi.org/10.1007/978-1-4614-3137-4_1 [ Links ]
22.Stancova V, Plhalova L, Bartoskova M, Zivna D, Prokes M, Marsalek P, et al. Effects of mixture of pharmaceuticals on early life stages of tench (Tinca tinca). Biomed Res Int. 2014;2014: 253-468. http://dx.doi.org/10.1155/2014/253468 [ Links ]
23.Memmert U, Peither A, Burri R, Weber K, Schmidt T, Sumpter JP, et al. Diclofenac: New data on chronic toxicity and bio-concentration in fish. Environ Toxicol Chem. 2013;32(2):442-452. https://doi.org/10.1002/etc.2085 [ Links ]
24.Schoenfuss HL, Furlong ET, Phillips PJ, Scott TM, Kolpin DW, Cetkovic-Cvrlje M, et al. Complex mixtures, complex responses: Assessing pharmaceutical mixtures using field and laboratory approaches. Environ Toxicol Chem. 2016;35(4):953-965. https://doi.org/10.1002/etc.3147 [ Links ]
25.Gonzalez-Doncel M, Garcia-Maurino JE, San Segundo L, Beltran EM, Sastre S, Torija CF. Embryonic exposure of medaka (Oryzias latipes) to propylparaben: Effects on early development and post-hatching growth. Environ Pollut. 2014;134:360-369. https://doi.org/10.1016/j.envpol.2013.09.022 [ Links ]
26.Holden KK, Bruton MN. A life-history approach to the early ontogeny of the Mozambique tilapia Oreochromis mossambicus (Pisces, Cichlidae). Afr Zool. 1992;27:173-191. https://doi.org/10.1080/02541858.1992.11448279 [ Links ]
27.Sadauskas-Henrique H, Sakuragui MM, Paulino MG, Fernandes MN. Using condition factor and blood variable biomarkers in fish to assess water quality. Environ Monit Assess. 2011;181:29-42. https://doi.org/10.1007/s10661-010-1810-z [ Links ]
28.Froese R. Cube law, condition factor and weight-length relationships: History, meta-analysis and recommendations. J Appl Ichthyol. 2006;22:241-253. https://doi.org/10.1111/j.1439-0426.2006.00805.x [ Links ]
29.Sparre P, Venema SC. Introduction to tropical fish stock assessment. Part 1: Manual. FAO Fisheries Technical Paper no. 306.1, Rev. 2. Rome: FAO; 1998. Available from: http://www.fao.org/docrep/W5449E/w5449e00.htm [ Links ]
30.Pena R, Dumas S. Development and allometric growth patterns during early larval stages of the spotted sand bass Paralabrax maculatofasciatus (Percoidei: Serranidae). In: Clemmesen C, Malzahn AM, Peck MA, Schnack D, editors. Advances in early life history study of fish. Barcelona: Scientia Marina; 2009. p. 183-189. Available from: http://hdl:10013/epic.36540 [ Links ]
31.Sumpter JP. Pharmaceuticals in the environment: Moving from a problem to a solution. In: Kummerer K, Hempel M, editors. Green and sustainable pharmacy. Berlin: Springer-Verlag; 2010. p. 11-22. Available from: https://www.springer.com/us/book/9783642051982 [ Links ]
32.Kruger T. Effects of zinc, copper and cadmium on Oreochromis mossambicus free embryos and randomly selected mosquito larvae as biological indicators during acute toxicity testing [thesis]. Johannesburg: Rand Afrikaans University; 2002. [ Links ]
33.Fujimura K, Okada N. Development of the embryo, larva and early juvenile of the Nile tilapia Oreochromis niloticus. Developmental staging system. Develop Growth Differ. 2007;49:301-324. https://doi.org/10.1111/j.1440-169X.2007.00926.x [ Links ]
34.Morrison CM, Miyake T, Wright JR Jr. Histological study of the development of the embryo and early larvae of Oreochromis niloticus (Pisces: Cichlidae). J Morphol. 2001;247:172-195. https://doi.org/10.1002/1097-4687(200102)247:2<172::AID-JMOR1011>3.0.CO;2-H [ Links ]
35.Carlander KD. Handbook of freshwater fishery biology. 3rd ed. Ames, IA: Iowa State University Press; 1969. Available from: https://UnivofPretoria.on.worldcat.org/oclc/934888916 [ Links ]
36.Bagenal TB, Tesch FW. Age and growth. In: Bagenal TB, editor. Method assessment of fish production in freshwater. 3rd ed. Oxford: Blackwell Scientific Publication; 1978. p. 101-136. [ Links ]
37.Organisation for Economic Co-operation and Development (OECD). Test no. 210: Fsh, early-life stage toxicity test. OECD guidelines for the testing of chemicals, section 2. Paris: OECD Publishing; 2013. https://doi.org/10.1787/9789264203785-en [ Links ]
38.Ferrer I, Thurman EM. Analysis of 100 pharmaceuticals and their degradates in water samples by liquid chromatography/quadrupole time-of-flight mass spectrometry. J Chromatogr A. 2012;1259:148-157. https://doi.org/10.1016/j.chroma.2012.03.059 [ Links ]
39.Tachihara K, Obara E. Morphological development of embryos and juveniles in the Mozambique tilapia, Oreochromis mossambicus as a direct developmental fish under rearing conditions. Suisan Zoshoku. 2003;51(3):295-306. [ Links ]
40.Makori AJ, Abuon PO, Kapio R, Anyona DN, Dida GO. Effects of water physico-chemical parameters on tilapia (Oreochromis niloticus) growth in earthen ponds in Teso North Sub-County, Busia County. Fish Aquat Sci. 2017;20, Art. #30, 10 pages. https://doi.org/10.1186/s41240-017-0075-7 [ Links ]
41.Weyl OLF, Hecht T. The biology of Tilapia rendalli and Oreochromis mossambicus (Pisces: Cichlidae) in a subtropical lake in Mozambique. Afr Zool. 1998;33(3):178-188. https://doi.org/10.1080/02541858.1998.11448469 [ Links ]
 Correspondence:
Correspondence:
Ina Wagenaar
inaw@uj.ac.za
Received: 21 Aug. 2018
Revised: 31 Jan. 2019
Accepted: 01 Feb. 2019
Published: 27 Mar. 2019














