Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.113 no.5-6 Pretoria may./jun. 2017
http://dx.doi.org/10.17159/sajs.2017/20160259
RESEARCH ARTICLE
The medical device development landscape in South Africa: Institutions, sectors and collaboration
Kylie de Jager; Chipo Chimhundu; Trust Saidi; Tania S. Douglas
Division of Biomedical Engineering, University of Cape Town, Cape Town, South Africa
ABSTRACT
A characterisation of the medical device development landscape in South Africa would be beneficial for future policy developments that encourage locally developed devices to address local healthcare needs. The landscape was explored through a bibliometric analysis (2000-2013) of relevant scientific papers using co-authorship as an indicator of collaboration. Collaborating institutions thus found were divided into four sectors: academia (A); healthcare (H); industry (I); and science and support (S). A collaboration network was drawn to show the links between the institutions and analysed using network analysis metrics. Centrality measures identified seven dominant local institutions from three sectors. Group densities were used to quantify the extent of collaboration: the A sector collaborated the most extensively both within and between sectors; local collaborations were more prevalent than international collaborations. Translational collaborations (AHI, HIS or AHIS) are considered to be pivotal in fostering medical device innovation that is both relevant and likely to be commercialised. Few such collaborations were found, suggesting room for increased collaboration of these types in South Africa.
SIGNIFICANCE:
• Results could inform the development of strategies and policies to promote certain types of medical device development.
• Further studies could identify drivers and barriers to successful medical device development in South Africa.
Keywords: bibliometrics; co-authorship; social network analysis; translational collaboration; foreign influence
Introduction
Medical device innovation requires contributions from multiple disciplines, as well as collaboration and knowledge transfer across sectors. The three main sectors identified as playing a role in medical device development are academia (in the form of higher education institutions), healthcare and industry.1-3
The knowledge base, resources and roles vary considerably across these sectors. Academic institutions often possess specialised instrumentation and equipment; are responsible for local education and training at all tertiary levels; and add to the stock of codified knowledge through publications, patents, and software and hardware prototypes.4 The healthcare sector best understands the patients' needs; and has access to currently available medical devices and knowledge about their shortcomings within the local context. Industry has knowledge of, and access to, current technologies used in the manufacture and development of medical devices; and is responsible for making the devices accessible to the general public.
Collaboration between organisations from the different sectors is a key component of innovation in the medical field.5 The benefits of academia-healthcare collaborations include increased awareness of technologies used in clinical practice and clinical input into research projects. Industry-academia collaboration increases the problem-solving capacity of industry by enabling access to university equipment and specialised knowledge.4 Universities benefit from this collaboration through awareness of current technologies used in industry6 and access to new funding opportunities7.
Of particular interest for medical device innovation are so-called translational collaborations - defined here as collaborations between all three sectors (academia, healthcare and industry) considered essential to the biomedical innovation system1,8 - which enable biomedical discoveries to translate into clinical practice.2 Translation is possible as a result of the different, yet complementary, perspectives and social capital5 provided by each collaborator.
Collaborations between organisations can be visualised using network graphics. Such networks have been developed using co-authorship of scientific publications as a proxy for collaboration between individual researchers, their affiliated institutions and the countries in which they are based.3,9-15 These networks provide a way to ascertain the extent and nature of collaboration between different categories of organisation.
The main aim of this paper was to characterise the broad medical device development landscape, particularly with regard to collaboration, in South Africa. This characterisation was accomplished using a bibliometric network analysis of scientific publications to generate a collaboration network. The network was then used to: identify the institutions active in the field and to which sectors they belong; determine the dominant institutions (and sectors); quantify the extent of intra- and cross-sector collaboration; investigate the presence of foreign influence; and explore the prevalence of various collaboration types, with a particular focus on translational collaborations.
Co-authorship as a proxy for scientific collaboration
Scientific collaboration is a fundamental aspect of research activity, which generates knowledge flows between different groups of partners.16 It is driven by globalisation and the emergence of new communication technologies, which make it possible for different institutions to work together.17,18 Through collaboration, institutions complement their expertise, share available resources and create social networks, which often result in the integration of knowledge, efforts and capabilities as well as enhancement of productivity.19 Scientific collaboration can be measured by co-authorship - which is one of the most tangible and well-documented forms of partnership.20,21 As scientific collaboration evolves, the focus is shifting from authors to the different institutions involved, with a trend, since the 1960s, towards interdisciplinarity.22
In analysing scientific collaboration networks, centrality measures (which include degree, betweenness and closeness centrality)23, can be used to identify dominant institutions which have greater influence within the network. Each centrality measure uses a different definition of the 'centre' of action in the network, although all of them analyse patterns of relations in the network.24 Degree centrality uses the number of direct contacts of an actor as an indicator of the quality of their interconnectedness.25,26 Usually institutions or nodes with a higher degree are more central to the structure and tend to have a greater ability to influence others.25 Betweenness centrality measures the influence of an individual over information flow between others27; those with high betweenness have the potential to influence others near them in a network through both direct and indirect pathways.28 Thus an actor with high betweenness centrality can potentially influence the spread of information through the network, by facilitating, hindering, or even altering the communication between others.29 Closeness centrality gives a measure of how well connected an actor is by considering the shortest paths between them and the other actors23, thereby quantifying how long the information takes to spread from a given actor to others in the network. As such, the extent of the influence of the actor over the entire network can be investigated.30,31
Lander3 suggests that, for biomedical research and development, academia is the key network sector, followed by healthcare and government organisations, with the industry sector playing a weaker role. This ranking is further supported by the findings of Chimhundu et al.32 who showed that universities and healthcare facilities were the dominant sectors in cross-sector collaboration for cardiovascular medical device development in South Africa. Nonetheless, collaboration between all three sectors is essential within the biomedical innovation system to facilitate the translation of resources.2,8 Globally, medical research has high degrees of 'extramural domestic' collaboration between different institutions in the same country.33 In African countries, collaboration in the medical field is characterised by the dominance of international institutions, with collaboration driven by foreign funding.34 The economic and/or political dependence of a country or geopolitical region and the presence of special equipment that is shared in large multinational projects, influences the degree of co-operation.35 In a study on cardiovascular device development in South Africa, foreign institutions were found to play a role in connecting local institutions that would otherwise have remained isolated.32
Method
Regulatory control of medical devices has necessitated a single definition that would allow for their inherent diversity. Such a definition was proposed by the Global Harmonization Task Force (GHTF)36, a consortium formed in 1992 consisting of regulatory authorities and representatives from the medical device industry, now known as the International Medical Device Regulators Forum. The GHTF definition of a medical device has been widely accepted and is as follows37:
A medical device is any instrument, apparatus, implement, machine, appliance, implant, in vitro reagent or calibrator, software, material or other similar or related article that does not achieve its primary intended action in or on the human body solely by pharmacological, immunological or metabolic means and that is intended for human beings for:
• the diagnosis, prevention, monitoring, treatment or alleviation of disease;
• the diagnosis, monitoring, treatment, alleviation of, or compensation for an injury;
• the investigation, replacement, modification, or support of the anatomy or of a physiological process;
• supporting or sustaining life;
• controlling conception;
• disinfecting medical devices; and
• providing information for medical or diagnostic purposes by means of in vitro examination of specimens derived from the human body.
Publication search methodology
Because medical devices incorporate numerous technologies, it is difficult to define a search based on device types. Consequently, the publication search was structured around the three sectors - academia, healthcare and industry - known to be crucial elements of biomedical innovation.1,2
A list was generated of South African institutions involved in medical device development (see Supplementary table 1). The list consisted of 23 universities that were in existence in South Africa at the end of 2013, 10 academic hospitals, and a set of companies known to be active in the medical device field. The list of companies comprised those registered as part of the MDMSA (Medical Device Manufacturers South Africa) - an umbrella corporation for companies in South Africa that manufacture medical devices; MDMSA had 15 members when the search was executed. The list was further expanded with select members of SAMED (South African Medical Device Industry Association) - an association overseeing medical device policies, innovation, ethical principles and practices within South Africa. From the list of 121 SAMED members, 17 companies were selected according to their location (based in South Africa) and their engagement in product development.
Table 1 outlines the search method employed using both websites and search engines. This exploratory search was intended to generate a broad overview of the South African medical device development landscape that would allow key players to be identified and aspects of collaboration to be investigated.
Filtering the search results
Articles published between January 2000 and December 2013 were retained. Individual publications were manually scrutinised to determine whether their content was related to medical device development, and either accepted or rejected according to the following criteria: publication type, affiliation and indicators of medical device development.
Publication type
Journal or conference proceeding publications were retained, as were articles published on the Research Space (https://researchspace.csir.co.za/dspace/) of the Council for Scientific and Industrial Research (CSIR). The CSIR is a government commissioned science council that provides an online archive of all their research; such outputs were considered to be of similar credibility to publications in conference proceedings. Other research-related documentation (e.g. magazine articles, internal reports) was excluded.
Affiliation
At least one co-author was required to be affiliated with a South African institution.
Indicators of medical device development
Publications were required to explicitly mention a medical device (as determined by the GHTF definition), and to relay information that would aid the (further) development of said device.
Publications that discussed the use of medical devices to examine medical conditions, and did not aid the development of the device, were excluded. Typically such publications mentioned the device only in the methods section. Review papers were excluded as they do not contribute directly to device development. Animal studies that did not contribute to device development intended for human use, were excluded.
Publications that presented non-medical technological advances without making a direct link to healthcare and a prospective medical device were excluded. However, if the focus of the publication was on the non-medical technology and a medical device was discussed as an application, then the publication was included. The publication of Booysen et al.38 serves as an example: advancements in rapid prototyping technology are discussed with reference to a secure airway clamp case study.
Device development was also understood to mean novel applications of existing devices. For example, Saleh et al.39 proposed using MRI to diagnose ventricular wall remodelling, while Bosanquet and John40 discussed a method to extract patterns in EEG recordings during exercise-fatigue experiments. Although MRI and EEG devices are not new, the applications presented were; in addition, when considering the GHTF definition37, both papers presented the development of 'software intended for the investigation of a physiological process'.
Only synthetic implants, grafts and/or replacements were considered to be acceptable. Biological samples (for instance vein grafts used in heart bypass surgery) were excluded.
Extracting collaboration data
A list was extracted of the institutions with which co-authors were affiliated. Four sectors were identified:
1. Academia (A): higher education institutions involved in academic research for the development of medical devices; predominantly consisting of universities, polytechnics and colleges.
2. Healthcare (H): clinics, hospitals and medical facilities whose primary function is patient care; essential for identifying healthcare needs.
3. Industry (I): companies, firms, organisations and individuals involved in medical device development for purposes of commercialisation.
4. Science and support (S): any organisation, not belonging to one of the other sectors, that contributes to, or utilises, the scientific body of knowledge through research, education and training, clinical services and/or community services. This includes science councils, other research facilities, non-government organisations (NGOs), non-profit organisations (NPOs) and designated special interest groups.
Science councils and facilities concentrate on performing social, scientific and technological research in accordance with their commission by the South African government under the Scientific Research Council Act 46 of 1988. NGOs and NPOs may serve as a source of research and information and offer assistance and educational opportunities. Special interest groups are recognised as such by the South African Medical Association, and promote the practice and study of a particular field of medicine.
The institutions were further assessed by applying the following filters:
• Departmental affiliations were omitted and only the affiliation with the parent institution was retained. An exception to this rule was in the case of academic hospitals. For instance an author affiliated with Onderstepoort Veterinary Academic Hospital (OVAH), which is associated with the University of Pretoria (UP), was recorded as having a H sector affiliation. If the author was jointly affiliated with a UP department, a second A sector affiliation was recorded.
• Private hospitals belonging to a larger conglomerate (e.g. MediClinic, Life Health and Netcare) were still considered as individual hospitals. This was thought to better reflect collaboration at the institutional level.
• Branches of large multinationals in different locations were considered separately, e.g. Siemens Healthcare (Atlanta, GA, USA), versus Siemens Medical Solutions (Baltimore, MD, USA).
After filtering, the country in which each institution was based was recorded.
Collaboration network generation and analysis
A collaboration network was generated and analysed using UCINET (version 6.474)41 and NetDraw (version 2.131)42. Within such networks, each institution is represented by a network node, while edges (ties between nodes) represent publications on which the institutions collaborated. Edge thickness was weighted according to the number of collaborative publications; edges were undirected, as collaboration is a reciprocal relationship.
NetDraw's spring-embedding graph layout algorithm was used to draw the network, followed by manual manipulation of node positions as necessary to ensure labels were legible. The size, shape and colour of the nodes were used to highlight features of interest, namely connectedness to other institutions, sector classification and institutional location, respectively.
Structural analysis
The following centrality measures24,43 were used to identify the central nodes (i.e. dominant institutions):
• Degree: The number of connections or edges that a node has to other nodes.
• Betweenness: The number of times a node falls on the geodesic distance between all pairs of nodes in the network, normalised with respect to the maximum possible betweenness a node could have.
• Closeness: Calculated for a node (Nn) by dividing the number of remaining nodes in the network (n−1) by the sum of all distances between node Nn and each of the remaining nodes. Closeness values were calculated for the largest component within the network.27
The centrality measures were normalised using the number of nodes present in the network.44-46
Collaboration analysis
Network density measures the speed of information diffusion among nodes24 and is defined as the sum of edges present in a network between groups of nodes, divided by the maximum number of edges that could possibly exist between the nodes. When calculated for different groups within a network, density provides insight into the exchange of information within and between the groups. In this paper, group densities are used to evaluate the extent of collaboration at both the sectoral and international levels.
Additionally, the various types of sectoral collaboration and their prevalence within the network were considered. For instance, if a publication has two co-authors, one affiliated with a university (sector A) and the other with a company (sector I), the publication represents an AI type of cross-sectoral collaboration. Translational collaborations include three possible groupings of the four sectors: AHI, HIS and AHIS. Each sector contributes different, yet complementary, resources. Typically, the A sector provides a research/development component, the H sector may be responsible for identifying the patient needs to be addressed, and the I sector may be responsible for getting the product to market. Recalling that in this study, the S sector has similar resources to those of the A sector, translational collaborations are consequently considered to comprise the A and/or the S sector, in combination with the H and I sectors.
Limitations of the data set
The data set presented in this paper has three inherent shortcomings. First, even though bibliometric studies often make use of co-authorship as an indicator of collaboration, co-authorship can at best only be considered a partial indicator as not all collaborations are formally acknowledged through a co-authorship.47 This is especially true at the micro-level, when considering individual collaborators; however, the problem is lessened when considering collaborations at the macro-level (i.e. the institutional level). Second, because of the exploratory nature of the publication search, not all relevant publications would have been found. However, by the very nature of medical devices, conducting an exhaustive search would not be practicable. Third, the type of publications considered (namely journal articles and conference proceedings) are the preferred output for the A sector. The other three sectors would not necessarily use these forums to document their medical device development activities. As such the data set may be biased towards activity within the A sector.
The data set was considered suitable for an initial investigation into collaboration activities and the parties involved.
Results and discussion
After filtering the search results, a total of 171 publications remained, comprising 781 authors affiliated with 116 institutions from the four sectors (45 A; 36 H; 21 I; 14 S).
Collaboration network
Figure 1 shows the collaboration network for institutions (nodes) found to be active in medical device development during the period 2000-2013. Both local (South African) and foreign (international) institutions are shown; node colour is used to differentiate between the two locations. Furthermore, node shape is used to represent the sector to which each institution belongs (A, diamond; H, circle; I, square; S, triangle), while node size is scaled according to the node degree. Abbreviations used for the institution names are listed in Supplementary tables 2-5.
The collaboration network provides a first step in characterising the medical device development landscape in South Africa. Of the 116 institutions, 56 are local and 60 are foreign. These institutions can be further broken down to the sectoral level: A(11 local, 34 foreign); H(24 local, 12 foreign); I(10 local, 11 foreign); and S(11 local, 3 foreign).
Comparing the proportion of institutions (both local and foreign) within each sector (A-39%; H-31%; I-18%; S-12%) against those found by Lander3 for the infection and immunity network in Canada (university-37%; hospital-23%; government-18%; firm-14%; NGO-8%) echoes the dominance of the academia sector. However, more healthcare institutions were present in the South African landscape, while the industry sector proportions are similar. The government and NGO sectors identified by Lander3 are similar in nature to the institutions belonging to the S sector of this study. More institutions from these sectors were present in the network in Canada than in South Africa.
In the academia sector, nearly half (11/23) of the South African universities were found to be active in medical device development. These local institutions, however, were far outnumbered by the foreign institutions. Foreign universities therefore make a substantial intellectual contribution to medical device development activity in South Africa, although their role cannot be determined from the current analysis. However, the network does show that the foreign nodes have low degree, which would imply low influence for the individual nodes.
Far more local than foreign healthcare institutions are present in the network. Such local collaborations are critical if medical device development is to target local healthcare needs; however, further analysis would be required to ascertain if there is alignment between the types of devices being developed and the healthcare needs.
Dominant institutions
The dominant institutions within the network were identified by calculating three centrality measures (degree, closeness and betweenness) and listing the 10 highest ranking nodes for each measure (Table 2). These measures indicate different ways in which institutions exhibit dominance in the network. Nodes with high degree have a greater ability to influence others, while high betweenness measures the institution's ability to spread information through the network, and closeness indicates which nodes could potentially facilitate the efficient spread of knowledge in the network.
According to Goh et al.48, a network typically constitutes a small number of influential individuals and many peripheral actors. The findings in Table 2 agree with this observation. Seven local nodes are common to all three centrality measures: UCT (University of Cape Town), GSH (Groote Schuur Hospital), SUN (Stellenbosch University), UP (University of Pretoria), WITS (University of the Witwatersrand), TH (Tygerberg Hospital) and NHLS (National Health Laboratory Services). The order in which these institutes appear varies for each measure, although UCT is consistently the highest ranked node, while GSH and SUN share the second and third highest rankings. Four of the seven institutions are from the A sector, two from the H sector and one from the S sector. It should also be noted that both of the H sector institutions are academic hospitals. This dominance exhibited by the A and H sectors is consistent with the findings of Lander3.
Universities are seen to form hubs that facilitate the exchange of information within the network; they are typically well connected and are considered to be influential in the development of medical devices. Because of their position in the network, universities have the potential to help establish new collaborations with other institutions in the network.
The industry sector is absent from Table 2, except for two foreign organisations. This finding may be as a result of the bias inherent in the network, namely that journal article publication is not imperative for industry. However, it could also indicate that local industry is not a dominant figure in medical device innovation within South Africa. Further investigation, possibly through patent analysis, could produce more insight. Another possible explanation of the low industry presence in the South African co-publication landscape could be that the academic research being generated is not finding a market. Lander and Atkinson-Grosjean2 described a number of translational pathways necessary for biomedical innovation, one of which was the commercial pathway which characterised movement between academia and the marketplace, typically facilitated by the I sector. Further analysis would be needed to investigate the extent of this pathway in South Africa.
Sectoral and international collaboration
Extent of collaboration
Table 3 shows the group densities (ρ) that were calculated for the four sectors. Within-group densities lie on the diagonal of the table, are shown in bold, and represent intra-sectoral collaboration. The remaining entries in the table are the between-group densities, which represent cross-sectoral collaboration. The table is symmetric, because of the reciprocal nature of collaboration in the network. The sum of edge weights (∑ew) used to calculate the densities is also shown in the table; these values indicate the number of edges present in the network, taking into account the weight of the edges, which connect all the nodes belonging to the various sectors. For instance, the summed weight of all edges in the network that connect institutions from the A sector to those of the H sector is 114.
Considering only intra-sectoral collaboration, from highest density to lowest we can rank the sectors as follows: A, H, S, I. The fact that universities are seen to collaborate extensively with one another (the density of 0.088 seen for AA collaboration is the highest in the entire table), is to be expected. Low intra-sector collaboration within the I sector (ρ=0.014; one of the lowest values in the entire table) is evident, perhaps because local companies perceive little commercial gain in II collaboration. The relatively high density seen for the H sector (ρ=0.065) could stem from the fact that many of the hospitals in the network are academic hospitals and may reflect university research practices.
With regard to cross-sectoral collaboration, the three highest ranking collaboration types are AH (ρ=0.070; ∑ew=114), AS (ρ=0.063; ∑ew=40) and AI (ρ=0.049; ∑ew=46), while the two lowest ranking collaboration types are IS (ρ=0.014; ∑ew=4) and HS (ρ=0.012; ∑ew=6).
AH has the highest cross-sectoral collaboration density - a finding in agreement with that of Chimhundu et al.32 for cardiovascular devices. The next highest cross-sector density was found for AS collaborations, even though the S sector (14 institutions) is considerably smaller than the H sector (36 institutions). When comparing AS and AI, even though AS has the higher ρ value, the ∑ew value is lower than that of AI. This result is explained by the fact that there are fewer S sector (14) than I sector (21) institutions. What these observations indicate, is that small sectors like the S sector can still be extensively involved in collaboration activities, and conversely, that larger sectors do not necessarily engage in more instances of collaboration.
The A sector is seen to collaborate extensively both intra- and cross-sectorally as it is present in the highest ranking collaboration types for both cases, and is seen to readily collaborate with all other sectors. Interestingly, the S sector is found to be present in one of the highest ranking cross-sectoral collaboration types (AS), as well as the two lowest (IS and HS).
A similar analysis was carried out to investigate the extent of international collaboration, the results of which are also shown in Table 3. Local only (l-l) collaboration is shown in bold text, while local-foreign (l-f) collaboration is shown in normal text. The number of local institutions in the network is about the same as the number of foreign institutions (56 and 60 respectively). Nonetheless the l-l density is almost three times greater than that of the l-f collaborations. Even though foreign institutions do have a presence in the South African medical device development landscape, local institutions collaborate far more extensively with one another than with foreign institutions.
Collaboration type prevalence
Publications were sorted according to the type of sectoral collaboration each represented. The number of publications thus found was further divided to show collaborations that involved only local institutions; mixed collaborations with more than one local institution and additional foreign involvement; and, foreign collaborations (a single local institution collaborating with foreign institutions). The proportions of the local, mixed and foreign collaborations are indicated in Figure 2.
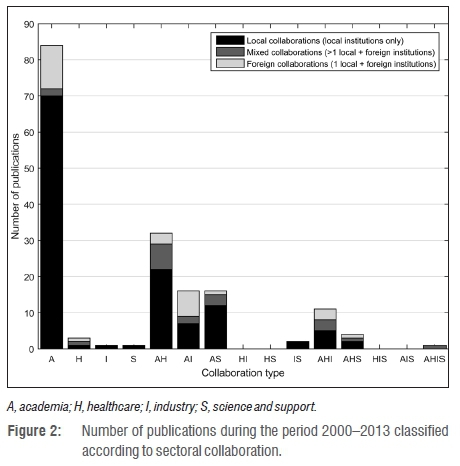
Even though the number of foreign institutions (60) present in the network is slightly greater than the number of local institutions (56), 123 of the total 171 publications (72%) were found to represent local collaboration. This proportion far exceeded the 20 publications (12%) representing mixed collaboration and 28 publications (16%) showing foreign collaboration. Thus local institutions collaborate far more locally than internationally. This observation is in agreement with the findings of Sooryamoorthy49 who investigated partnership trends evident in South African medical research (1975-2005). Sooryamoorthy49 showed that out of 5642 medical research publications, only 20.6% involved international partnerships in which at least one co-author was affiliated with an institution from a foreign country.
In Figure 2, translational collaboration types are represented by the columns labelled AHI, HIS and AHIS. Of these three collaboration types, AHI was the most active, HIS had no publications, and AHIS only had mixed collaborations. The low number of publications (7%; 12 out of 171) involving translational collaborations may be indicative of the biomedical innovation chain in South Africa not fully utilising the pathways presented by Lander and Atkinson-Grosjean2. Even though there is evidence of medical device development activity and cross-sectoral collaboration occurring locally within the country, the lack of translational collaborations could indicate that the devices being developed may not be relying on evidence of healthcare needs from the H sector, or are not reaching the market (lack of I sector involvement). However, the fact that there are institutions from the various sectors engaging in medical device development, means that collaborative opportunities exist. A study by Chinchilla-Rodríguez et al.50 investigating medical research collaboration in Latin America and the Caribbean (2003-2007) showed that through the implementation of policies to promote desired intra-regional collaboration, the growth of select collaboration types can indeed be fostered.
Summary and conclusion
We examined the South African medical device development landscape for the period 2000-2013. Through a bibliometric analysis in which co-authorship on scientific papers was interpreted as an indicator of collaboration, a collaboration network of the medical device development field was produced. Four sectors - academia, healthcare, industry, and science and support - were identified as being active in device development. The dominant institutions within the network were identified according to their influence on other institutions, their ability to quickly disseminate information through the network, and their ability to broker the exchange of information between institutions. Of these, seven local institutions, from three of the four sectors (4-A, 2-H, 1-S), were found to be the most dominant. The three highest-ranking dominant institutions were the University of Cape Town (UCT), Groote Schuur Hospital (GSH) and Stellenbosch University (SUN).
Collaboration activities at the sectoral level were investigated. With regard to intra-sector collaboration, the A sector collaborated the most, followed by the H sector and then the S sector.
About the same number of local and foreign institutions (56 and 60, respectively) were present in the network. The local institutions, however, were seen to collaborate far more with each other (72% of all collaborations) than with international institutions (28%).
There were far fewer translational collaborations - involving at least three different sectors (A or S, H and I) - present in the South African medical device field, compared with intra-sectoral collaborations, or cross-sectoral collaborations involving two different sectors. Thus there is room for increased translational collaboration within South Africa, potentially for greater health impact. Policies structured to help engender more cross-sector interaction could benefit medical device development, by creating opportunities for more translational collaborations.
Acknowledgements
We thank Mr Mohammed Esmail (Cape Peninsula University of Technology), Ms Katharina Hauprich (University of Applied Sciences Hamm-Lippstadt) and Dr Robyn May (University of Cape Town), for their contributions to the collection, filtering and exploration of the data. This work was supported by the Community Engagement Programme of the South African National Research Foundation (NRF) [82624]; the South African Research Chairs Initiative of the Department of Science and Technology and the NRF [98788]; the Andrew W. Mellon Foundation; and the Programme for the Enhancement of Research Capacity (PERC) at the University of Cape Town.
Authors' contributions
T.S.D. was the project leader and was responsible for funding acquisition. K.d.J., C.C. and T.S.D. were responsible for conceptualisation and developed the bibliometric and network analysis methodology employed. K.d.J. oversaw the data collection and analysis. K.d.J. and C.C. were responsible for data curation. T.S. developed the theoretical framework. K.d.J. wrote the initial draft of the manuscript. C.C., T.S. and T.S.D. contributed to the interpretation of results and reviewed and edited the manuscript.
References
1. Hicks D, Katz JS. Hospitals: The hidden research system. Sci Public Policy. 1996;23(5):297-304. https://doi.org/10.1093/spp/23.5.297 [ Links ]
2. Lander B, Atkinson-Grosjean J. Translational science and the hidden research system in universities and academic hospitals: A case study. Soc Sci Med. 2011;72(4):537-544. https://doi.org/10.1016/j.socscimed.2010.11.019 [ Links ]
3. Lander B. Sectoral collaboration in biomedical research and development. Scientometrics. 2013;94(1):343-357. https://doi.org/10.1007/s11192-012-0776-8 [ Links ]
4. Lester RK. Universities, innovation, and the competitiveness of local economies: A summary report from the Local Innovation Systems Project - Phase I. Working Paper 05-010 [document on the Internet]. [ Links ] c2005 [cited 2016 Jul 13]. Cambridge, MA: Industrial Performance Center, Massachusetts Institute of Technology. Available from: http://web.mit.edu/lis/papers/LIS05-010.pdf
5. Lander B. The role of institutions and capital in intersectoral collaboration: Infection and immunity research and development collaboration in Vancouver. Rev Policy Res. 2014;31(5):390-407. https://doi.org/10.1111/ropr.12086 [ Links ]
6. Fries RC, Glave SA, Radick MK. The benefits of industry-university interaction. In: Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2008 August 20-25; Vancouver, Canada. IEEE; 2008. p. 1598-1601. https://doi.org/10.1109/IEMBS.2008.4649477 [ Links ]
7. Douglas TS. Drivers and restrainers of relevance in graduate BME education - A South African study. In: Proceedings of the 34th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC); 2012 August 28 - September 01; San Diego, CA, USA. IEEE; 2012; p. 5054-5057. https://doi.org/10.1109/EMBC.2012.6347129 [ Links ]
8. The Academy of Medical Sciences FORUM. Academia, industry and the NHS: Collaboration and innovation - Meeting report. c2010 [cited 2014 Nov 27]. [ Links ] Available from: http://www.acmedsci.ac.uk/viewFile/publicationDownloads/Collabor.pdf
9. Breschi S, Catalini C. Tracing the links between science and technology: An exploratory analysis of scientists' and inventors' networks. Res Policy. 2010;39(1):14-26. https://doi.org/10.1016/j.respol.2009.11.004 [ Links ]
10. Meyer M, Bhattacharya S. Commonalities and differences between scholarly and technical collaboration. Scientometrics. 2004;61(3):443-456. https://doi.org/10.1023/B:SCIE.0000045120.04489.80 [ Links ]
11. Abbasi A, Altmann J. On the correlation between research performance and social network analysis measures applied to research collaboration networks. In: Proceedings of the 2011 44th Hawaii International Conference on System Sciences (HICSS); 2011 January 4-7; Kauai, HI, USA. IEEE; 2011. p. 1-10. https://doi.org/10.1109/HICSS.2011.325 [ Links ]
12. Gazni A, Sugimoto CR, Didegah F. Mapping world scientific collaboration: Authors, institutions, and countries. J Am Soc Inf Sci Tec. 2012;63(2):323-335. https://doi.org/10.1002/asi.21688 [ Links ]
13. Ding Y. Scientific collaboration and endorsement: Network analysis of coauthorship and citation networks. J Informetr. 2011;5(1):187-203. https://doi.org/10.1016/j.joi.2010.10.008 [ Links ]
14. Newman M. Scientific collaboration networks I: Network construction and fundamental results. Phys Rev E. 2001;64(1), Art. #016131, 8 pages. https://doi.org/10.1103/PhysRevE.64.016131 [ Links ]
15. Ramlogan R, Mina A, Tampubolon G, Metcalfe JS. Networks of knowledge: The distributed nature of medical innovation. Scientometrics. 2007;70(2):459-489. https://doi.org/10.1007/s11192-007-0212-7 [ Links ]
16. Abramo G, D'Angelo CA, Solazzi M. Are researchers that collaborate more at the international level top performers? An investigation on the Italian university system. J Informetr. 2011;5(1):204-213. https://doi.org/10.1016/j.joi.2010.11.002 [ Links ]
17. Hoekman J, Frenken K, Tijssen RJ. Research collaboration at a distance: Changing spatial patterns of scientific collaboration within Europe. Res Policy. 2010;39(5):662-673. https://doi.org/10.1016/j.respol.2010.01.012 [ Links ]
18. Ynalvez MA, Shrum WM. Professional networks, scientific collaboration, and publication productivity in resource-constrained research institutions in a developing country. Res Policy. 2011;40(2):204-216. https://doi.org/10.1016/j.respol.2010.10.004 [ Links ]
19. Powell WW, Koput KW, Smith-Doerr L. Interorganizational collaboration and the locus of innovation: Networks of learning in biotechnology. Admin Sci Quart. 1996;41(1):116-145. https://doi.org/10.2307/2393988 [ Links ]
20. Franceschet M, Costantini A. The effect of scholar collaboration on impact and quality of academic papers. J Informetr. 2010;4(4):540-553. https://doi.org/10.1016/j.joi.2010.06.003 [ Links ]
21. Glänzel W, Schubert A. Double effort = double impact? A critical view at international co-authorship in chemistry. Scientometrics. 2001;50(2):199-214. http://dx.doi.org/10.1023/A:1010561321723 [ Links ]
22. Qin J. An investigation of research collaboration in the sciences through the philosophical transactions 1901-1991. Scientometrics. 1994;29(2):219-238. https://doi.org/10.1007/BF02017974 [ Links ]
23. Yan E, Ding Y. Applying centrality measures to impact analysis: A coauthorship network analysis. J Am Soc Inf Sci Tec. 2009;60(10):2107-2118. https://doi.org/10.1002/asi.21128 [ Links ]
24. Hanneman RA, Riddle M. Introduction to social network methods. Riverside, CA: University of California; 2005. [ Links ]
25. Landherr A, Friedl B, Heidemann J. A critical review of centrality measures in social networks. Bus Inf Syst Eng. 2010;2(6):371-385. https://doi.org/10.1007/s12599-010-0127-3 [ Links ]
26. Valente TW, Coronges K, Lakon C, Costenbader E. How correlated are network centrality measures? Connect (Tor). 2008;28(1):16-26. [ Links ]
27. Opsahl T, Agneessens F, Skvoretz J. Node centrality in weighted networks: Generalizing degree and shortest paths. Soc Networks. 2010;32(3):245-251. https://doi.org/10.1016/j.socnet.2010.03.006 [ Links ]
28. Abbasi A, Hossain L, Leydesdorff L. Betweenness centrality as a driver of preferential attachment in the evolution of research collaboration networks. J Informetr. 2012;6(3):403-412. https://doi.org/10.1016/j.joi.2012.01.002 [ Links ]
29. Dolev S, Elovici Y, Puzis R. Routing betweenness centrality. J Acm. 2010;57(4):1-27. https://doi.org/10.1145/1734213.1734219 [ Links ]
30. Okamoto K, Chen W, Li XY. Ranking of closeness centrality for large-scale social networks. In: Preparata FP, Wu X, Yin J, editors. Frontiers in algorithmics. Lecture notes in computer science volume 5059. Berlin: Springer; 2008. p. 186-195. https://doi.org/10.1007/978-3-540-69311-6 [ Links ]
31. Wehmuth K, Ziviani A. DACCER: Distributed Assessment of the Closeness CEntrality Ranking in complex networks. Comput Netw. 2013;57(13):2536-2548. https://doi.org/10.1016/j.comnet.2013.05.001 [ Links ]
32. Chimhundu C, De Jager K, Douglas T. Sectoral collaboration networks for cardiovascular medical device development in South Africa. Scientometrics. 2015;105(3):1721-1741. https://doi.org/10.1007/s11192-015-1743-y [ Links ]
33. Thijs B, Glänzel W. A structural analysis of collaboration between European research institutes. Res Evaluat. 2010;19(1):55-65. https://doi.org/10.3152/095820210X492486 [ Links ]
34. Pouris A, Ho YS. Research emphasis and collaboration in Africa. Scientometrics. 2013;98(3):2169-2184. https://doi.org/10.1007/s11192-013-1156-8 [ Links ]
35. Glänzel W, Schubert A. Analysing scientific networks through co-authorship. In: Moed HF, Glänzel W, Schmoch U, editors, Handbook of quantitative science and technology research. Dordrecht: Springer Netherlands; 2005. p. 257-276. https://doi.org/10.1007/1-4020-2755-9_12 [ Links ]
36. Global Harmonization Task Force Study Group 1. Information document concerning the definition of the term "medical device". c2005 [cited 2014 Jul 07]. [ Links ] Available from: http://www.imdrf.org/docs/ghtf/final/sg1/technical-docs/ghtf-sg1-n29r16-2005-definition-medical-device-050520.pdf
37. World Health Organization (WHO). Medical devices: Managing the mismatch: An outcome of the priority medical devices project. Geneva: WHO; 2010. Available from: http://apps.who.int/iris/bitstream/10665/44407/1/9789241564045_eng.pdf [ Links ]
38. Booysen G, De Beer D, Truscott M, Combrinck J, Mosimanyane D. Combining additive fabrication and conventional machining technologies to develop a hybrid tooling approach. In: Proceedings of the 21st International DAAAM Symposium volume 21. Vienna: DAAAM International; 2010. p. 1563-1564. Available from: http://www.daaam.info/Downloads/Pdfs/proceedings/proceedings_2010/24988_Annals_1_head.pdf [ Links ]
39. Saleh MG, Sharp SK, Alhamud A, Spottiswoode BS, Van der Kouwe AJW, Davies NH, et al. Long-term left ventricular remodelling in rat model of nonreperfused myocardial infarction: Sequential MR imaging using a 3T clinical scanner. J Biomed Biotechnol. 2012;2012, Art. #504037, 10 pages. https://doi.org/10.1155/2012/504037 [ Links ]
40. Bosanquet D, John L. A method for investigating alpha band phase synchronization in the cortex during a fatiguing muscle contraction using EEG. In: The 2006 International Conference on Scientific Computing (CSC). USA. 2006. Available from: https://www.semanticscholar.org/paper/A-Method-For-Investigating-Alpha-Band-Phase-Bosanquet-John/b6b6a00a836a21f6af7c3480d5ed6c3f57934d0a [ Links ]
41. Borgatti SP, Everett MG, Freeman LC. Ucinet for Windows: Software for social network analysis. Harvard, MA: Analytic Technologies; 2002. Available from: https://sites.google.com/site/ucinetsoftware/home [ Links ]
42. Borgatti SP. NetDraw software for network visualization. Lexington, KY: Analytic Technologies; 2002. Available from: https://sites.google.com/site/netdrawsoftware/home [ Links ]
43. De Prato G, Nepelski D. Global technological collaboration network: Network analysis of international co-inventions. J Technol Transf. 2014;39(3):358-375. https://doi.org/10.1007/s10961-012-9285-4 [ Links ]
44. Abbasi A, Altmann J, Hossain L. Identifying the effects of co-authorship networks on the performance of scholars: A correlation and regression analysis of performance measures and social network analysis measures. J Informetr. 2011;5(4):594-607. https://doi.org/10.1016/j.joi.2011.05.007 [ Links ]
45. Cimenler O, Reeves KA, Skvoretz J. A regression analysis of researchers' social network metrics on their citation performance in a college of engineering. J Informetr. 2014;8(3):667-682. https://doi.org/10.1016/j.joi.2014.06.004 [ Links ]
46. Rodriguez Miramontes J, Gonzalez-Brambila CN. The effects of external collaboration on research output in engineering. Scientometrics. 2016;109(2):661-675. https://doi.org/10.1007/s11192-016-2054-7 [ Links ]
47. Laudel G. What do we measure by co-authorships? Res Evaluat. 2002;11(1):3-15. https://doi.org/10.3152/147154402781776961 [ Links ]
48. Goh KI, Oh E, Jeong H, Kahng B, Kim D. Classification of scale-free networks. Proc Natl Acad Sci USA. 2002;99(20):12583-12588. https://doi.org/10.1073/pnas.202301299 [ Links ]
49. Sooryamoorthy R. Medical research in South Africa: A scientometric analysis of trends, patterns, productivity and partnership. Scientometrics. 2010;84(3):863-885. https://doi.org/10.1007/s11192-010-0169-9 [ Links ]
50. Chinchilla-Rodríguez Z, Benavent-Pérez M, De Moya-Anegón F, Miguel S. International collaboration in medical research in Latin America and the Caribbean (2003-2007). J Am Soc Inf Sci Tec. 2012;63(11):2223-2238. https://doi.org/10.1002/asi.22669 [ Links ]
 Correspondence:
Correspondence:
Kylie de Jager
kylie.dejager@uct.ac.za
Received: 30 Aug. 2016
Revised: 01 Dec. 2016
Accepted: 31 Jan. 2017
FUNDING: Andrew W. Mellon Foundation; Community Engagement Programme of the National Research Foundation (South Africa); Programme for the Enhancement of Research Capacity, University of Cape Town; South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation (South Africa)
ARTICLE INCLUDES: ✓ Supplementary material














