Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.114 n.11-12 Pretoria Nov./Dec. 2018
http://dx.doi.org/10.17159/sajs.2018/5152
RESEARCH ARTICLE
Remains of a barn owl (Tyto alba) from the Dinaledi Chamber, Rising Star Cave, South Africa
Ashley Kruger; Shaw Badenhorst
Evolutionary Studies Institute, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Excavations during November 2013 in the Rising Star Cave, South Africa, yielded more than 1550 specimens of a new hominin, Homo naledi. Four bird bones were collected from the surface of the Dinaledi Chamber during the first phase of the initial excavations. Although mentioned in the initial geological and taphonomic reports, the bird remains have not been formally identified and described until now. Here we identify these remains as the extant barn owl (Tyto alba) which is today common in the region and which is considered to have been an important agent of accumulation of microfaunal remains at many local Plio-Pleistocene sites in the Cradle of Humankind. Based on the greatest length measurement and breadth of the proximal articulation of the tarsometatarsus specimen, it is suggested that a single (female) individual is represented, despite the small sample sizes available for comparison. Although it is unclear how the remains of this female owl came to be accumulated in the remote Dinaledi Chamber, we suggest several possible taphonomic scenarios and hypothesise that these remains are not directly associated with the Homo naledi remains.
Significance:
•Owl bones from the Dinaledi Chamber are the only other macro-vertebrate remains from this Chamber.
•The other remains discovered are that of more than 15 individuals of the enigmatic Homo naledi.
•The remains of the Dinaledi Chamber owl further our understanding of the contents of the important material contained within the Dinaledi system as they are the only more recent fossils to be recovered from this area of the Rising Star Cave system and are therefore important in and of themselves as an indicator that more proximal parts of the Rising Star Cave system have been suitable for use by barn owls at greater time depths than the present.
Keywords: Homo naledi; Cradle of Humankind; faunal remains; Pleistocene; vertebrate taphonomy
Introduction
The Rising Star site
The Rising Star Cave system is located in the Cradle of Humankind UNESCO World Heritage Site, 50 km west-northwest of Johannesburg, South Africa (Figure 1). It is known that amateur cavers had periodically been visiting the cave system for a number of years (see Dirks et al.1); however, it was not until September 2013 that this system was formally investigated and fossil hominin remains were discovered in a very remote chamber named the Dinaledi Chamber.1-3 Several excavations in the Chamber and adjacent spaces have yielded 1681 fossil hominin remains attributed to the new species Homo naledi.1,2,4 Important to this study, approximately 300 bone specimens were collected from the cave surface of the Dinaledi Chamber and a further 1250 numbered fossil specimens were recovered from a small excavation pit in the cave floor no larger than 1 m2 and less than 300 mm deep. This assemblage is the largest single collection of fossil hominin material found on the African continent to date, and the Rising Star Cave system is the only current location of remains of the hominin taxon, H. naledi.1,2,4,5
Dated to between 236 kya and 335 kya5, geologically the Dinaledi Chamber and its fossil contents present an anomalous depositional environment in comparison to the 'classic' sites of the Cradle of Humankind in Gauteng Province, South Africa. Sites such as Sterkfontein, Kromdraai, Swartkrans and Malapa are noted for yielding fossil remains typically contained in lithified breccias, or found in decalcified sedimentary units derived ultimately from clastic lithified breccia.6-11 In the majority of Cradle of Humankind fossil-bearing caves, it is hypothesised that skeletal material was brought into the system through a variety of agents, before being lithified.12 Such agents can be biotic or abiotic, and include processes such as the effects of gravity (for example a fatal fall into a natural death trap, or downslope movement on talus slopes), vertebrate accumulation (predation or scavenging by carnivores, or accumulation by rodents), mass movement of sediments, fluvial transportation, or animal movement into the systems, or a combination of such processes. These processes generally produce taphonomic markers within a fossil assemblage, the role in site formation of which can be inferred from factors such as body-part representation, bone breakage patterns, or traces of surface modification including those of weathering, tooth marks or insect damage.13-18
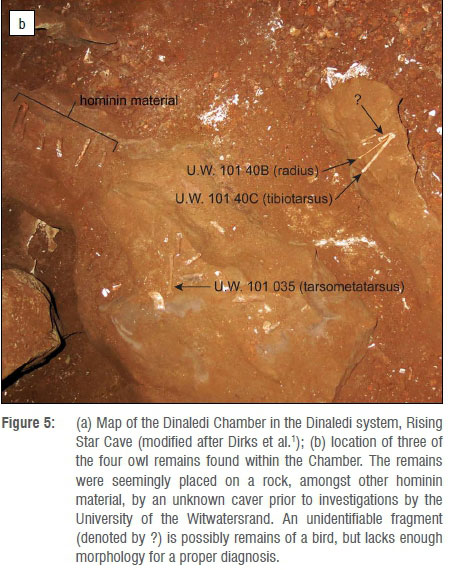
Although a large number of fossil specimens of H. naledi have been recovered from the Dinaledi Chamber, surprisingly no definitive contemporaneous fauna1 has been found to date, apart from four bird bones which were collected from the surface of the Dinaledi Chamber during excavations in 2013. It is clear from the first pictures taken by the exploration teams upon entering the Chamber in September of 2013 that these bones were placed together on a raised stone in the Chamber with a few other bones, indicating likely human agency (by explorers) in their positioning prior to discovery by scientists.
Based upon the physical state of the bones themselves as well as the clear lack of fossilisation as is typical on the hominin bones also found on the surface of the cave, we hypothesise that these remains are modern, or much closer to the present time, and not directly associated temporally with the H. naledi remains, likely being considerably younger. The appearance of preservation of these bones is clearly different from the hominin material found in the chamber.
While initially mentioned in the geological and taphonomic descriptions1, the Dinaledi Chamber bird remains have not been formally identified or described until now. In this paper, we describe the four bird remains from the Dinaledi Chamber, using several possible explanations, expressed as hypotheses, to try explain how these bird bones were introduced into the Dinaledi Chamber.
Methods and results
The Dinaledi Chamber sample contains four bird specimens. Taxonomic diagnosis was made using comparative collections housed at the Ditsong National Museum of Natural History (formerly the Transvaal Museum) in Pretoria, South Africa. The measurements follow procedures given by Von den Driesch19.
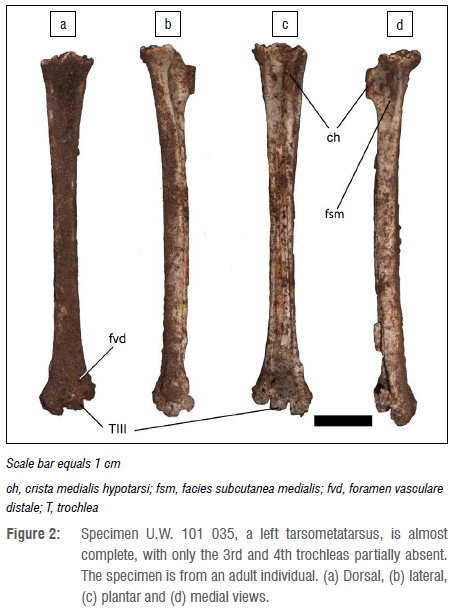
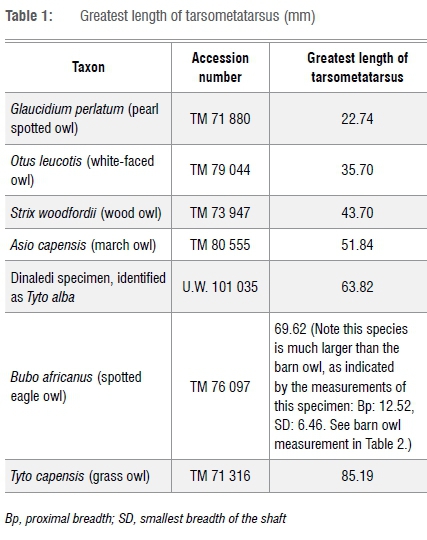
The most complete of these specimens, specimen U.W. 101 035 (Figure 2), a left tarsometatarsus, was used for skeletal measurements. This specimen is almost complete, with only the 3rd and 4th trochleas absent. The specimen is from an adult individual. The morphology of the proximal articulation distinctly places the specimen in the Strigiformes order, which consists of various species of owls. The morphology of the proximal articulation is identical to that of the extant barn owl (Tyto alba). This is supported by the greatest length of the specimen, which is also most similar to the barn owl (Table 1) among Strigiformes examined in this study. Very large- and small-sized owl species are not included because of the substantial adult size differences in these bones. In owls, the distal trochlea is similar in proportions. The presence of the 2nd trochlea is a reflection of maximum length.
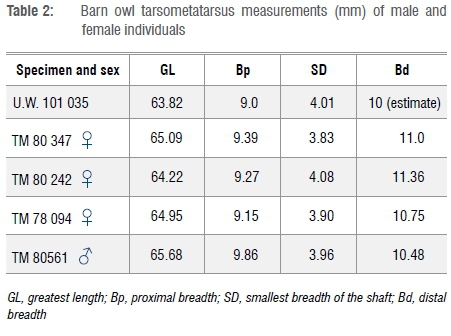
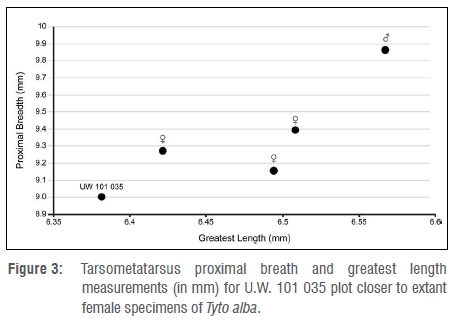
Based on measurement and morphology of the tarsometatarsus of the specimen from the Dinaledi Chamber, it is concluded that a barn owl (Tyto alba) is represented. In many bird species, male individuals are larger than female individuals, and this dimorphism can be reflected in anatomical measurements.20 In the case of the barn owl, there is marked sexual dimorphism in terms of wing length (290-298 mm in male and 235-287 mm in female individuals21). Dimensions of the tarsometatarsus of specimen U.W. 101 035 from the Dinaledi Chamber appear to be more similar in size to that of female owls, but the comparative samples are small (Table 2; Figure 3).
A few other specimens accompanied the tarsometatarsus (Figure 4). These include: (1) a left tibiotarsus fragment of a bird, with the mid-distal shaft present, and part of the distal condyle (U.W. 101 40C; Figure 4a); (2) a right radius fragment of a bird, consisting of the distal, mid and proximal shaft, with the distal styloid process absent (U.W. 101 40B; Figure 4b); and (3) a right ulna fragment of a bird, consisting of the proximal, mid and distal shaft, with a small portion of the distal articulation present (U.W. 101 965 and 822; Figure 4c). In all cases, these specimens are similar in size and morphology to that of a barn owl, and are proportionally correct in size for an individual slightly smaller than the TM 80 242 and TM 78 094 specimens, and we conclude that they are most probably from the same individual as U.W. 101 035. However, the specimens are too fragmented to attempt to make an identification based on morphology alone. In this instance, the minimum number of individuals for T. alba is 1.

Discussion and conclusion
The common barn owl (Tyto alba)
Tyto alba is the most widely distributed species of owl in the world, and one of the most widespread of all birds, occupying many ecological areas, except Antarctica and parts of the Sahara Desert.22 While almost exclusively nocturnal, in rare cases, T. alba is known to hunt diurnally.23-25 As for most owls, the diet of T. alba comprises mainly small vertebrates, with a large majority represented by rodents. Undigested remains of the consumed prey in the stomachs of owls form into pellets, which are regurgitated, and are often rich in bone and teeth.26-29 These regurgitated pellets are often found on the ground, underneath the diurnal resting area occupied by the owl.27 A study done by Duke et al.23 showed a striking difference in the bone composition of pellets when comparing those found in owls (48%) with those of hawks (6.5%) based on weight. This richness and affinity for micromammal bone accumulation in the form of pellets are often found at palaeontological and archaeological sites around the world.
As the owl pellets fall to the ground, they slowly start disintegrating and start to be incorporated in the sediments. It is for this reason that owls are widely recognised as accumulating agents, and much work has been undertaken to examine the pellets of both modern and fossil owls26,28,29, as the contents of these pellets serve as good palaeoenvironmental indicators30-37. In addition to serving as palaeoenvironmental indicators, owl pellets are also used in the study of stratigraphy at a particular site and the study of evolution of fauna.27
Barn owls are known to roost in a variety of habitats, occupying different types of cavity roosts. These habitats may include the twilight regions of rock fissures or hollow interiors of tree trunks (both dead and alive); owls are seldom found roosting in exposed roosts.38 It is widely known that, in southern Africa, the protected openings and entrances of caves are often frequented by barn owls and used for roosting.36,38-40
Tyto alba is widespread in southern Africa and makes use of a variety of habitats ranging from woodlands to deserts, but excluding forests21, and is known to roost in an assortment of places including cliffs, buildings, wells, mineshafts and caves. Owls have contributed microfauna remains to many Plio-Pleistocene sites in the Cradle of Humankind41,42 including Gladysvale, Kromdraai, Sterkfontein and Swartkrans29,37-39,43-48.
The Dinaledi Owl
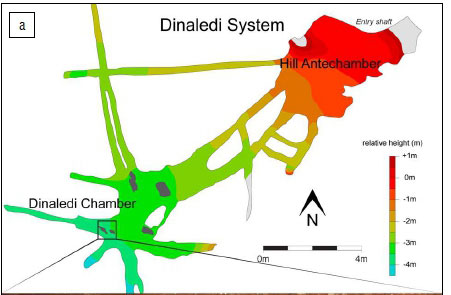
Figure 5a shows the Dinaledi Chamber and the location, where recovered, of three of the four bird remains discussed here. As noted earlier, the remains had apparently been picked up and placed on a rock in the distal section of the Dinaledi Chamber (Figure 5b) by one or more cavers prior to the exploration of the Chamber by our scientific team. As noted by Dirks et al.1, the owl remains are taphonomically distinct from the rest of the hominin assemblage as they lack surface modification and stain patterns seen on the hominin remains; in addition, they are covered in an adhesion of a thin film of calcite crystals. These crystals cover much of the surface of the owl bone, and thus suggest that they may have been deposited relatively recently.
There are several possible scenarios to explain the placement of these bird remains. A modern owl may have become lost in the system and, by way of flying around in the dark, found its way into the Dinaledi Chamber (Hypothesis 1). It is also possible that the remains fell down the narrow chute - a 12-m drop immediately above the Dinaledi system, and the only currently known accessible route into this system (Hypothesis 2). If this were the case, it is possible that more remains from this individual are still to be recovered, possibly from the base of the chute, in what is now called the Hill Antechamber of the Dinaledi system49, although in this scenario, human agency would be required for the bones to find their way into the more distal Dinaledi Chamber.
Alternatively, but less likely, the remains of this modern owl could have been introduced into the system by a caver carrying them in, possibly from the main entrance of the Rising Star Cave (Hypothesis 3). This hypothesis is supported by the fact that the four remains were found on a rock within the Dinaledi Chamber, although why a caver would carry remains into such a difficult to access area and then leave them there is not obvious. In this scenario, it is possible that the owl died at the entrance of the Rising Star Cave, as this area has a constructed entrance (created by limestone miners at some point in the 1930s) and a natural cave roof opening, both of which open up into a rocky cave wall approximately 6 m high. This rock face is a suitable roosting area, and is currently inhabited by bats, swallows and other bird species including occasionally barn owls. This open area has a relative abundance of light, and as is commonly known, barn owls will seek out roosts which are dark and enclosed, even when roosting in trees or on the ground.38
An alternative entrance into the Dinaledi Chamber, as suggested for example by Thackeray50, is another possible scenario for the introduction of the modern owl remains (Hypothesis 4). However, extensive and exhaustive exploration by cavers from the University of the Witwatersrand has, to date, failed to identify another entrance to the Dinaledi system.
We favour one of the first two hypotheses as the most likely origin of this material in the position of their discovery: the owl became lost in the system and found its way either into the Dinaledi Chamber, or to the top of the chute, before perishing.
The owl remains from the Dinaledi Chamber help further our understanding of the contents of the important material contained within the Dinaledi system. They are also the only more recent fossils to be recovered from this area of the Rising Star Cave system and are therefore important in and of themselves as an indicator that more proximal parts of the Rising Star Cave system have been suitable for use by barn owls at greater time depths than the present. Once described, some of these bones will be subjected to radiocarbon dating to establish if they might be useful in placing an uppermost date on the bone content of the Dinaledi Chamber as perhaps they date the last depositional event to occur within the relatively closed Dinaledi system.
Acknowledgements
We acknowledge funding from: DST-NRF Centre of Excellence in Palaeosciences (CoE-Pal); Lee R Berger Foundation for Exploration; Lyda Hill Foundation; National Geographic Society; National Research Foundation (South Africa); and University of the Witwatersrand. The support of the CoE-Pal towards this research is hereby acknowledged. Opinions expressed, and conclusions arrived at, are those of the authors and are not necessarily to be attributed to the CoE-Pal. We thank B. Zipfel and S. Jirah for access to collections at the Evolutionary Studies Institute. We thank the Ditsong National Museum of Natural History for access to their faunal collection.
Authors' contributions
A.K. conducted the research and wrote the original draft of the manuscript. S.B. took all measurements and undertook the identification. Both authors contributed equally to manuscript revisions and editing.
References
1.Dirks PHGM, Berger LR, Roberts EM, Kramers JD, Hawks J, Randolph-Quinney PS, et al. Geological and taphonomic context for the new hominin species Homo naledi from the Dinaledi Chamber, South Africa. eLife. 2015;4, e09561, 37 pages. https://doi.org/10.7554/eLife.09561 [ Links ]
2.Berger LR, Hawks J, De Ruiter DJ, Churchill SE, Schmid P, Delezene LK, et al. Homo naledi, a new species of the genus Homo from the Dinaledi Chamber, South Africa. eLife. 2015;4, e09560, 35 pages. https://doi.org/10.7554/eLife.09560 [ Links ]
3.Kruger A, Randolph-Quinney PS, Elliott M. Multimodal spatial mapping and visualisation of Dinaledi Chamber and Rising Star Cave. S Afr J Sci. 2016;112(5/6), Art. #2016-0032, 11 pages. http://dx.doi.org/10.17159/sajs.2016/20160032 [ Links ]
4.Hawks J, Elliott M, Schmid P, Churchill SE, Ruiter DJd, Roberts EM, et al. New fossil remains of Homo naledi from the Lesedi Chamber, South Africa. eLife. 2017;6, e24232, 63 pages. https://doi.org/10.7554/eLife.24232 [ Links ]
5.Dirks PHGM, Roberts EM, Hilbert-Wolf H, Kramers JD, Hawks J, Dosseto A, et al. The age of Homo naledi and associated sediments in the Rising Star Cave, South Africa. eLife. 2017;6, e24231, 59 pages. https://doi.org/10.7554/eLife.24231 [ Links ]
6.Clarke RJ. On some new interpretations of Sterkfontein stratigraphy. S Afr J Sci. 1994;90:211-214. [ Links ]
7.Partridge TC. Re-appraisal of lithostratigraphy of Sterkfontein hominid site. Nature. 1978;275:282-287. https://doi.org/10.1038/275282a0 [ Links ]
8.Berger LR, Menter CG, Thackeray JF. The renewal of excavation activities at Kromdraai, South Africa. S Afr J Sci. 1994;90:209-210. [ Links ]
9.Kuman K, Field AS, Thackeray JF. Discovery of new artefacts at Kromdraai. S Afr J Sci. 1997;93:187-193. [ Links ]
10.Brain CK. Structure and stratigraphy of the Swartkrans Cave in the light of the new excavations. In: Brain CK, editor. Monograph 8: Swartkrans. Pretoria: Transvaal Museum; 1993. p. 23-34. [ Links ]
11.De Ruiter DJ. Revised faunal lists for Members 1-3 of Swartkrans, South Africa. Ann Transv Mus. 2003;40:29-41. [ Links ]
12.Dirks PHGM, Berger LR. Hominin-bearing caves and landscape dynamics in the Cradle of Humankind, South Africa. J Afr Earth Sci. 2013;78:109-131. https://doi.org/10.1016/j.jafrearsci.2012.09.012 [ Links ]
13.Lyman R. Vertebrate taphonomy. Cambridge: Cambridge University Press; 1994. https://doi.org/10.1017/CBO9781139878302 [ Links ]
14.Lyman R, Fox G. A critical evaluation of bone weathering as an indication of bone assemblage formation. In: Haglund W, Sorg M, editors. Forensic taphonomy: The post-mortem fate of human remains. Boca Raton, FL: CRC Press; 1997. p. 223-247. [ Links ]
15.Lyman RL. What taphonomy is, what it isn't, and why taphonomists should care about the difference. J Taphonomy. 2010;8(1):1-16. [ Links ]
16.Pokines JT. Faunal dispersal, reconcentration, and gnawing damage to bone in terrestrial environments. In: Pokines J, Symes S, editors. Manual of forensic taphonomy. Boca Raton, FL: CRC Press; 2013. p. 201-248. [ Links ]
17.Pokines JT, Baker JE. Effects of burial environment on osseous remains. In: Pokines J, Symes S, editors. Manual of forensic taphonomy. Boca Raton, FL: CRC Press; 2013. p. 73-114. https://doi.org/10.1201/b15424-6 [ Links ]
18.Bristow J, Simms Z, Randolph-Quinney PS. Taphonomy. In: Black S, Ferguson E, editors. Forensic anthropology 2000-2010. Boca Raton, FL: CRC Press; 2011. p. 279-318. [ Links ]
19.Von den Driesch A. A guide to the measurement of animal bones from archaeological sites: As developed by the Institut für Palaeoanatomie, Domestikationsforschung und Geschichte der Tiermedizin of the University of Munich. Cambridge, MA: Peabody Museum Press; 1976. [ Links ]
20.Badenhorst S, Lyle R, Merewether J, Driver J, Ryan S. The potential of osteometric data for comprehensive studies of turkey (Meleagris gallopavo) husbandry in the American Southwest. Kiva. 2012;78(1):61-78. https://doi.org/10.1179/kiv.2012.78.1.61 [ Links ]
21.Maclean GL. Robert's birds of southern Africa. Cape Town: John Voelcker Bird Book Fund; 1985. [ Links ]
22.Shawyer CR. The barn owl. London: Hamlyn; 1994. [ Links ]
23.Duke G, Jegers A, Loff G, Evanson O. Gastric digestion in some raptors. Comp Biochem Physiol A Physiol. 1975;50(4):649-656. https://doi.org/10.1016/0300-9629(75)90121-8 [ Links ]
24.Bunn D. Regular daylight hunting by barn owls. Brit Birds. 1972;65:26-30. [ Links ]
25.Harte K. Barn owl hunting by daylight. The Wilson Bulletin. 1954:270-270. [ Links ]
26.Dodson P, Wexlar D. Taphonomic investigations of owl pellets. Paleobiology. 1979;5(3):275-284. https://doi.org/10.1017/S0094837300006564 [ Links ]
27.Culver DC, White WB. Encyclopedia of caves. Amsterdam: Elsevier; 2005. [ Links ]
28.Kusmer KD. Taphonomy of owl pellet deposition. J Paleontol. 2015;64(4):629-637. https://10.1017/S0022336000042669 [ Links ]
29.Levinson M. Taphonomy of microvertebrates - from owl pellets to cave breccia. Ann Transv Mus. 1982;33(6):115-121. [ Links ]
30.Winkler AJ. Neogene paleobiogeography and East African paleoenvironments: Contributions from the Tugen Hills rodents and lagomorphs. J Hum Evol. 2002;42(1-2):237-256. https://doi.org/10.1006/jhev.2001.0501 [ Links ]
31.Jaeger J, Wesselman H. Fossil remains of micromammals from the Omo Group deposits. Earliest man and environments in the Lake Rudolf Basin. Chicago, IL: University of Chicago Press; 1976. p. 351-360. [ Links ]
32.Fernandez-Jalvo Y, Andrews P. Small mammal taphonomy of Gran Dolina, Atapuerca (Burgos), Spain. J Archaeol Sci. 1992;19(4):407-428. https://doi.org/10.1016/0305-4403(92)90058-B [ Links ]
33.Denys C. Fossil rodents (other than Pedetidae) from Laetoli. In: Leakey MD, Harris JM, editors. Laetoli: A Pliocene site in northern Tanzania. Oxford: Oxford University Press; 1987. p. 118-170. [ Links ]
34.De Graaff G. On the fossil mammalian microfauna collected at Kromdraai by Draper in 1895. S Afr J Sci. 1961;57(9):259-260. [ Links ]
35.Avery D. The environment of early modern humans at Border Cave, South Africa: Micromammalian evidence. Palaeogeogr Palaeoclim Palaeoecol. 1992;91(1-2):71-87. https://doi.org/10.1016/0031-0182(92)90033-2 [ Links ]
36.Davis D. The barn owl's contribution to ecology and palaeoecology. Ostrich. 1959;30(S1):144-153. https://doi.org/10.1080/00306525.1959.9633322 [ Links ]
37.Avery D. The Plio-Pleistocene vegetation and climate of Sterkfontein and Swartkrans, South Africa, based on micromammals. J Hum Evol. 2001;41(2):113-132. https://doi.org/10.1006/jhev.2001.0483 [ Links ]
38.Reed DN. Taphonomic implications of roosting behavior and trophic habits in two species of African owl. J Archaeol Sci. 2005;32(11):1669-1676. https://doi.org/10.1016/j.jas.2005.05.007 [ Links ]
39.Reed DN. Micromammal paleoecology: Past and present relationships between African small mammals and their habitats. Stony Brook, NY: State University of New York; 2003. [ Links ]
40.McCrae C. A comparative study of Late Holocene and Plio-Pleistocene-aged micromammalian owl accumulations from South Africa. Palaeontol Afr. 2009;44:190-191. [ Links ]
41.Brain CK. The hunters or the hunted? Chicago, IL: Chicago University Press; 1981. [ Links ]
42.Watson V. Form, function and fibres: A preliminary study of the Swartkrans fossil birds. Koedoe. 1991;34(1):23-29. https://doi.org/10.4102/koedoe.v34i1.410 [ Links ]
43.Glue DE. Avian predator pellet analysis and the mammalogist. Mammal Rev. 1970;1(3):53-62. https://doi.org/10.1111/j.1365-2907.1970.tb00320.x [ Links ]
44.Vernon C. An analysis of owl pellets collected in southern Africa. Ostrich. 1972;43(2):109-124. https://doi.org/10.1080/00306525.1972.9632586 [ Links ]
45.Pocock T. Plio-Pleistocene fossil mammalian microfauna of southern Africa - A preliminary report including description of two new fossil muroid genera (Mammalia: Rodentia). Palaeontol Afr. 1987;26(7):69-71. [ Links ]
46.Avery D. A preliminary assessment of the micromammalian remains from Gladysvale Cave, South Africa. Palaeontol Afr. 1995;32:1-10. [ Links ]
47.Avery D. Micromammals as palaeoenvironmental indicators of the southern African Quaternary. T Roy Soc S Afr. 2007;62(1):17-23. https://doi.org/10.1080/00359190709519193 [ Links ]
48.Leichliter JN. Micromammal paleoecology: Theory, methods, and application to modern and fossil assemblages in the Cradle of Humankind World Heritage Site, South Africa. Boulder, CO: University of Colorado Boulder; 2011. [ Links ]
49.Berger LR, Elliott MC, Peixotto B, Morris H, Feuerriegel EM, Tucker SJ, et al. A new naming scheme for the Dinaledi Chamber System and associated antechambers and passages of the Rising Star Cave System, South Africa. Paper presented at: The 87th Annual Meeting of the American Association of Physical Anthropologists; 2018 April 11-14; Austin, Texas, USA. Am J Phys Anthropol. 2018;165(S66):25-26. [ Links ]
50.Thackeray JF. The possibility of lichen growth on bones of Homo naledi: Were they exposed to light? S Afr J Sci. 2016;112(7-8), Art. #a0167, 5 pages. http://dx.doi.org/10.17159/sajs.2016/a0167 [ Links ]
 Correspondence:
Correspondence:
Ashley Kruger
ashleykruger@gmail.com
Received: 06 June 2018
Revised: 27 July 2018
Accepted: 13 Sep. 2018
Published: 27 Nov. 2018