Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.113 n.3-4 Pretoria Mar./Apr. 2017
http://dx.doi.org/10.17159/sajs.2017/20160105
RESEARCH ARTICLE
Characterisation of vumba and ubumba clays used for cosmetic purposes
Refilwe Morekhure-MphahleleI, II; Walter W. FockeIII; Wiebke GroteIV
IDepartment of Chemistry, Tshwane University of Technology, Pretoria, South Africa
IIDepartment of Chemical Engineering, University of Pretoria, Pretoria, South Africa
IIIInstitute of Applied Materials, Department of Chemical Engineering, University of Pretoria, Pretoria, South Africa
IVDepartment of Geology, University of Pretoria, Pretoria, South Africa
ABSTRACT
Two traditional cosmetic clays bear similar names in different local South African languages: vumba (Tshivenda) and ubumba (isiZulu). The wet clays are applied topically for cosmetic purposes by the respective indigenous peoples. Six samples from two South African provinces were characterised using X-ray diffraction, X-ray fluorescence spectroscopy, Fourier transform infrared spectroscopy, thermal gravimetric analysis and scanning electron microscopy. It was found that the samples differed widely with respect to mineralogy and chemical composition. This finding raises the possibility that texture characteristics during application on the skin override composition effects. Of concern is the high levels of quartz found in all the samples as it might pose a health hazard; the lowest value for quartz was 11 wt% for vumba, while values for ubumba ranged from 26 wt% to 85 wt%. All samples contained varying amounts of silicates in the form of smectite, kaolin, chlorite and plagioclase. Minor amounts of anatase and rutile were present in some samples. Three samples also contained goethite. All samples were essentially free from the toxic elements As, Pb, Hg, Cd, Se and Sb. However, they did contain low levels of chromium and heavy metals such as Cu, Zn and Ni. The pH values of ubumba slurries were slightly basic, while those of a vumba slurry were slightly acidic.
SIGNIFICANCE:
• Wide ranges of composition appear to be acceptable.
• The clays do not contain highly toxic or radioactive elements.
• The high levels of quartz present may pose a human health risk.
Keywords: quartz; tradition; chemical composition; mineral composition
Introduction
Throughout human history, clays and clayey soils have been used for religious, artistic, cosmetic and therapeutic purposes. For example, the utilisation of ochre, a clay stained by iron oxides, dates back to the Middle Stone Age. South Africa has a rich tradition in this regard. Evidence of human exploitation, also dating back to the Middle Stone Age, has been found at Klein Kliphuis1, the Blombos Cave2, Diepkloof Rock Shelter3 and the Sibudu Cave4. The practice of using ochre-like substances for topical cosmetic applications and other purposes persists to the present day. The red clay pastes are known as letsoku in Sotho culture and ibomvu among Nguni people and are used by both women and men in traditional ceremonies. This and other lighter-coloured clays apparently also serve as sunscreens.5
Clays find application as cosmetic products, i.e. applications in which the preparation is placed in contact with the outside of the human body, e.g. the skin, hair and lips.6 In this context 'clay' can refer to a mineralogical term. However, it may also denote a natural material composed of very fine-grained minerals that show some plasticity when mixed with an appropriate amount of water.7 The layered structure and colloidal size of the particles constituting the clay give rise to desired rheological characteristics and sorptive abilities, among others.7 Natural clays typically contain other minerals such as quartz, feldspars, carbonates, sulfates and iron, aluminium or titanium oxides. These minerals may affect the chemical (e.g. stability, purity), physical (e.g. texture, moisture content, particle size) and toxicological requirements specified for each clay application.7 Particular attention is paid to the presence of silica as there is sufficient evidence to suggest that it is a potential carcinogen. To avoid requirements for labelling that provides safety information, the silica content must be less than 0.1%.7
Traditional clays, used among indigenous people in the Eastern Cape Province of South Africa, have been studied extensively.5,6,8-11 However, there are few reports dealing with the nature and properties of the yellowish-grey clays that are also used primarily for cosmetic purposes. Interestingly, letsoku is usually supplied as a dry powder, whereas other clays, colloquially known as vumba and ubumba in the rural areas, are supplied in a wet form and provide a skin moisturising effect. This communication provides information on the nature and chemical composition of the latter two clays sourced from KwaZulu-Natal and Limpopo Provinces, respectively. This information is relevant for assessing the suitability of these cosmetic clays for more widespread commercial distribution.12
Materials and methods
Materials and sample preparation
The wet light brown to grey clay samples were sourced from three different sites in two South African provinces. The clays were supplied by traditional healers and are reportedly sold locally for topical cosmetic applications, although health benefits are also claimed. In some cases, different samples with distinct properties were supplied from the same general deposit. Details of the sampling locations are given in Table 1.
The wet clay samples were supplied packaged in clear polyethylene bags by the traditional healers who sell the products for topical applications. They were transferred into polyethylene bottles. Portions were dried at 50 °C overnight before being milled into fine powders (<75 μηι) in a milling unit fitted with a tungsten carbide vessel.
Characterisation
X-ray diffraction (XRD) measurements were performed at the Department of Geology (University of Pretoria) on a PANalytical X'Pert PRO X-ray diffractometer in q-q configuration, equipped with Fe-filtered Co-Ka radiation (λ = 1.789 Å) and an X'Celerator detector and variable divergence and fixed receiving slits. Samples were prepared according to the standardised PANalytical back-loading system, which provides nearly random distribution of the particles. The data were collected in the angular range 5°<2Θ<90° with a step size of 0.008° 2θ and a 10-s scan step time. The phases were identified using X'Pert Highscore plus software.
The relative phase amounts (weight %) were estimated by the Rietveld method using Autoquan/BGMN software (GE Inspection Technologies; Kleeberg & Bergmann) which employs the fundamental parameter approach. Autoquan combines the analytical potential of BGMN with an 'easy to operate' user surface. In the Rietveld method, an observed data pattern is compared with a calculated pattern. By varying all the parameters the difference between the calculated and observed patterns is minimised by a least squares procedure, until the best possible fit is obtained. The background was fitted by the polynomial order which was determined automatically, depending on the angular range.
X-ray fluorescence spectroscopy (XRF) analyses were performed at the Department of Geology (University of Pretoria) on powders milled to a fine particle size (<75 μm). The moisture content was determined by weighing an accurate mass of approximately 3 g of powder into an alumina crucible. The crucible was then heated in an oven at 100 °C for 2 h. Thereafter the dehydrated samples were roasted at 1000 °C overnight. A sample mass of 1 g of the residue was fused with about 6 g of lithium tetraborate at 1050 °C for metal oxide determination. The trace metals were determined on samples bound with poly(vinyl alcohol) and pressed into powder briquettes.
Scanning electron microscopy (SEM) was performed in the Laboratory for Microscopy and Microanalysis (University of Pretoria), on the powders. Sample powders were lightly sprinkled onto small stubs of double-sided adhesive carbon tape. Compressed air was used to remove any excess powder. The morphology of the carbon-coated powders was studied with a Zeiss Ultra-55 field emission scanning electron microscope fitted with an InLens detector at an acceleration voltage of 1 kV.
Attenuated total reflection Fourier transform infrared (ATR-FTIR) spectra were recorded on an in-house Perkin Elmer Spectrum 100 Series instrument. The spectra represent averages of 32 scans over the wave number range 500-4000 cm-1.
Thermogravimetric analysis (TGA) was performed on an in-house Perkin Elmer TGA 4000 unit. Alumina crucibles were used and the sample size was about 40 mg. Temperature was scanned from 25 °C to 950 °C at 10 °C/min with air flowing at 50 mL/min.
The cation exchange capacity of the clays was determined using the pH-dependent methylene blue halo method adapted from Kahr and Madsen13.
Results and discussion
The X-ray diffractograms presented in Figure 1 indicate that the materials are complex mixtures of various minerals. The mineral compositions derived from the XRD data are summarised in Table 2. According to the main mineral phases present, samples V1 and U1 are smectitic clays with major admixtures of quartz and minor amounts of kaolin and plagioclase. In contrast, all the other samples are composed primarily of quartz with intermediate amounts of kaolinite and plagioclase. All samples contain minor amounts of the recalcitrant titanium dioxide, i.e. rutile and anatase. Only samples U1, U3 and W1 contained goethite, which also explains their yellowish colour. An unexpected and surprising observation is the high compositional variability of the samples. This variability extends to the samples sourced from effectively the same locations. This finding leads one to speculate that factors other than mere compositional aspects determine the suitability of the clays for their intended purposes. Perhaps the subjective texture experience during skin application and use might be more important. In the wet, as-supplied form, all samples had a smooth creamy feel when rubbed between the thumb and index finger. However, they also featured varying degrees of grittiness. Sample W2 was particularly gritty, probably because of the presence of silica particles.
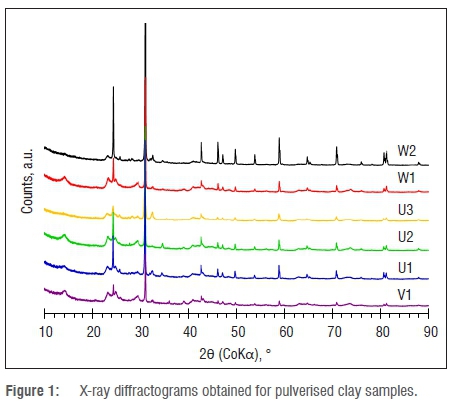
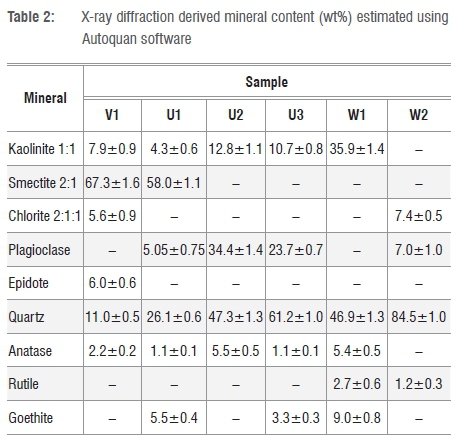
A significant finding is that all samples contained substantial amounts of quartz. The quartz content varied from 11.4 wt% in sample V1 to 84.5 wt% in sample W2. According to Khiari et al.14, a quartz content above 15 wt% is cause for concern, even for topical applications, as there is sufficient evidence of quartz carcinogenicity.
The chemical composition results in Table 3 confirm the high silicon content expected in view of the silica and the silicate minerals present in the samples. In particular, it confirms the very high quartz content of sample W2. Sample V1 shows the highest iron content, despite the fact that it apparently contains no goethite. At first this appears to be a contradiction. However, iron-rich smectites containing even higher amounts of iron have been reported.15
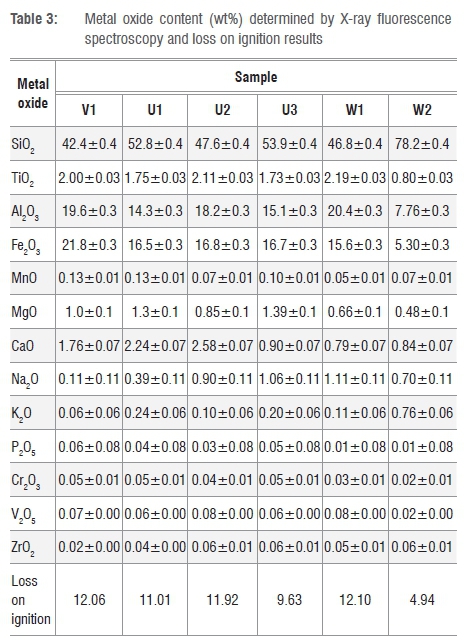
The trace metals detected by XRF are listed in Table 4. Fortunately, all the samples are essentially free of the toxic elements As, Pb, Hg, Cd, Se and Sb. However, they do contain low levels of chromium and other heavy metals such as Cu, Zn and Ni. They do not contain detectable levels of the radioactive elements uranium and thorium. Strontium (Sr) (30-98 ppm), zirconium (Zr) (172-387 ppm) and yttrium (Y) (10-44 ppm) are present in substantial amounts. The stable form of Sr is not radioactive and it does not pose a significant human health risk at the levels detected. However, Zr and Y do pose a radioactive risk. Future studies should investigate whether the potential toxic elements are readily exchangeable or strongly bound to the mineral structures.
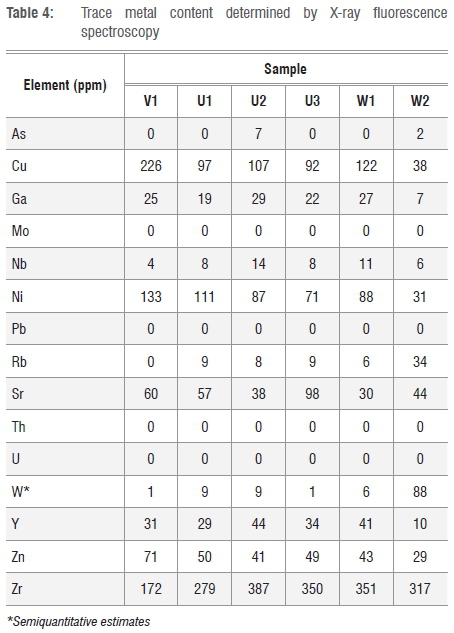
Additional data on the materials are presented in Table 5. Dispersion of sample V1 in water rendered it slightly acidic, but all the other samples produced a slightly basic pH. Clays used in cosmetics are usually fairly basic with the pH ranging from pH 7 to 10.5.7 Literature reports indicate skin pH values ranging from pH 4 to 7, but 'natural' skin actually has a surface pH just below 5.6,16 Apparently this pH is beneficial for the natural resident flora.7 Application of clay-based cosmetics could make it too alkaline which could cause the skin to become too dry and sensitive, and may even result in eczema. So care is advised when using the present clays in skin applications for extended periods of time.
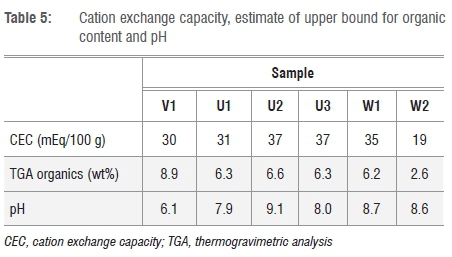
The fairly significant loss on ignition values (9-12%) (see Table 3) suggest the presence of organic material. In some cases, when soil samples were heated in air, mass loss was observed in the TGA traces over the temperature range 200-500 °C (Figure 2). This mass loss often is associated with the oxidation of any organic substances present.17,18 However, goethite decomposes into haematite at about 300 °C.19 The mass loss resulting from the corresponding loss of water is 10.0 wt%. Taking this loss into account, an upper bound for the organic content of the materials was determined and these values are listed in Table 5. The apparent organic content varied from 2.6 wt% for sample W2 to 8.9 wt% for sample V1.
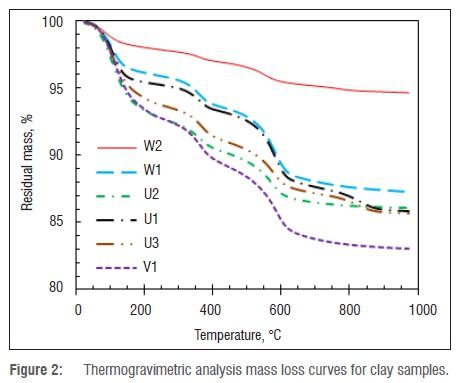
However, the absence of any bands near 3000 cm-1 in the FTIR spectra shown in Figure 3 suggests that very little, if any, organic material is actually present. The spectra can be fully explained by the presence of inorganic components in terms of previous observations.20-23 The O-H asymmetric stretching occurs around 3700 cm-1, probably as a result of the presence of the surface and internal OH groups of the Al-OH in the octahedral sheets. This band is lacking in the spectrum of sample W2, confirming the XRD result of the absence of kaolinite. The band at 3660 cm-1 is attributed to water OH bending vibrations23, but there is only a very shallow dip in the spectrum of sample W2. The absence of a band located at 1230-1280 cm-1 implies the absence of amorphous silica. Si-O stretch vibrations are observed at 1000 cm-1.23 The bands near 920 cm-1 are typical for Si-O stretching and Al2O-H deformations in the aluminium silicate, i.e. kaolinite.20 The bands at 1100 cm-1 are assigned to Si-O and those at 1025 cm-1 to Si-O planar stretching. The bands around 775 cm-1 and 750 cm-1 can be attributed to the Al-O-Si inner surface vibration. The bands at 800 cm-1, 775 cm-1 and 690 cm-1 in the spectrum of sample W2 are typical for quartz.21
The powder particles present in each individual clay sample exhibited various morphologies. The scanning electron micrographs in Figure 4 show only some selected particle morphologies found in each of the clay samples. However, in all cases, flake-shaped particles and highly agglomerated structures comprising small roundish sub-particles are present. The former reflect the presence of the phyllosilicates (kaolin, smectites, etc.), while the latter represent the other minerals present (silica, goethite, anatase, rutile, etc.).
Conclusion
We analysed clay samples sourced via traditional healers from Mtubatuba and Nongoma in KwaZulu-Natal and from Mbaleni in Limpopo, South Africa. These clays, known as vumba and ubumba, are supplied in a wet state and are reportedly used as topical cosmetics with health benefits. XRD analysis revealed a surprising variability in the mineralogical composition of the clays, even in samples taken from the same deposit. This implies that the actual application is robust with respect to clay composition. XRF analysis showed that the samples were free from radioactive elements and the toxic elements As, Pb, Hg, Cd, Se and Sb. However, they did contain low levels of chromium and other heavy metals, e.g. Cu, Zn, Ni. A disconcerting discovery is the high silica content, ranging from 11 to 85 wt%. Even though the intended purpose relates to topical applications, these levels may pose a certain health risk.
Acknowledgements
We thank Prof. T.S. Ramukumba and Ms V. Simelani-Ramela for help with the sourcing of the samples and Tshwane University of Technology for financial support including a sabbatical stay for R.M-M.
Authors' contributions
R.M-M. collected the samples, performed most of the experiments and managed the characterisation done by others, notably the XRD and XRF investigations. She also analysed the results and drafted the manuscript. W.W.F. supervised R.M-M., assisted in evaluating the experimental results and edited the manuscript. W.G. assisted with sample preparation (milling) and performed the XRD analyses.
References
1. Mackay A, Welz A. Engraved ochre from a Middle Stone Age context at Klein Kliphuis in the Western Cape of South Africa. J Archaeol Sci. 2008;35(6):1521-1532. http://dx.doi.org/10.1016/j.jas.2007.10.015 [ Links ]
2. Henshilwood CS, D'Errico F, Van Niekerk KL, Coquinot Y Jacobs Z, Lauritzen SE, et al. A 100,000-year-old ochre-processing workshop at Blombos Cave, South Africa. Science. 2011;334(6053):219-222. http://dx.doi.org/10.1126/science.1211535 [ Links ]
3. Dayet L, Texier PJ, Daniel F, Porraz G. Ochre resources from the Middle Stone Age sequence of Diepkloof Rock Shelter, Western Cape, South Africa. J Archaeol Sci. 2013;40(9):3492-3505. http://dx.doi.org/10.1016/j.jas.2013.01.025 [ Links ]
4. Wadley L. Cemented ash as a receptacle or work surface for ochre powder production at Sibudu, South Africa, 58,000 years ago. J Archaeol Sci. 2010;37(10):2397-2406. http://dx.doi.org/10.1016/j.jas.2010.04.012 [ Links ]
5. Dlova NC, Nevondo FT, Mwangi EM, Summers B, Tsoka-Gwegweni J, Martincigh BS, et al. Chemical analysis and in vitro UV-protection characteristics of clays traditionally used for sun protection in South Africa. Photodermatol Photoimmunol Photomed. 2013;29(3):164-169. http://dx.doi.org/10.1111/phpp.12042 [ Links ]
6. Mpako MP Matike EM, Ekosse GI, Ngole VE. Ceremonial usage of clays for body painting according to traditional Xhosa culture. Indilinga. 2011;10(2):235-244. [ Links ]
7. López-Galindo A, Viseras C, Cerezo P. Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Appl Clay Sci. 2007;36(1-3):51-63. http://dx.doi.org/10.1016/j.clay.2006.06.016 [ Links ]
8. Matike DME, Ekosse GI, Ngole VM. Indigenous knowledge applied to the use of clays for cosmetic purposes in Africa: An overview. Indilinga. 2010;9(9):2138-2150. [ Links ]
9. Matike DME, Ekosse GIE, Ngole VM. Physico-chemical properties of clayey soils used traditionally for cosmetics in Eastern Cape, South Africa. Int J Phys Sci. 2011;6(33):7557-7566. http://dx.doi.org/10.5897/IJPS11.1249 [ Links ]
10. Mpuchane SF, Ekosse GIE, Gashe BA, Morobe I, Coetzee SH. Microbiological characterisation of southern African medicinal and cosmetic clays. Int J Environ Health Res. 2010;20(1):27-41. http://dx.doi.org/10.1080/09603120903254025 [ Links ]
11. Jumbam ND. Mineralogical and geochemical analyses of the healing elements in clayey soils from Isinuka traditional spa in Port St Johns, South Africa. Trans Roy Soc S Afr. 2014;68(1):25-31. http://dx.doi.org/10.1080/0035919X.2012.756075 [ Links ]
12. Mattioli M, Giardini L, Roselli C, Desideri D. Mineralogical characterization of commercial clays used in cosmetics and possible risk for health. Appl Clay Sci. 2016;119:449-454. http://dx.doi.org/10.1016/j.clay.2015.10.023 [ Links ]
13. Kahr G, Madsen FT. Determination of the cation exchange capacity and the surface area of bentonite, illite and kaolinite by methylene blue adsorption. Appl Clay Sci. 1995;9(5):327-336. http://dx.doi.org/10.1016/0169-1317(94)00028-O [ Links ]
14. Khiari I, Mefteh S, Sánchez-Espejo R, Cerezo P Aguzzi C, López-Galindo A, et al. Study of traditional Tunisian medina clays used in therapeutic and cosmetic mud-packs. Appl Clay Sci. 2014;101:141-148. http://dx.doi.org/10.1016/j.clay.2014.07.029 [ Links ]
15. Brigatti MF. Relationships between composition and structure in Fe-rich smectites. Clays Clay Miner. 1983;18(2):177-186. http://dx.doi.org/10.1180/claymin.1983.018.2.06 [ Links ]
16. Lambers H, Piessens S, Bloem A, Pronk H, Finkel P Natural skin surface pH is on average below 5, which is beneficial for its resident flora. Int J Cosmet Sci. 2006;28(5):359-370. http://dx.doi.org/10.1111/j.1467-2494.2006.00344.x [ Links ]
17. Kristl M, Mursec M, Sustar V Kristl J. Application of thermogravimetric analysis for the evaluation of organic and inorganic carbon contents in agricultural soils. J Therm Anal Calorim. 2016;123(3):2139-2147. http://dx.doi.org/10.1007/s10973-015-4844-1 [ Links ]
18. Smidt E, Lechner P. Study on the degradation and stabilization of organic matter in waste by means of thermal analyses. Thermochim Acta. 2005;438(1-2):22-28. http://dx.doi.org/10.1016/j.tca.2005.08.013 [ Links ]
19. Song S, Jia F, Peng C. Study on decomposition of goethite/siderite in thermal modification through XRD, SEM and TGA measurements. Surf Rev Lett. 2014;21(1), Art. #1450019, 6 pages. http://dx.doi.org/10.1142/S0218625X1450019X [ Links ]
20. Fadil-Djenabou S, Ndjigui P-D, Mbey JA. Mineralogical and physicochemical characterization of Ngaye alluvial clays (Northern Cameroon) and assessment of its suitability in ceramic production. J Asian Ceram Soc. 2015;3(1):50-58. http://dx.doi.org/10.1016/j.jascer.2014.10.008 [ Links ]
21. Akyuz S, Akyuz T, Basaran S, Bolcal C, Gulec A. Analysis of ancient potteries using FT-IR, micro-Raman and EDXRF spectrometry. Vibrat Spec. 2008;48(2):276-280. http://dx.doi.org/10.1016/j.vibspec.2008.02.011 [ Links ]
22. Russell J, Fraser A, Wilson M. Clay mineralogy: Spectroscopic and chemical determinative methods. London: Chapman Hall; 1994. [ Links ]
23. Boulingui JE, Nkoumbou C, Njoya D, Thomas F, Yvon J. Characterization of clays from Mezafe and Mengono (Ne-Libreville, Gabon) for potential uses in fired products. Appl Clay Sci. 2015;115:132-144. http://dx.doi.org/10.1016/j.clay.2015.07.029 [ Links ]
 Correspondence:
Correspondence:
Walter Focke
Walter.Focke@up.ac.za
Received: 06 Aug. 2016
Revised: 06 Oct. 2016
Accepted: 08 Oct. 2016
FUNDING: Tshwane University of Technology














