Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.114 no.11-12 Pretoria nov./dic. 2018
http://dx.doi.org/10.17159/sajs.2018/4874
RESEARCH ARTICLE
Potential of marula (Sclerocarya birrea subsp. caffra) waste for the production of vinegar through surface and submerged fermentation
Tumisi B.J. MolelekoaI; Thierry RegnierI; Laura S. da SilvaI; Wilma A. AugustynII
IDepartment of Biotechnology and Food Technology, Tshwane University of Technology, Pretoria, South Africa
IIDepartment of Chemistry, Tshwane University of Technology, Pretoria, South Africa
ABSTRACT
Although there is an abundance of indigenous fruits in South Africa, knowledge of their potential uses is mainly restricted to within communities. In this study, marula fruit-processing waste by-products (fruit pulp residue and skin) were used as substrates in surface culture and submerged fermentation methods to produce vinegar (acetic acid) using spontaneous and starter culture techniques. The study revealed the possibility of producing vinegar through both methods of fermentation, with yields of acetic acid ranging between 41 000 mg/L and 57 000 mg/L (surface culture method) and between 41 000 and 54 000 mg/L (submerged culture method). Furthermore, the physicochemical property analyses revealed marula vinegar to be a potential source of bioactive compounds (total phenolics 0.289-0.356 mg/L GAE and total flavonoids 0.146-0.153 mg/L CAE) which displayed a potent antiradical activity against DPPH•: 78.85% for surface culture and 73.03% submerged culture, respectively. The sensory panel recommended application of the vinegar in products such as salad dressing and mayonnaise. Finally, we have demonstrated that the surface culture method using the inoculation technique is more suitable for the production of high-quality vinegar, with possible consideration for commercialisation.
Significance:
•Marula fruit has high economic importance for South Africa, particularly for the Limpopo Province.
•Marula waste can be a source of bioactive compounds, yet comparatively little is reported on the potential use of the waste to produce vinegar.
•Self-development of communities through viable and easy to produce commodities from marula fruit needs to be implemented and prioritised in the Limpopo Province.
Keywords: acetic fermentation; commercialisation; fruit; high quality; sensory attributes
Introduction
Apart from the commercial production of common fruits such as apples, peaches, pears and oranges, there is a growing trend to domesticate indigenous fruit-bearing trees in Africa such as the marula (Sclerocarya birrea subsp. caffra) and the kei apple (Dovyalis caffra)1 for fruit production. Marula is particularly well known for its fruit2, which abscise before ripening while still green and then ripen rapidly within 8 days3. Subsequently, the colour of the fruit changes from green to yellow, the aroma develops and the flesh softens.3 The tree is highly appreciated by rural communities for its fruit; the edible flesh of the fruit is eaten raw or is used to prepare juices, jams, conserves, dry fruit rolls and alcoholic beverages.4 The fruit's kernels are consumed raw or roasted, and/or used to extract oil using cold-press methods. The oil is used for cooking and is renowned for its cosmetic application.5,6 Thus, the marula is considered a multipurpose tree in rural communities.
The resulting by-product is also further processed into value-added products. The popularity of marula is growing locally and internationally as a consequence of the well-known Amarula Cream Liqueur, manufactured locally by Distell (Stellenbosch, South Africa). Processing the fruit creates valuable waste by-products which are discarded, and as such are underutilised.
On a commercial scale, alcoholic beverage production (Amarula Cream Liqueur) and oil production for cosmetic uses are the primary commercial applications for the marula fruit. During harvest, rural communities collect the fruit and deliver it to central locations in and around the town of Phalaborwa in the Limpopo Province. Distell SA requires only 30% of the harvest for their production facility. Consequently, a significant percentage of the harvested fruit, as well as substandard fruits, are not utilised and become waste. Oelofse7 has reported that in South Africa alone, waste generated from fruit- and vegetable-processing was 45% of various product commodities in 2012. Historically, these by-products are not considered to have commercial value because of a lack of available and affordable processes to convert the by-products into value-added commodities.
Fermentation using various microorganisms such as yeast and lactic acid bacteria is one of the oldest methods used for food preservation. Fermented foods are popular throughout the world, and make a significant contribution to the diet of millions of individuals.8 Fermentation is a cheap and energy efficient method of preserving perishable raw materials, such as fruits and vegetables.
Vinegar is defined as a sharp sour-tasting liquid containing acetic acid obtained by fermentation of especially sour wine, malt or cider using acetic acid bacteria. It is an important condiment and typically contains ±6% (60 000 mg/L) acetic acid, carbohydrates, organic acids, alcohols and polyols, amino acids and peptides.9
Commercially, vinegar is produced mainly from alcoholic stock solutions such as apple ciders and grape wine, using a variety of fermentation methods. Methods include submerged and surface culture fermentation.10 A less common approach is the use of raw agricultural crops, such as sorghum, in a solid-state fermentation type method.11 Vinegar production is a two-stage process, and the submerged culture fermentation method is by far the most common method in commercial production.10 The aim of this study was to evaluate the feasibility of marula fruit waste as a substrate for vinegar production.
Materials and methods
Three batches of marula waste by-products were received from The Marula Company (Phalaborwa, Limpopo Province). The fruit waste was transported frozen in 20-L buckets to Tshwane University of Technology (Tshwane, South Africa). Upon receipt, the fruit waste was thawed in a refrigerator and working samples of 500 g were transferred into plastic Ziploc® bags, labelled MRPS1 and MRPS2 for season 1 and season 2, respectively, and stored in a freezer (Snijderg, United Scientific, Goodwood, South Africa) at -80 °C until further use.
Isolation of yeast from marula pulp
Frozen marula substrates (25 g) were subsampled from the 500 g frozen waste and defrosted under laminar flow. A 1:10 (w:v) dilution was prepared by dissolving 25 g substrate in 250 mL sterile Ringer's solution (Merck, Johannesburg, South Africa) and placed in a sterile stomacher bag and macerated with a stomacher machine (Seward, Worthing, West Sussex, England) for 5 min at 450 rpm. A 10-fold dilution series was prepared and 100 μL of each aliquot was transferred to Petri plates containing Rose Bengal Chloramphenicol Agar (Merck, Johannesburg, South Africa).
Pure yeast isolates were sub-cultured in sterile Sabouraud 2% dextrose broth. The culture was incubated at 25 °C until an optical density of 0.5 (equivalent to 1 x 106 cfu/mL) was obtained. Aliquot samples were taken every 24 h and optical density was measured at λ=600 nm using a spectrophotometer (Helios-Gamma, ThermoFischer Scientific, Johannesburg, South Africa). The isolates were preserved according to the method of Nyanzi12 for further use as inoculum in alcoholic fermentation and for molecular identification. The 18S internally transcribed spacer was used to identify the yeast at species level, as recommended by Guillómon and Mas13. The consensus sequence was used to obtain the identity of the yeast from the UK National Centre for Biotechnology Information.
Fermentation and vinegar processing
Two fermentation methods were studied: submerged culture fermentation and surface culture fermentation. In addition, both natural fermentation and inoculated fermentation techniques were used. For the inoculation method, yeast isolated from the marula substrate was used for the alcoholic fermentation stage, and a pure culture of Acetobacter aceti (Anatech Cultures, Johannesburg, South Africa) was used for the acetic acid fermentation stage.
Fermentation medium
The fermentation medium was used for both methods (submerged and surface culture methods). Instant active dry yeast (Saccharomyces cerevisiae) (0.05 g) was added to 250 mL of warm (30±2 °C) water and left for 10 min. One tenth of the yeast was inoculated to a sterilised yeast extract peptone dextrose broth (1000 mL) and incubated at 28 °C under aeration for 24 h prior to fermentation. The vinegar processing medium consisted of 30% (w/v) marula substrate (MRPS1 or MRPS2), anhydrous glucose (in concentrations of 8%, 16% and 32% (w/v)), and yeast extract (5 mL of 2% w/v). The entire volume of each mixture was made up to 250 mL with sterile distilled water and mixed by swirling. Fermentation medium formulations yielding higher concentrations of acetic acid were considered for further physicochemical analyses.
Submerged culture fermentation
The prepared MRPS1 and MRPS2 fermentation media were inoculated with 5 mL of the naturally occurring yeast culture at an optical density of 0.5 and incubated anaerobically at 25 °C for 6 days to initiate the alcoholic fermentation stage. Subsequently, each flask was further incubated at 30 °C for 12 days and shaken at 80 rpm to aerate the medium with atmospheric air, allowing the growth of naturally occurring acetic acid bacteria (AAB) for the acetic acid fermentation stage. Fermentation was stopped by pasteurisation as follows: the resulting vinegar was aseptically transferred to a sterile 500 mL round bottom flask fitted to a Rotavapor (BÜCHI Labotechnik-AG, Flawil, Switzerland) and attached to a water bath and rotated at 80 rpm at 70 °C for 30 min. The system was closed to avoid loss of volatile compounds, and rotation ensured even distribution of heat. Subsequently the vinegar was cooled to ambient temperature and transferred into 50-mL Falcon centrifuge tubes (Eppendorf, Johannesburg, South Africa) and centrifuged at 15 810 rcf (15 °C for 10 min) (Sorvall RC 6 Centrifuge, Johannesburg, South Africa). The vinegar supernatant was collected and the pellets discarded. The vinegar was clarified by filtration (4-µm syringe filter), bottled and stored until further analyses. Analyses conducted during the fermentation process and on the final product include: pH measured on Days 6, 9, and 12 of the fermentation period, alcohol concentration measured on Day 6 using a glass alcoholmeter, and acetic acid concentration quantified using high performance liquid chromatography (HPLC) at the end of fermentation (Day 12).
Surface culture fermentation
This fermentation method followed the same procedure as stated for the submerged culture method. However, during the acetic acid fermentation stage, the flasks were not agitated, leading to the atmospheric oxygen diffusing slowly into the fermenting medium. Ethanol utilised by the AAB (in the pellicle) was quantified over time. Once depleted, fermentation was stopped by pasteurisation. The resulting vinegar was analysed as described for the submerged culture fermentation method.
Inoculated fermentation
The above-mentioned fermentation methods were mainly mediated by naturally occurring microflora, i.e. yeast and AAB. In this experiment, acetic fermentation (bioconversion of ethanol to acetic acid) was achieved by inoculation with a pure Acetobacter aceti (ATCC 15973) starter culture (ANATECH Cultures, Johannesburg, South Africa). Briefly, lyophilised Acetobacter aceti (±0.5 g) was regrown in sterile glucose yeast extract broth (250 mL) consisting of 1% (w/v) glucose, 1% (w/v) yeast extract powder, 6% (v/v) ethanol, 0.05% MgSO4 and 0.05% KH2PO4, and incubated in a rotary incubator at 30 °C until an optical cell mass density of 0.5 was obtained. On Day 6, the fermenting medium obtained in both fermentation methods (submerged culture and surface culture methods) was inoculated with 10 mL culture broth. Alcohol, acetic acid concentration and pH were determined as previously stated.
Physicochemical analyses of the vinegar
The physicochemical analyses of the prepared vinegar solutions included: HPLC, colour assessment and determination of total phenolic content, total flavonoid content, antiradical activity and antimicrobial activity.
High-performance liquid chromatography
Organic acids (acetic, propionic and lactic acid) in the prepared vinegar solutions using both surface and submerged culture methods were quantified as described by de Sena Aquino et al.14 Analysis of the produced organic acids was carried out through high performance liquid chromatography (Agilent Technologies 1200 Infinity, Chemetrix, Johannesburg, South Africa) equipped with an Inertsil C-18 reversed phase column (250 mm x 4.6 mm i.d. x 5 μm particle size) and a UV/Vis fixed wavelength detector at 220 nm. A mobile phase solution consisting of 0.02 mol/L KH2PO4 (Merck, Johannesburg, South Africa) buffer solution, adjusted to a pH (2.88 ± 0.02) was used to separate the organic acids. For calibration curves, standard solutions containing 400 μL/L, 200 μL/L, 100 μL/L and 40 μL/L of 99% organic acids (acetic, propionic and lactic) (Merck, Johannesburg, South Africa) were made up in distilled water, and the solutions filtered through 0.45 μm cellulose filter (Millipore, Johannesburg, South Africa) to remove any solid particles. The HPLC separation was performed by isocratic elution with 100% buffer at a flowrate of 1 mL/min. The total time of analysis was 10 min. The quantity of each acid present in the vinegar was determined using the following linear regression equations obtained through the standard curve calibrations:
y = 1.4045x for acetic acid;
y = 1.1563x for lactic acid and
y = 0.5237x - 12.926 for propionic acid.
Colour measurement
The colour of each vinegar at room temperature was determined using a Minolta Chromometer (Konica Minolta-CR-410, Osaka, Japan) on the basis of the CIE L*, a*, b* system.15 Chroma and °hue angle were calculated according to Zhang et al.16
Total phenolic content
The concentration of phenolic compounds present in the vinegar samples was determined using the Folin-Ciocalteau method described by Du Plooy et al.17 and expressed as gallic acid equivalent (GAE) per litre.
Total flavonoid content
The total flavonoid content of the prepared vinegars was determined following a method described by Ozturk et al.18 and expressed as catechin acid equivalent (CAE) per litre.
Anti-radical activity
The anti-radical activity was determined as free DPPH· (2,2-diphenyl-1-picrylhydrozyl) radical scavenging capacity as described by Ozturk et al.18, and the percentage anti-radical activity (%ARA) determined by:
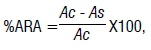
where Ac is the absorbance of the control and As is the absorbance of the sample.
Sensory evaluation
The sensory description of the different marula vinegars was based on a 9-point hedonic scale (1 = least like and 9 = strongly like) as described by Ubeda et al.19 The untrained panel consisted of 23 male and female consumer science students from the Department of Hospitality Management (Tshwane University of Technology, Pretoria, South Africa) between the ages of 20 and 23 years old. White and red grape commercial vinegar samples (Wellington's®, Heinz Foods Pty Ltd, Cape Town, South Africa) were included for comparative purposes.
Statistical analysis
Each analysis was conducted in triplicate and means and standard deviations were calculated using Microsoft Excel (Microsoft Corporation, USA). The statistical significance (p≤0.05) of the data sets was evaluated using Geostats® data analysis and statistical software.
Results and discussion
Natural yeast identification
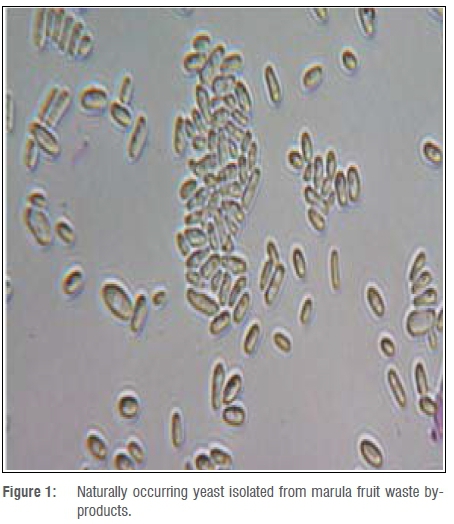
Non-Saccharomyces yeast (Figure 1) isolated from marula fruit by-products was identified as Pichia kudriavzevi. This yeast strain has been isolated worldwide from various substrates, including soil, fruits and fermentation must. This yeast species has been reported by Del Monaco et al.20 as ideal for alcoholic fermentation in vinegar.
pH measurement
Figure 2 illustrates pH measurements for submerged and surface culture using spontaneous and inoculated bacterial cultures. In naturally fermented media, pH is controlled by the growth of a specific microorganism group (AAB or lactic acid bacteria), and yeasts by the secretion of metabolic by-products such as organic acids or organic alcohols.13 In vinegar production, pH is the primary means of assessing the accumulation of acetic acid as a metabolic by-product in the fermentation medium.
Although acetic acid is the principal component of concern in vinegar production, other organic acids such as lactic acid, propionic acid and succinic acid are also produced. Collectively, these organic acids are responsible for lowering the pH to below 6.0 in the fermenting medium. Wai-Ho et al.21 described the optimal pH of a commercially produced vinegar to be in the range 2.0-3.5. This range is also the optimal proliferation range for AAB responsible for producing acetic acid in vinegar. The naturally fermented 8% (w/v) glucose fermentation medium had relatively lower pH values (pH 3.36-3.39) than the 16% and 32% (w/v) glucose media (pH 3.49-3.84), using both submerged and surface culture methods.
A similar trend was observed for the inoculated fermentations using 8%, 16% and 32% (w/v) glucose fermentation media. However, the inoculated fermentation method yielded lower pH (pH 2.60-3.14) for both submerged and surface culture methods. Overall, the pH values obtained in this study are higher than those reported for apple cider vinegars.21
It is important to remember that the functional alcohol and aldehyde dehydrogenase enzyme complexes, responsible for the bioconversion of ethanol into acetic acid, are optimally active at a pH of 2.0 to 3.5 in less than 8% alcohol (w/v).21,22 The recorded alcohol levels for 8%, 16% and 32% (w/v) glucose media on Day 6 of alcoholic fermentation were 8%, 11% and 14% (w/v), respectively. Consequently, the higher alcohol content became inhibitory to the oxidising bacteria, resulting in vinegar with a low titre strength.
HPLC and bioactive compounds
Active compounds such as phenols and flavonoids in naturally fermented vinegars have drawn much consumer interest because of their health-conferring benefits, which include appetite suppression and free radical stabilisation. Apple cider and balsamic vinegars are by far the most renowned for these characteristics. Naturally, fermented vinegars are heterogeneous in nature, with a variety of organic acids including acetic, succinic, butyric and lactic acid.11 This phenomenon is attributed to various pathways used by the fermentation microorganisms secreting distinct by-products and fermentation intermediates, such as acetyl aldehyde.13
Table 1 summarises the organic acid profile of the marula vinegar produced by the various methods. The acetic acid produced accounted for more than 99% of the organic acids. The high concentration of acetic acid is primarily because of the dominant microorganism (AAB) present in the vinegar. These organisms are well known to produce acetic acid as a metabolic by-product.22 In addition, more acetic acid was produced using the surface culture method combined with the inoculation technique (57 611 mg/L; Table 1) than using the submerged culture method and/or natural fermentation techniques (ranging from 41 000 to 54 000 mg/L acetic acid). The higher concentration of acetic acid produced in the marula vinegar using the surface culture fermentation method is in accordance with the findings of Tan10, who reported that this method yields a higher quality cherry vinegar. The surface culture method is generally preferred for vinegar production, as it is non-destructive to the fermenting microorganisms, and the condition of fermentation optimises their metabolic processes.
The submerged culture method, on the other hand, requires stringent monitoring of oxygen and the replenishing of alcohol during fermentation to ensure continuous acetic acid production.11 Moreover, the US Food and Drug Administration (FDA)23 stipulates that the minimum threshold of acetic acid in low strength vinegars should be at least 40 000 mg/L while the Korean Ministry of Food and Drug Safety recommends a minimum of 50 000 to 85 000 mg/L acetic acid in high titre vinegars.
In the present study, the average concentration of acetic acid (ranging from 40 626 to 57 611 mg/L) in marula vinegar falls within the low strength bracket as described by the FDA.23 Therefore, for commercialisation purposes, the optimisation of fermentation conditions (formulations and processing parameters) to achieve higher titre strength marula vinegar (≥50 000 mg/L acetic acid) will be required. This optimisation could be achieved through a rational feeding strategy of amino acids (as opposed to the addition of fermentable sugars) in the fermentation medium, as described by Zhengliang et al.24 Nitrogenous compounds improve the enzyme complexes (ADH and ALDH) of AAB during fermentation; hence, the bioconversion of ethanol to acetic acid by this enzyme complex becomes more efficient.
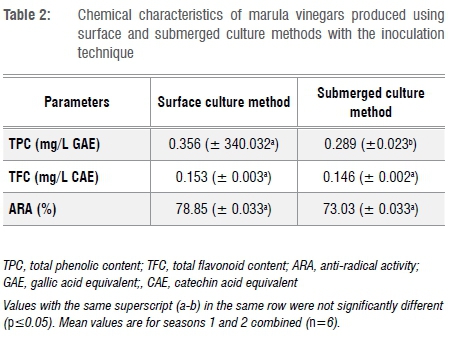
Table 2 summarises the chemical characteristics of marula vinegars produced in this study. The physicochemical properties reported in this study are in accordance with those reported by Ozturk et al.18 for Turkish homemade vinegars. With an average phenolic content of 0.323 mg/L GAE and an anti-radical activity of ±75%, the produced vinegars contain a significant amount of secondary metabolites displaying potential anti-radical properties. These bioactive compounds are generally considered to have health benefits, as they have the ability to quench free radicals in biological systems.25
Colour
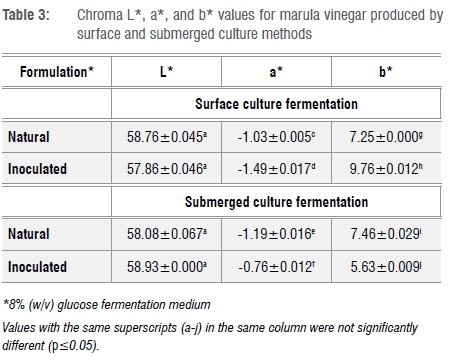
Agricultural food commodities, especially fruit and vegetables, contain several colour compounds including carotene (yellow to reddish), chlorophyll (green), flavonoids (white) and anthocyanins (blue to purple).26,27 However, adverse pH change, temperature (heat in particular), physical bruising including cutting, and processing, all influence the final colour of the destined product.27 Marula fruit is known to contain chlorophyll and carotenes.3 However, the colour of the marula vinegars was pale amber to deep amber. The transition from light to dark is a result of the oxidation of phenolic compounds catalysed by polyphenol oxidase. Table 3 summarises the colour values of each marula vinegar produced. It is important to notice that the fermentation methods used did not significantly change the chroma of the two vinegars. However, the marula vinegar is lighter than apple cider vinegar with an L* value of 58. The presence of negligible green (chlorophyll) colour compounds is indicated by a low negative a* value and a positive b* value. These values are in accordance with those described by Ozturk et al.18 for Turkish homemade vinegars.
Sensory evaluation
Sensory evaluation is pivotal in the marketability of any new product. The acceptability of the new vinegars was not different from their commercial counterparts (Figure 3). While most panellists equally liked all four vinegars in term of appearance, the testers most preferred the aroma of the marula vinegar produced using the submerged fermentation method. Although the aroma of the two marula vinegars was deemed acceptable, it was described as 'uncommon' and sweet, which may be associated with the natural flavour of the indigenous fruit. According to Bauer et al.28, the presence of butyl acetate can be linked to the sweet flavour of any food product. It is important to note that the taste of the marula vinegars was described as 'sweet sour' in comparison to the commercial vinegars, which were found to be 'too burny' and 'strong'. The strong taste of the commercial vinegars could be attributed to their low pH (2.57 and 2.49) and titratable acidity (7.5% acetic acid w/v) (data not illustrated) compared with the marula vinegars (pH 2.90 and 3.00, and titratable acidity 5.01% and 5.74%, respectively).
When asked about the potential use of the marula vinegars, the majority of the panellists recommended the marula vinegar to be used for the production of salad dressing or even mayonnaise. Based on the consumer's evaluation, the marula-based vinegars obtained the same consumers' purchase rating as the commercial vinegars.
Conclusion
We have shown that marula fruit-processing by-products could serve as a suitable substrate for acetic acid production. This application will add value to such products (skins, pips and pulp residues) to be utilised in tailored niche areas such as fermentation, and as such add value to the bio-economy. However, optimisation of processing (nutrient feeding strategy, temperature and aeration) parameters is necessary to ensure production of high titre marula vinegar. The surface culture method is more suitable than submerged culture fermentation for the preservation of the bioactive compounds in the vinegar. Finally, it is recommended that, with the support of the Department of Science and Technology, communities explore the production of such commodities.
Acknowledgements
We acknowledge financial support from the Tshwane University of Technology and the National Research Foundation of South Africa.
Authors' contributions
T.B.J.M. performed all the laboratory experiments as part of his master's degree and worked on the original concept of the manuscript. L.S.d.S. gave scientific inputs during the project and provided significant contributions to the final version of the manuscript. T.R. provided guidance, inputs during the research and edited the manuscript. W.A.A. provided scientific input and technical advice for the chemical analysis and edited the manuscript.
References
1.Van Wyk BE. The potential of South African plants in the development of new food and beverage products. S Afr J Bot. 2011;77:857-868. https://doi.org/10.1016/j.sajb.2011.08.003 [ Links ]
2.Wynberg RP, Laird SA, Shackleton S, Mander C, Shackleton C, Du Plessis P, et al. Marula commercialization for sustainable and equitable livelihoods. For Trees Livelihoods. 2003;13(3):203-215. https://doi.org/10.1080/14728028.2003.9752458 [ Links ]
3.Van Hal HP. Processing of marula (Sclerocarya birrea subsp. caffra) fruits. A case study on health promoting compounds in marula pulp (PhD thesis). Wageningen: Wageningen University; 2013. [ Links ]
4.Nerd A, Mizrahi Y. Domestication and introduction of marula (Sclerocarya birrea subsp. caffra) as a new crop for the Negev desert of Israel. In: Janick J, Simon JE, editors. New crops. New York: Wiley; 1993. p. 496-499. [ Links ]
5.Du Plessis P. Promoting indigenous fruit in Namibia. CRIAA SA-DC. Windhoek: Namibia; 2002. [ Links ]
6.Mojeremane W, Tshwenyane SO. The resource role of marula (Sclerocarya birrea): A multipurpose indigenous tree of Botswana. J Biol Sci. 2004;4:771-775. https://doi.org/10.3923/jbs.2004.771.775 [ Links ]
7.Oleofse S. Food waste in South Africa/Africa: Opportunities and challenges. Pretoria: Council for Scientific and Industrial Research; 2013. [ Links ]
8.Montville TJ, Matthews KR. Food microbiology: Fundamentals and frontiers. Principles, which influence microbial growth, survival and death in foods. 2nd ed. Washington DC: ASM Press; 2001. [ Links ]
9.Li T, Lo YM, Moon B. Feasibility of using Hericium erinaceus as the substrate for vinegar fermentation. LWT - Food Sci Technol. 2014;55:323-328. https://doi.org/10.1016/j.lwt.2013.07.018 [ Links ]
10.Tan SC. Vinegar fermentation (MSc thesis). New Orleans, LA: Louisiana State University; 2005. [ Links ]
11.Li S, Li P, Feng F, Xin L. Microbial diversity and their roles in the vinegar fermentation process. Appl Microbiol Biotechnol. 2015;99(12):4997-5024. https://doi.org/10.1007/s00253-015-6659-1 [ Links ]
12.Nyanzi R. Identification and properties of potential probiotic bacteria for application in mageu (DTech thesis). Pretoria: Tshwane University of Technology; 2007. [ Links ]
13.Guillamòn JM, Mas A. Acetic acid bacteria. In: Carrascosa AV, Muñoz R, González R, editors. Molecular wine microbiology. New York: Elsevier; 2011. p. 227-255. https://doi.org/10.1016/B978-0-12-375021-1.10009-8 [ Links ]
14.De Sena Aquino AC, Azevedo MS, Ribeiro DH, Costa AC, Amante ER. Validation of HPLC and CE methods for determination of organic acids in sour cassava starch wastewater. Food Chem. 2015;172:725-730. https://doi.org/10.1016/j.foodchem.2014.09.142 [ Links ]
15.Hunterlab. Insight on color: CIE L*a*b* color scale. Reston, VA: Hunterlab; 2008. [ Links ]
16.Zhang Y, Wang SY, Wang CY, Zheng W. Change in strawberry phenolics, anthocyanins, and antioxidant capacity in response to high oxygen treatments. LWT - Food Sci Technol. 2007;49(1):49-57. https://doi.org/10.1016/j.lwt.2005.08.013 [ Links ]
17.Du Plooy GW, Combrinck S, Regnier T, Botha BM. Linking lenticel discolouration of mango (Mangifera indica L.) fruit to reversed-phase HPLC profiles of phenolic compounds. J Hortic Sci Biotechnol. 2009;84:421-426. https://doi.org/10.1080/14620316.2009.11512543 [ Links ]
18.Ozturk I, Caliskan O, Tornuk F, Ozcan N, Yalcin H, Balsa M, et al. Antioxidant, antimicrobial, mineral, volatile, physicochemical, and microbial characteristics of traditional home-made Turkish vinegars. LWT - Food Sci Technol. 2015;63 (1):144-151. https://doi.org/10.1016/j.lwt.2015.03.003 [ Links ]
19.Ubeda C, Callejón RM, Troncoso AM, Morales ML. Consumer acceptance of new strawberry vinegars by preference mapping. Int J Food Prop. 2017;20:2760-2771. https://doi.org/10.1080/10942912.2016.1252388 [ Links ]
20.Del Mónaco SM, Rodríguez ME, Lopes CA. Pichia kudriavzevii as a representative yeast of North Patagonian wine making terroir. Int J Food Microbiol. 2016;230:31-39. https://doi.org/10.1016/j.ijfoodmicro.2016.04.017 [ Links ]
21.Wai-Ho C, Lazim AM, Fazry S, Zaki UKH, Lim SJ. Varieties, production, composition and health benefits of vinegars: A review. Food Chem. 2017;489:1621-1630. https://doi.org/10.1016/j.foodchem.2016.10.128 [ Links ]
22.Gullo M, Verzelloni E, Canonico M. Aerobic submerged fermentation by acetic acid bacteria for vinegar production: Process and biotechnological aspects. Process Chem. 2014;49:1571-1579. https://doi.org10.1016/j.procbio.2014.07.003 [ Links ]
23.US Food and Drug Administration (FDA). CPG Sec. 525.825 vinegar, definitions - adulteration with vinegar eels [document on the Internet]. c1995 [cited 2018 Aug 14]. Available from: https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-afda-ice/documents/webcontent/ucm074471.pdf [ Links ]
24.Zhengliang-Qi Dong D, Yang H, Xia X. Improving fermented quality of cider vinegar via rational nutrient feeding strategy. Food Chem. 2017;224:312-319. https://doi.org/10.1016/j.foodchem.2016.12.078 [ Links ]
25.Moo-Huchi VM, Estrada-Mota I, Estrada-León R, Cuevas-Glory L, Ortiz-Vázquez E, Vargas MD-LVY, et al. Determination of some physicochemical characteristics, bioactive compounds and antioxidant activity of tropical fruits from Yucatan, Mexico. Food Chem. 2014;152:508-515. https://doi.org/10.1016/j.foodchem.2013.12.013 [ Links ]
26.Coultate TP. Food: The chemistry of its components. 5th ed. Cambridge, UK: The Royal Society of Chemistry; 2009. [ Links ]
27.Vagiri M, Jensen M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017;217:409-417. https://doi.org/10.1016/j.foodchem.2016.08.121 [ Links ]
28.Bauer K, Garbe D, Surburg H. Natural raw materials in the flavor and fragrance industry. In: Bauer K, Garbe D, Surburg H, editors. Common fragrance and flavor materials: Preparation, properties and uses. 3rd ed. London: WILEY-VCH; 2007. p. 161-219. [ Links ]
 Correspondence:
Correspondence:
Thierry Regnier
regniert@tut.ac.za
Received: 09 Apr. 2018
Revised: 14 Aug. 2018
Accepted: 14 Aug. 2018
Published: 27 Nov. 2018














