Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.115 n.1-2 Pretoria Jan./Feb. 2019
http://dx.doi.org/10.17159/sajs.2019/4391
RESEARCH ARTICLE
Genotoxicity of aqueous extracts of Tulbaghia violacea as determined through an Allium cepa assay
Lerato N. Madike; Samkeliso Takaidza; Cornelius Ssemakalu; Michael Pillay
Department of Biotechnology, Vaal University of Technology, Vanderbijlpark, South Africa
ABSTRACT
Tulbaghia violacea (wild garlic) is commonly used in traditional medicine for the treatment of various ailments including fungal infections, gastrointestinal ailments, asthma, fever, colds and pulmonary tuberculosis. We assessed the potential genotoxic effects of water extracts from the leaves, stems and roots of T. violacea using the Allium cepa assay. Extracts at concentrations of 100, 250, 500 and 1000 μg/mL were tested on root meristems of A. cepa. Ethidium bromide was used as a positive control whereas distilled water acted as a negative control. The results reveal that as the concentrations of the water extracts of T. violacea increased, the mitotic indices decreased. Similarly, the percentage of chromosomal aberrations was dependent on the concentration as well as on which part of the plant was used. The six most common chromosome aberrations included laggard chromosomes, chromosome bridges, c-mitosis, sticky chromosomes, formation of binuclei and formation of trinuclei. The presence of micronucleated cells at interphase also increased as the concentration of the water extracts increased. The results confirm that water extracts of T. violacea exert significant genotoxic effects at higher concentrations, with the stem extracts being more toxic than the leaf and root extracts at similar concentrations.
SIGNIFICANCE:
•Water extracts of T. violacea - a plant commonly used in traditional medicine - were found to have significant genotoxic effects at higher concentrations
Keywords: traditional medicines; chromosome aberrations; ethidium bromide; genotoxic; mitotic index
Introduction
Recently, there has been a significant increase in the number of herbal medicinal products globally. It is estimated that the world's population will be greater than 7.5 billion in the next 10-15 years, primarily in the southern hemisphere where approximately 80% of the population still relies on a traditional system of medicine based on herbal drugs for primary health care.1-3 There are over 1.5 million medicinal plants that have been investigated and most of them are reported to contain toxic substances.4 Therefore, it should be stressed that the use of any plant for medicinal purposes is not guaranteed to be safe.5 This raises the need for further research on the mode of preparation and toxicology of medicinal plants to gather reliable information on their safety and effective use.6Tulbaghia violacea Harv., a member of the family Amaryllidaceae (formerly Alliaceae), is commonly known as 'wild garlic or society garlic'.7-9 The plant is found in the Eastern Cape, KwaZulu-Natal and northern Gauteng in South Africa, and even as far north as Zimbabwe.10Tulbaghia violacea has traditionally been used extensively in South African traditional medicine for the treatment of HIV/Aids and oral fungal infections. It has also found diverse application in the treatment of gastrointestinal ailments, including as a purgative for treatment of constipation; asthma; fever; colds; and pulmonary tuberculosis10-13; and as an anti-helminthic 14. It has been reported that the odour of T. violacea deters moles.12 Zulu communities in South Africa grow this plant around their homes as it is believed to repel snakes and the bulbs are used to prepare an aphrodisiac.10-12
However, like any other drug, extensive consumption of medication prepared from T. violacea has been associated with a variety of undesirable symptoms such as abdominal pain, inflammation, gastroenteritis, acute inflammation and sloughing of the intestinal mucosa, cessation of gastrointestinal peristalsis, contraction of the pupils, subdued reactions to stimuli and even fatality.8,11,15 The aim of this study was to evaluate the genotoxicity of the leaf, stem and root extracts of T. violacea by using the Allium cepa assay.
Materials and methods
Plant collection
Tulbaghia violacea was collected from different indigenous plant nurseries in the Gauteng Province of South Africa, and grown in a greenhouse at the Vaal University of Technology (Gauteng, South Africa). Identification of this plant was done with the assistance of Professor Stefan Siebert, a botanist at AP Goossen's Herbarium, North-West University, where a unique voucher specimen number ST0008 was deposited.
Preparation of plant extracts
Whole plants of T. violacea were uprooted carefully and washed with tap water to remove the soil and debris. The leaves, stems and roots were then separated from each other, cut into small pieces, frozen at -20 °C, lyophilised and eventually pulverised into a fine powder. Crude water extracts from the different parts of the plant were prepared by mixing 10 g of the pulverised plant material with 200 mL distilled water. The mixture was then boiled for 10 min and allowed to cool to room temperature. Thereafter, the mixture was filtered through 0.45 µm Whatman® filter paper. The resultant filtrate was then frozen and lyophilised. A stock solution of each part of the lyophilised crude water extract was prepared at a concentration of 10 mg/mL using distilled water and stored at -20 °C in opaque vessels until they were required. The crude extracts of 10 mg/mL (0.1 g in 10 mL) from the leaves, stems and roots of T. violacea were each reconstituted by dissolving in distilled water to concentrations of 100, 250, 500 and 1000 µg/mL.
Pre-treatment
The Allium cepa L. (onion) bulbs were grown in distilled water at room temperature for 2-3 days. When the roots were 2-6 mm in length, the bulbs were treated with different concentrations of the crude extracts (100, 250, 500 and 1000 μg/mL; n=4 for each) for 24 h. Another set of the onions was placed in ethidium bromide (100, 250, 500 and 1000 μg/mL; n=4 for each) to serve as the positive control while a further set (n=4) was grown in distilled water to serve as the negative control. The root growth was measured, and the solutions were changed daily. The onion with the poorest growth from every concentration (100, 250, 500 and 1000 μg/mL) and controls (positive and negative) was excluded from the experiment and the remaining three onions were prepared for microscopy.16,17
Slide preparation
For each bulb, five root tips at approximate lengths of 10 mm were harvested and fixed in ethanol/glacial acetic acid solution 3:1 (v/v) for 10 min. After fixation, the root tips were washed a few times with distilled water. They were then hydrolysed with 1 N HCl at 60-70 °C for 5 min. The roots were then washed a few times with distilled water. The terminal 1-2 mm of the root tip was cut and placed on a glass slide. The excess liquid was sucked up using blotting paper. A drop of freshly prepared acetocarmine was placed on the root tips and left for 5-10 min at room temperature. Using a glass coverslip, the stained root tips were squashed to form a smear and the excess stain was blotted with paper towel. The sides of the coverslip were sealed with clear fingernail polish. Three slides were prepared per bulb with a total of nine slides per concentration.
Observation of slides
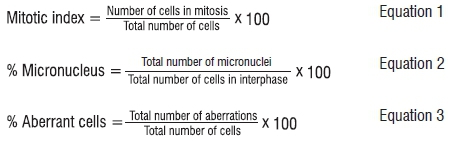
The slides were observed under a light microscope at 200x and 400x magnification. A light microscope (Olympus, Tokyo, Japan) with a digital camera was used to obtain images of the chromosome aberrations. Photomicrographs (10 images per slide) were prepared and a minimum of 1000 cells per slide were analysed (nine slides were observed for each treatment). The mitotic index, presence of micronuclei and chromosome aberrations in mitotic phases were calculated by examining and counting a minimum of 1000 cells per slide (nine slides were observed for each treatment). The experiment was replicated three times with three roots for each replicate. Therefore, nine slides were prepared for each treatment group. The mitotic index, percentage cells with micronuclei and aberrant cells were obtained using Equations 1, 2
ImageJ analysis
Images obtained from the light microscope were converted to 8-bit greyscale using ImageJ software (version 1.46r, Bethesda, MD, USA). The thresholds of the images were then adjusted to obtain the best fit for different particle aggregates in each cell and the total number of cells was calculated. A total number of nine slides for each concentration of plant extract (100, 250, 500 and 1000 μg/mL) was analysed. From each slide, 10 images were assessed, amounting to a total of 90 images per concentration of plant extract. The mean data for each concentration were used for further analysis.
Data analysis
The mean and standard error of the mean for each of the treatment groups were calculated. Data obtained from the microscopic and ImageJ analyses were analysed using a multiple t-test to determine significant differences between treatment groups and the negative control (p<0.05).
Results
Root growth, mitotic index and chromosomal aberration analysis
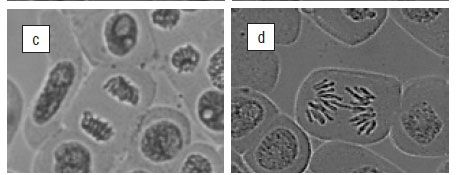
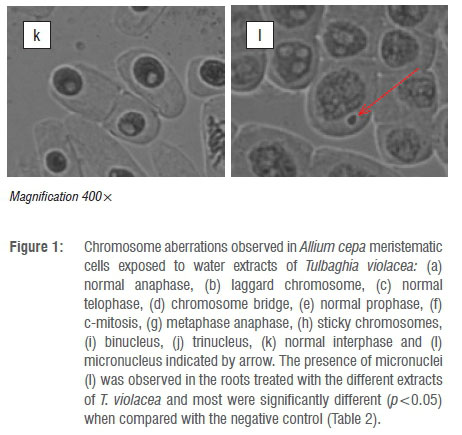
The root length, mitotic indices (MI) and chromosomal aberrations for the various concentrations of the water extracts of the leaves, stems and roots of T. violacea on A. cepa are shown in Table 1. The results show that as the concentration of the crude extract increased, there was a significant (p<0.05) decrease in mean root length, mitotic indices and percentage of aberrant chromosomes. The mitotic index decreased significantly (p<0.05) with the leaf extracts at 500 μg/mL and 1000 μg/mL with values of 27.78% and 24.54%, respectively. Leaf extracts at 100 μg/mL and 250 μg/mL produced mitotic indices of 43.71% and 58.66%, respectively. For the stem extracts, the higher concentrations (250 500, 1000 μg/mL) produced a significant reduction in mitotic indices with values of 33.64%, 32.57% and 19.69%, respectively, when compared to the non-significant 40.56% for 100 µg/mL. Similarly, water extracts of the roots at 250, 500 and 1000 µg/mL significantly decreased mitotic indices to 37.24%, 31.08% and 22.59%, respectively, whereas the 100 µg/mL treatment produced no significant change (MI=58.88%). These values were low when compared to the mitotic index for the negative control (distilled water) which was 61.83%. For the positive control (ethidium bromide), all the tested concentrations produced a significant reduction in the mitotic index, with the lowest MI value of 1.48% produced by the highest concentration of 1000 μg/mL. Chromosome aberrations were observed in all stages of mitosis (prophase, metaphase, anaphase and telophase). Figure 1a-l depicts illustrations of normal phases of cell division as well as the six common aberrations observed in the A. cepa assay. These aberrations include laggards (Figure 1b), chromosome bridges (Figure 1d), c-mitosis (Figure 1f), sticky chromosomes (Figure 1h), binuclei (Figure 1i) and trinuclei (Figure 1j). The most common chromosomal aberrations were represented by c-mitosis, binuclei formation and sticky chromosomes (Figure 1e-j). The percentage of aberrant cells was 4.72% for the negative control (distilled water), when compared to 21.91% for the highest concentration of 1000 µg/mL for the positive control (ethidium bromide).
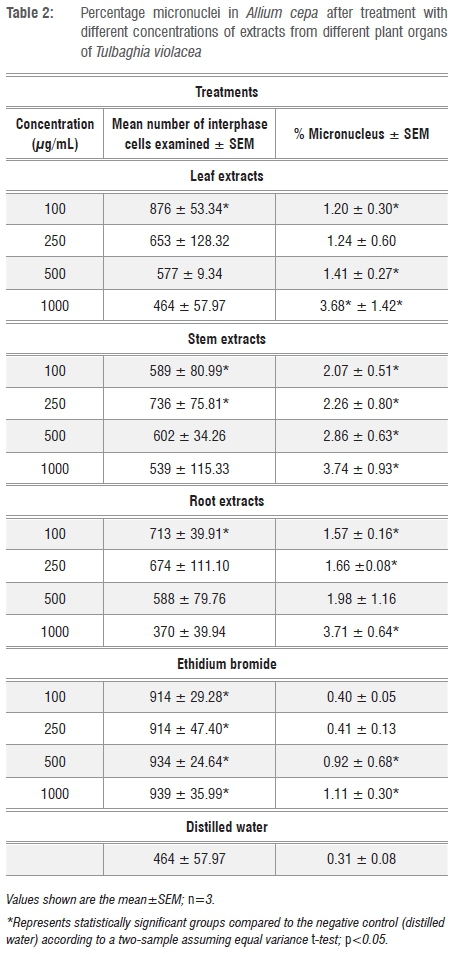
The percentage of micronucleated cells was generally higher at 1000 µg/mL for the stem (3.74%), followed by the root (3.71%) and finally the leaf (3.68%) crude extracts (Table 2).
Discussion
Root length and mitotic indices
A decrease of over 45% in root length indicates the presence of toxic substances18, which can be regarded to have a sub-lethal effect on plants19. In our study, the decrease of over 45% in root length was dependent on the concentration of the extracts and was variable for the different plant organs. For example, inhibition of root growth by the leaf and root extracts occurred at concentrations above 250 μg/mL, whereas that of the stem extracts occurred at concentrations above 100 μg/mL (Table 1). Therefore, although the leaf and root extracts showed potential toxicity at concentrations above 250 µg/mL, the stems may be considered toxic at concentrations above 100 µg/mL.
Similarly, any substance that reduces the mitotic index to below 22% of the negative control is considered to cause lethal effects on test organisms while a reduction below 50% has sub-lethal effects20 and is called the cytotoxic limit value. We have adopted this system of categorisation in this study. In general, treatment of the A. cepa roots with distilled water (negative control) was non-toxic whereas that with ethidium bromide (positive control) was toxic at all tested concentrations with MI values below 22%. Ethidium bromide has been found to be an extremely effective cytoplasmic mutagen which results in the loss or alteration of DNA and RNA.21 It was found that a low concentration of 100 µg/mL for the leaf extracts produced no toxic effect on the roots as shown by the relatively high mitotic index of 58.66% (Table 1). However, concentrations higher than 100 µg/mL produced a sub-lethal effect as the mitotic indices were below 50% (Table 1). Similar results were obtained for the root extracts (Table 1). For the stem extracts, concentrations of 100, 250 and 500 µg/mL produced a sub-lethal effect on A. cepa whereas the 1000 µg/mL concentration produced a lethal effect with an MI value of 19.69%. The lower mitotic indices, especially for the higher concentrations of the crude extracts, may be as a result of the direct genotoxic effects of the extracts. Several factors can decrease the mitotic index, such as obstruction of the onset of prophase, the arrest of one or more mitotic phases, or a slowing of the rate of cell progression through mitosis.22 Similar reasons may be applicable for the decreased mitotic indices observed in this study. Microscopic analysis revealed a concentration-dependent reduction in mitotic indices (Table 1) with significant differences (p<0.05) between the treated groups and the positive control (ethidium bromide) and the negative control (distilled water) value of 61.83%.
Most of the chromosomal aberrations observed were in the metaphase and anaphase stages. This finding is in agreement with the results of Armbruster et al.23 who treated wheat root tips with the herbicide dithiopyr and with those of Kaymak and Pinar24 for the herbicide tebuconazole in A. cepa. Both studies concluded that structural aberrations of spindle formation may result in cell division disturbances. The most common aberrations that were present in all the tested plant extracts and controls included binucleated cells, sticky chromosomes and c-mitosis, followed in frequency by laggards, chromosomal bridges and trinucleated cells (Figure 1a-j). The occurrence of aberrant cells was concentration and extract dependent, because as the concentration increased, the number of aberrant cells also increased and varied depending on the extract used. Overall, the highest percentages of aberrant cells were observed after treatment with the stem extracts in comparison with treatment with leaf and root extracts. Laggard chromosomes (Figure 1b) are usually a result of the failure of the chromosomes to attach to the spindle fibre and to move to either of the two opposite poles.25 Chromosome bridges were observed in the anaphase and telophase stages and were more frequent at higher concentrations of T. violacea crude extracts, with the highest number of bridges observed after treatment with stem extracts (Figure 1d). The formation of chromosome bridges may be attributed to chromosomal stickiness and the subsequent failure of chromosome separation during anaphase.26,27 C-mitosis (Figure 1f) results when dissociating disulfide bonds prevent spindle microtubules from assembling28 and is an indication of a weak toxic effect which may be reversible18,29. The number of cells with c-mitosis after treatment with extracts from all plant parts surpassed that of the negative control for all concentrations (100, 250, 500 and 1000 µg/mL) of T. violacea treatment, which confirms the mitodepressive effect of T. violacea crude extracts on spindle fibres. Sticky chromosomes (Figure 1h) are usually a consequence of a physiological effect resulting from depolymerisation of DNA, partial dissolution of nucleoproteins, breakage and exchanges of the basic folded fibre units of chromatids and the stripping of the protein covering of DNA in chromosomes.30 Their presence is an indication of a highly toxic and irreversible effect, probably leading to cell death.31,32 The formation of binucleated or trinucleated (Figure 1i and 1j) cells may be attributed to the inhibition of cytokinesis.33 It is clear from this study that the crude water extracts from the leaves, stems and roots of T. violacea possess chromotoxic and mitodepressive properties. These properties are evident from the reduction in the active mitotic index and manifestation of spindle formation, respectively.34
The percentage of aberrant cells (Table 1) was dependent on the concentration of the extract and from which plant part it was derived. The highest proportion (20.91%) of aberrations occurred with the highest concentration of the stem extracts whilst the lowest proportion (16.39%) occurred with the highest concentration (1000 μg/mL) of the leaf extracts. The aberrations as a result of the root extracts were intermediate between those observed for the stem and leaf extracts. In our previous study35 we found that most of the phytocompounds were present in the leaves of T. violacea and recommended use of the leaves as a way of conserving the species. The results of this study suggest that, to prevent genotoxic effects, concentrations lower than 1000 ug/mL should be used for therapeutic purposes. One of the anomalies observed in this study is that the negative control (water) also induced a low number of chromosomal aberrations. There are thousands of cells in the meristematic zone of the roots that are undergoing mitosis at any one time. It is possible that errors in cell division may be expected under these circumstances. The presence of ions in the water may also cause minor chromosomal aberrations.18 The presence of ions in the negative control was overlooked in this research and should be considered in future research. Nonetheless, there was a significant difference in the number of chromosomal aberrations after treatment with the plant extracts compared to that after treatment with the negative control (distilled water).
The presence of micronuclei-bearing cells (Figure 1l) may be a consequence of clastogenic (chromosome breakage) or aneugenic (chromosome lagging and interference on the spindle behaviour) effects.36,37 The higher percentage of micronucleated cells after treatment with the stem extracts than that after treatment with the leaf and root extracts (Table 2) confirms that the stem extracts had the greatest genotoxic effect on the cells as also indicated by the number of chromosomal aberrations. The positive control (ethidium bromide) which was expected to produce the highest percentage of micronucleated cells actually produced the lowest percentage. This result may have been because of more cells in interphase after treatment with the positive control than those observed after treatment with the T. violacea plant extracts. This observation may also be attributed to the fact that ethidium bromide prevents subsequent replication of DNA by arresting cell division.38
Conclusion
We assessed the potential genotoxic effects of water extracts of the leaves, stems and roots of T. violacea using the A. cepa assay. Generally, high concentrations of the extracts showed potential genotoxic effects, evidenced by the sub-lethal and lethal effects of the different concentrations of the extracts on the roots, the reduced mitotic indices, the abnormal chromosome behaviour and the presence of micronuclei. Extracts of the stem were generally more toxic than those of the leaves and roots. It can thus be concluded that T. violacea plant extracts cause mitodepressive and chromotoxic effects in plant genomes and induce various types of chromosomal aberrations which reveal potential toxicity of the plant, particularly at high concentrations. Although these results provide a good initial indication of the toxicity of T. violacea plant parts, a direct link to the toxicological effect of the extracts in humans was not established. There is thus a need to conduct in vivo cytogenetic studies to ascertain the mechanisms behind the in vitro findings of the A. cepa assay.
Acknowledgements
We acknowledge the Research Directorate at Vaal University of Technology, South Africa, for their financial support.
Authors' contributions
S.T. conceptualised the project and collected the plant; L.N.M. analysed the samples, undertook the data analysis and wrote the manuscript; C.C.S. was responsible for student supervision; M.P. was the project leader and was responsible for data curation and revisions.
References
1.Chan K. Some aspects of toxic contaminants in herbal medicines. Chemosphere. 2003;52(9):1361-1371. https://doi.org/10.1016/S0045-6535(03)00471-5 [ Links ]
2.Ramawat KG, Goyal S. The Indian herbal drugs scenario in global perspectives. In: Ramawat KG, Merillon JM, editors. Bioactive molecules and medicinal plants. Berlin: Springer; 2008. p. 325-347. https://doi.org/10.1007/978-3-540-74603-4_18 [ Links ]
3.Muhammad H, Gomes-Carneiro M, Poça K, De-Oliveira A, Afzan A, Sulaiman S, et al. Evaluation of the genotoxicity of Orthosiphon stamineus aqueous extract. J Ethnopharmacol. 2011;133(2):647-653. https://doi.org/10.1016/j.jep.2010.10.055 [ Links ]
4.Ishii R, Yoshikawa K, Minakata H, Komura H, Kada T. Specificities of bio-antimutagens in plant kingdom. Agric Biol Chem. 1984;48(10):2587-2591. https://doi.org/10.1271/bbb1961.48.2587 [ Links ]
5.Ukwuani AN, Abubakar MG, Hassan SW, Agaie BM. Toxicological studies of hydromethanolic leaves extract of Grewia crenata. Int J Pharm Sci Drug Res. 2012;4:245-249. [ Links ]
6.Mann RD, Andrews EF. Pharmacovigilance. Chichester: Wiley; 2002. https://doi.org/10.1002/0470853093 [ Links ]
7.Van Wyk B-E, Oudtshoorn Bv, Gericke N. Medicinal plants of South Africa. Pretoria: Briza Publications; 1997. [ Links ]
8.Van Wyk B-E, Gericke N. People's plants: A guide to useful plants of southern Africa. Pretoria: Briza Publications; 2000. [ Links ]
9.George S, Bhalerao SV, Lidstone EA, Ahmad IS, Abbasi A, Cunningham BT, et al. Cytotoxicity screening of Bangladeshi medicinal plant extracts on pancreatic cancer cells. BMC Complement Altern Med. 2010;10(1), Art. #52, 11 pages. https://doi.org/10.1186/1472-6882-10-52 [ Links ]
10.Dyson A. Discovering indigenous healing plants of the herb and fragrance gardens at Kirstenbosch National Botanical Garden. Cape Town: South African National Botanical Institute; 1998. [ Links ]
11.Burton SG. A chemical investigation of T. violacea. Grahamstown: Rhodes University; 1990. [ Links ]
12.Kubec R, Velíšek J, Musah RA. The amino acid precursors and odor formation in society garlic (Tulbaghia violacea Harv.). Phytochemistry. 2002;60(1):21-25. https://doi.org/10.1016/S0031-9422(02)00065-1 [ Links ]
13.Ncube B, Finnie JF, Van Staden J. Seasonal variation in antimicrobial and phytochemical properties of frequently used medicinal bulbous plants from South Africa. S Afr J Bot. 2011;77(2):387-396. https://doi.org/10.1016/j.sajb.2010.10.004 [ Links ]
14.Watt JM, Breyer-Brandwijk MG. The medicinal and poisonous plants of Southern and Eastern Africa. Edinburgh: E & S Livingstone Ltd; 1962. [ Links ]
15.Maoela MS. Studies on some biologically active natural products from Tulbaghia alliacea [MSc thesis]. Cape Town: University of the Western Cape; 2005. [ Links ]
16.Oyedare BM, Bakare AA, Akinboro A. Genotoxicity assessment of water extracts of Ocimum gratissimum, Morinda lucida and Citrus medica using the Allium cepa assay. Bol Latinoam Caribe Plantas Med Aromát. 2009;8(2):97-103. [ Links ]
17.Cuyacot AR, Mahilum JJM, Madamba M. Cytotoxicity potentials of some medicinal plants in Mindanao, Philippines. Asian J Plant Sci Res. 2014;4(1):81-89. [ Links ]
18.Fiskesjo G. The Allium test as a standard in environment monitoring. Hereditas. 1985;102:99-102. https://doi.org/10.1111/j.1601-5223.1985.tb00471.x [ Links ]
19.Wierzbicka M. The effect of lead on the cell cycle in the root meristem of Allium cepa L. Protoplasma. 1999;207:186-194. https://doi.org/10.1007/BF01282999 [ Links ]
20.Sharma S, Vig AP. Antigenotoxic effects of Indian mustard (Brassica juncea (L.) Czern.) aqueous seeds extract against mercury (Hg) induced genotoxicity. Sci Res Essays. 2012;7(13):1385-1392. https://doi.org/10.5897/SRE11.468 [ Links ]
21.Soslau G, Fuhrer JP, Nass MMK, Warren L. The effect of ethidium bromide on the membrane glycopeptides in control and virus transformed cells. J Biol Chem. 1974;249(10):3014-3020. [ Links ]
22.Christopher HB, Kapoor MB. The cytogenetic effects of sodium salicylate on the root meristem cells of Allium sativa. L. Caryologia. 1988;54:203-209. [ Links ]
23.Armbruster BL, Molin WT, Bugg MW. Effects of the herbicide dithiopyr on cell division in wheat root tips. Pestic Biochem Physiol. 1991;39(2):110-120. https://doi.org/10.1016/0048-3575(91)90131-5 [ Links ]
24.Kaymak F, Pinar GR. Genotoxic effects of Raxil on root tips and anthers of Allium cepa L. Caryologia. 2009;62(1):1-9. https://doi.org/10.1080/00087114.2004.10589659 [ Links ]
25.Tkalec M, Malaric K, Pavlica M, Pevalek-Kozlina B, Vidakovic-Cifrek Z. Effects of radiofrequency electromagnetic fields on seed germination and root meristematic cells of Allium cepa L. Mutat Res Genet Toxicol Environ Mutagen. 2009;672:76-81. https://doi.org/10.1016/j.mrgentox.2008.09.022 [ Links ]
26.Gömürgen AN. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Caryologia. 2005;70:119-128. https://doi.org/10.1508/cytologia.70.119 [ Links ]
27.Türkoglu S. Evaluation of genotoxic effects of sodium propionate, calcium propionate and potassium propionate on the root meristem cells of Allium cepa. Food Chem Toxicol. 2008;46:2035-2041. https://doi.org/10.1016/j.fct.2008.01.043 [ Links ]
28.Levan A. The effect of colchicine on root mitosis in Allium. Hereditas. 1938;24:471-486. https://doi.org/10.1111/j.1601-5223.1938.tb03221.x [ Links ]
29.Yuet PK, Darah I, Yusuf UK, Yeng C, Sasidharan S. Genotoxicity of Euphorbia hirta: An Allium cepa assay. Molecules. 2012;17(7):7782-7791. https://doi.org/10.3390/molecules17077782 [ Links ]
30.Mercykutty VC, Stephen J. Adriamycin induced genetic toxicity as demonstrated by the Allium test. Cytologia. 1980;45:769-777. https://doi.org/10.1508/cytologia.45.769 [ Links ]
31.Rencuzogullari E, Kayraldiz A, Ila HB, Cakmak T, Topaktas M. The cytogenetic effects of sodium metabisulfite, a food preservative in root tip cells of Allium cepa L. Turk J Biol. 2001;25:361-370. [ Links ]
32.Türkoglu S. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat Res Genet Toxicol Environ Mutagen. 2007(626):4-14. https://doi.org/10.1016/j.mrgentox.2006.07.006 [ Links ]
33.Khanna N, Sharma S. Allium cepa root chromosomal aberration assay: A review. Ind J Pharm Educ. 2013;1(3):105-119. https://doi.org/10.30750/ijpbr.1.3.15 [ Links ]
34.Kumar G, Shikha D. Mitodepressive and chromotoxic impact of nicotinamide on root meristems of Cyamopsis tetragonoloba L. Chromosome Bot. 2012;7:105-110. https://doi.org/10.3199/iscb.7.105 [ Links ]
35.Madike LN, Takaidza S, Pillay M. Preliminary phytochemical screening of crude extracts from the leaves, stems, and roots of Tulbaghia violacea. Int J Pharmacogn Phytochem Res. 2017;9(10):1300-1308. https://doi.org/10.25258/phyto.v9i10.10453 [ Links ]
36.Meng Z, Zhang L. Cytogenetic damage induced by sodium bisulfite. Mutat Res. 1992;298:63-89. https://doi.org/10.1016/0165-1218(92)90030-4 [ Links ]
37.Yi H, Meng Z. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat Res Genet Toxicol Environ Mutagen. 2003;537:109-114. https://doi.org/10.1016/S1383-5718(03)00054-8 [ Links ]
38.Brachet J, Bonotto S. Biology of Acetabularia. New York: Academic Press; 1970. [ Links ]
 Correspondence:
Correspondence:
Michael Pillay
mpillay@vut.ac.za
Received: 05 Feb. 2018
Revised: 29 Aug. 2018
Accepted: 30 Aug. 2018
Published: 30 Jan. 2019