Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.113 no.3-4 Pretoria mar./abr. 2017
http://dx.doi.org/10.17159/sajs.2017/20160251
RESEARCH ARTICLE
Co-infection with Schistosoma haematobium and soil-transmitted helminths in rural South Africa
Mari MolvikI, II; Elin HeilandI; Siphosenkosi G. ZuluIII; Elisabeth KleppaII, IV; Kristine LilleboII; Svein G. GundersenV, VI; Jane D. KvalsvigIII; Myra TaylorIII; Eyrun F. KjetlandII, III; Birgitte J. VennervaldI
IParasitology and Aquatic Diseases, University of Copenhagen, Copenhagen, Denmark
IINorwegian Centre for Imported and Tropical Diseases, Department of Infectious Diseases Ullevaal, Oslo University Hospital, Oslo, Norway
IIIDiscipline of Public Health Medicine, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IVFaculty of Medicine, University of Oslo, Oslo, Norway
VResearch Department, Sorlandet Hospital, Kristiansand, Norway
VIInstitute for Global Development and Planning, University of Agder, Kristiansand, Norway
ABSTRACT
Schistosomiasis and soil-transmitted helminthiasis are among the most prevalent neglected tropical diseases and may lead to severe consequences. We assessed the extent of co-infection between Schistosoma haematobium and the soil-transmitted helminths (STHs) Ascaris lumbricoides and Trichuris trichiura in schoolgirls in the rural areas of KwaZulu-Natal, South Africa. We also explored if S. haematobium can serve as a predictor for soil-transmitted helminths in this area. From 15 selected schools, 726 primary schoolgirls aged 10-12 years provided both urine and stool samples. The samples were examined for the presence of eggs using the urine sedimentation technique for S. haematobium and the Kato Katz technique for STHs. Pearson's chi-square test was used to calculate the association and Spearman's rank correlation was used for the correlation analysis. There was a highly significant correlation between S. haematobium and STHs at a school level (Spearman's correlation coefficient =0.93; p<0.001). The prevalences were found to be 36.9% and 38.8% for S. haematobium and STHs, respectively. A significant association was found between S. haematobium and STHs (odds ratio =2.05; confidence interval =1.58-2.93; p<0.001). Indirect indicators of urogenital schistosomiasis (e.g. water contact and haematuria) were significantly associated with A. lumbricoides and T. trichiura infection. We have demonstrated a highly significant correlation and overall association between urogenital schistosomiasis and A. lumbricoides and T. trichiura. We cautiously suggest that all S. haematobium endemic areas should be treated for STH infections.
SIGNIFICANCE:
• The prevalences of urogenital schistosomiasis and soil-transmitted helminth infections were highly significantly correlated.
• More than half (60%) of the investigated schools are in need of annual treatment for S. haematobium infection.
• Almost half of the infected schoolgirls had a heavy intensity of S. haematobium infection.
• Nearly all the schools investigated require treatment for soil-transmitted helminthiasis once or even twice per year.
• This study can contribute to the epidemiological planning process of the deworming programme.
Keywords: urogenital schistosomiasis; KwaZulu-Natal; Ascaris lumbricoides; Trichuris trichiura; schoolchildren
Introduction
Schistosomiasis and soil-transmitted helminthiasis represent the most common neglected tropical diseases and may cause acute and chronic illness.1 The most prevalent schistosome species in South Africa is Schistosoma haematobium, which causes urogenital schistosomiasis.2 It is estimated that 5.2 million people in South Africa are infected with S. haematobium.3The total number of people infected with soil-transmitted helminths (STHs) in South Africa is unknown, but according to the World Health Organization (WHO) approximately 3.2 million children require treatment in South Africa.4 These helminth infections may have serious consequences in children, which could lead to decreased growth and stunting, decreased cognitive development and school performance and increased school absenteeism.5,6 Furthermore, infection with STHs is associated with anaemia and malnutrition5,7 and urogenital schistosomiasis may also lead to anaemia, dysuria, haematuria, infertility and, in some instances, bladder cancer8 and may increase the risk of HIV infection in women6,9,10. The diseases may be controlled by periodic treatment with so-called preventive chemotherapy.11
Studies have demonstrated that deworming programmes significantly improve learning, growth and school attendance among schoolchildren1,12 and mass treatment programmes integrated with other health services have been found to be the most cost-effective approach to combat the helminth infections11.
Previous studies have shown that areas endemic for schistosomiasis are often also endemic for soil-transmitted helminthiasis.13 S. haematobium is transmitted to humans through infested fresh water and STHs are transmitted from contaminated soil in areas that lack adequate sanitation, as eggs are excreted in human urine and faeces, respectively.1 Improvements in water, sanitation and hygiene are important to prevent all helminth infections14 and in South Africa, sanitation programmes have been rolled out but have not yet reached all areas15. In KwaZulu-Natal, 14% of households have never had access to potable water.15 Furthermore, even people who have access to taps providing potable water must often use the river because of irregular water supply and/or long queues for drinking water at the communal taps.16
Soil-transmitted helminthiasis is most commonly diagnosed by stool collection and microscopy, requiring willing patients and trained laboratory staff.1 S. haematobium prevalence may be assessed in children by detecting macro- or microscopic haematuria through a questionnaire or by urine dipstick. The latter technique has demonstrated to be quick, easy to perform, and highly sensitive and specific in children17-19, although final diagnosis must be confirmed by microscopy or more advanced methods20.
The Integrated School Health Programme's policy in South Africa recommends treatment for all children in areas endemic for schistosomiasis and soil-transmitted helminthiasis, but this recommendation has still to be implemented21; and these areas have still to be identified22. In order to make decisions on where to treat, countrywide mapping of helminth infections is needed.
In this study, we aimed to explore the burden of water- and soil-transmitted helminth infections in a middle-income country like South Africa and to determine if infection with S. haematobium could serve as a predictor for STHs.
Methods
Study area
The study was conducted between September 2009 and November 2010 in Ugu District in the KwaZulu-Natal Province of South Africa (Figure 1). The study site is endemic for S. haematobium and STHs.2326 The total population of the District is 709 918; over 50% are female and 33% are under the age of 14.27
Study population
Of the 309 primary schools in the area, 18 schools in rural Ugu were randomly selected for inclusion. All girls between the ages of 10 and 12 were invited to participate, and a total of 1057 girls participated in the study. In three of the schools, fewer than 10 girls provided urine and stool samples, and the schools were therefore excluded from the calculations. From the 15 included schools, 726 individuals submitted both stool and urine samples. Girls who did not provide at least one urine and one stool sample were excluded. No mass treatment had been conducted in this cohort of children before the data collection.
Parasitological examination
Urine specimens were collected for three consecutive days between 10:00 and 14:00. Samples were transported to the laboratory in dark cooler boxes. After arrival, 1 mL of 2% tincture of merthiolate in 5% formalin solution was added to each 10-mL urine sample for preservation. Two samples from each participant (labelled A and B) were registered daily (six samples in total per girl). Within the same week, the samples were centrifuged and microscopically investigated for S. haematobium eggs. The egg counts were based on the 10 mL of urine and each slide was read independently by two technicians. One stool sample was provided by each girl and the stool samples were kept at 4 °C until they were processed within 24 h after collection. The Kato-Katz technique was used for diagnosing STHs.28,29 The stool sample was divided into two (A and B) and each sample of 41.9 mg was prepared on a slide and investigated by microscope by two laboratory technicians. Because of the duration between sampling and preservation, it was not possible to identify hookworm eggs in the stool samples.
Ethical considerations and treatment
Three ethics committees granted permission to undertake this study. The Biomedical Research Ethics Committee of the University of KwaZulu-Natal (reference BF029/07), the KwaZulu-Natal Department of Health (Pietermaritzburg, 3 February 2009, reference HRKM010-08) and the Regional Norwegian Ethics Committee gave ethical clearance (reference 469-07066a1.2007.535). Further, both the Departments of Health and Basic Education in Ugu District gave permission. Prior to the study, information meetings were held for the parents, principals, school governing bodies and teachers. Assent was given by each girl, and informed consent forms were signed by parents/guardians. Treatment with a single dose of praziquantel (40 mg/kg) for schistosomiasis was offered to all by the Department of Health and information about possible side effects was given. Treatment for STH was offered at the local clinics.
Data analysis
If at least one egg was found in the urine or stool sample, the person was registered as positive. Schools were categorised into risk groups according to WHO guidelines.1 Intensity of S. haematobium was expressed as eggs per 10 mL of urine, based on the maximum egg count of the three urine samples provided. 'Light infection' is defined as 1-50 eggs per 10 mL of urine and 'heavy infection' as more than 50 eggs per 10 mL of urine.1 The median egg count of the study population was calculated for S. haematobium. The egg counts for the STHs were stopped at 500 eggs per slide and so it was not possible to calculate median or intensity of egg excretion in the high excretors and therefore for the STHs.
Interviews
Research assistants interviewed each girl in the local language (isiZulu) using a pre-designed questionnaire, the results of which are described elsewhere.25 Interviews included questions on water contact and urogenital symptoms such as pain when urinating and having observed red urine.
Statistical analysis
Data were entered into MS Excel spreadsheets and exported to SPSS Statistics (IBM SPSS Version 22). When the data did not have a normal distribution, a non-parametric statistical test was used. A p-value lower than 0.05 was considered statistically significant. Pearson's chi-square test and odds ratios were used to calculate the association between the helminth species. Pearson's chi-square and Mann-Whitney U tests were used for comparison of age or household size and categorical data of included and excluded cases. The helminth prevalences found in each school were compared using Spearman's rank correlation.
Results
Table 1 shows the descriptive characteristics of the study population. Over a third (37%; 268/726) of the girls was infected with schistosomiasis and the prevalence of STHs (either A. lumbricoides or T. trichiura) was 38.8% (282/726). The egg counts for S. haematobium ranged from 1 to 624 eggs per 10 mL (with a median of 21). Heavy intensity of S. haematobium was found in 47.8% (128/268) of the girls positive for this infection. Infection with S. haematobium was not only significantly associated with STH infection (either ascariasis or trichuriasis) (odds ratio =2.15; 95% confidence interval =1.58-2.93; p<0.001), but also with each of the species (Table 1). Red urine, dysuria, washing blankets in the river and swimming in the river were associated with S. haematobium (data not shown). These variables were also investigated as a predictor for STHs and found to be significantly associated (Table 1). Age did not influence any of the associations. The included (n=726) and excluded (n=297) cases had similar exposures to risk water and similar family structures as those shown in Table 2.
School-level analysis
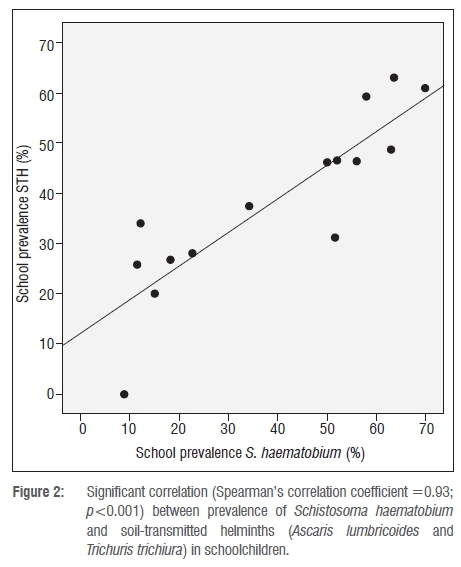
The prevalence of S. haematobium found in each investigated school ranged between 8.8% and 70.0% (median 50.0%) and the prevalence of STHs in the schools ranged between 0 and 63.0% (median 37.5%).
Figure 2 shows the correlation between S. haematobium and STHs based on school prevalences. The correlation coefficient was found to be 0.93 (p<0.001). Of the investigated schools in Ugu District, 60% (9/15) were found to have a prevalence of urogenital schistosomiasis above 50% and the same applied for 20% (3/15) of the schools for soil-transmitted helminthiasis. Just above 70% (11/15) of the schools had a prevalence of STH infection between 20% and 50%.
Discussion
At a school level, the prevalence of urogenital schistosomiasis and STH infections were highly significantly correlated. Overall prevalence of infection with S. haematobium and STHs was significantly associated. In addition, indirect indicators of urogenital schistosomiasis were significantly associated with A. lumbricoides and T. trichiura infection. Based on the WHO recommendations, this study shows that 60% of the investigated schools are in need of annual treatment for S. haematobium because they show a prevalence in excess of 50%.1 For soil-transmitted helminthiasis, the recommendation is treatment once per year if the school is classified as low risk (a prevalence of 20-50%) and twice a year if classified as high risk (a prevalence greater than 50%).1 Nearly all the schools investigated require treatment for soil-transmitted helminthiasis once or even twice per year. Almost half of the infected school girls showed a heavy intensity of S. haematobium infection.
The observed associations are consistent with previous studies13,30 and contribute to the evidence showing that co-infection occurs in areas endemic for S. haematobium. This present study shows that the prevalence of S. haematobium, A. lumbricoides and T. trichiura was within the same range as reported previously in the area.23,24,31 Our study only included participants who provided both urine and stool samples, but there was no difference between the included and excluded girls in terms of household size, water contact and age.
Intensity of infection has been shown to be positively correlated with morbidity, and measuring intensity allows quantification of the proportion of individuals with high intensity of infection who may suffer serious consequences.1 However, previous studies have shown that the majority of infected children harbour light infections and relatively few people suffer severe morbidity.32 The WHO recommends calculating the intensity of infection at community level to estimate the morbidity rate in the same area.1 This calculation can be done by estimation of the mean intensity, or more comprehensively by classes of intensity,33 as done in this paper. Our results showed that almost 50% of the girls had a heavy intensity S. haematobium infection (egg counts above 500). We did not investigate the intensity of STH infection. However, persons with a heavy intensity of one helminth species have been found to be more likely to harbour a heavy intensity of the other helminth species.34 It has been hypothesised that overdispersion may be explained by a predisposition of some individuals to helminth infections, although the underlying reasons remain poorly understood.32,35 Possible factors are differences in susceptibility and immunity against infection.32 Another possibility is that because of poor sanitation in general, infestation in only a few latrines can lead to contamination of the water and soil by these helminths, as there is often limited access to clean water. Jinabhai et al.31 reported this pattern of intensity for S. haematobium and T. trichiura in southern KwaZulu-Natal.
Saathoff et al.36 found that amongst 10- to 12-year-old pupils, the boys had about 10% lower prevalence of S. haematobium than the girls and the prevalence of A. lumbricoides, but not T. trichiura, also differed significantly between boys and girls. On the other hand, the opposite tendency was found among schoolchildren in Zanzibar: boys were slightly more affected by S. haematobium, both regarding prevalence and intensity, and significantly more heavily infected with T. trichiura than the girls.30 Hence, the results from this study on schoolgirls cannot be extrapolated to different populations because local gender-related habits can differ.
Low socio-economic status is intimately related to the presence of helminth infections.37 Members of poorer families are found to be more frequently infected with A. lumbricoides and T. trichiura and more likely to have both schistosomiasis and soil-transmitted helminthiasis.37 Poor economic growth has been shown to maintain high prevalences of STH infections.38 However, the connection between poverty and STHs is complex, because STH prevalence in turn is a contributing factor to poor economic growth and the anaemia caused by soil-transmitted helminthiasis is associated with reduced work output.38 It has been shown that children from poorer families are more likely to drop out of school.39 There is an 85% school enrolment in South Africa.40 This study only included enrolled schoolgirls and those present at the days of sample collections. Schoolgirls may be absent as a result of sickness caused by urogenital schistosomiasis or soil-transmitted helminthiasis or other health issues, or may stay home for family reasons. Hence, these prevalences and intensities of infection may be underestimated.
Different activities involving water contact are highly associated with S. haematobium infection,41 and washing hands with soap after defecation and before eating is protective against STHs (except hookworm) by limiting the faeco-oral route of infection.37 Access to clean fresh water has been associated with a significant reduction in STH infection.30 Furthermore, not using a tap as the source for drinking water is positively associated with urogenital schistosomiasis.37 The Ugu District Municipality Integrated Development Plan confirmed that constructions of sanitary facilities and water sources have taken place in the area in recent years, but improvements have not reached all areas.27 The prevalence of the helminth infections in the schools varied from 8.8% to 70.0% for S. haematobium and 0 to 63% for STHs. In order to explain the differences between schools, more background information about the socioeconomic status of the participants and their community, their access to water and sanitation facilities and local risk factors is needed.14,37,39,41
Because of both time and resource constraints, only one faecal sample was collected from each participant in this study, although three faecal samples on three consecutive days markedly raises the sensitivity.29,42 Because egg excretion has a day-to-day variation, there may have been an under- or overestimation of both prevalence and intensity of infection.42 Hookworm was not investigated, because of logistical constraints, although it would have been desirable as part of the total examination for STHs.
The difference in transmission patterns of S. haematobium and STHs is essential when it comes to prevention and control. Although the helminth infections have many of the same risk factors, the transmission cycle of S. haematobium requires fresh water with snails as the intermediate host.2 The distribution and transmission of schistosomiasis is therefore known to be highly focal - depending on different factors such as presence of water and local environmental conditions - whereas STHs are more widely distributed.1 In the southern part of South Africa, schistosomiasis is not found more than 300 m above sea level, and often not in urban areas. STHs, however, are found in the Cape Peninsula and in urban slums.43 However, because of the great burden of helminth infections, WHO recommends coordinated interventions to secure a joint and synergic control of the diseases.11 South Africa's first school-based helminth control programme in KwaZulu-Natal, from 1997 to 200023, showed a decrease in prevalence for all the helminths investigated (S. haematobium, A. lumbricoides, T. trichiura and hookworms) after treating the schoolchildren with chemotherapy.44 Despite the successful results, the programme was discontinued and it has been suggested that this discontinuation was a result of a shift in funding priorities consequent to the massive burden of HIV and tuberculosis.43 Newer studies have shown that patients with urogenital schistosomiasis may be at higher risk for HIV acquisition, which should be considered of importance for control of the helminth infection.45 The South African health authorities have recently launched an initiative to combat helminth infections in endemic areas and this study can contribute to the epidemiological planning process of the deworming programme.
Considering the significant correlation and association between urogenital schistosomiasis and STH infection shown in this study, using S. haematobium as a predictor for STHs, at least in rural parts of districts where urogenital schistosomiasis is endemic, may facilitate the processes of planning interventions.1 Stool sampling and analyses are laborious and the faecal collection may be challenging as a result of age and cultural differences of the study participants.1 Despite the fact that the investigated helminth infections have different transmission patterns and require different treatment interventions, this study shows that simple urine analyses, or possibly even water contact information, may indicate which persons and rural schools need intervention for both urogenital schistosomiasis and soil-transmitted helminthiasis. However, because of the focal nature of schistosomiasis transmission, ultimately, both stool and urine prevalence surveys should be done countrywide with a view of mapping disease distribution.
Acknowledgements
We warmly thank the schoolgirls who participated in this study and their carers. We are grateful for the help from the BRIGHT clinical staff for sample collection and laboratory work - this study could not have been done without their hard work and effort. We thank R.F. Manyaira for able assistance, S.D. Holmen for SPSS support and P.D. Ndhlovu, H.N. Galappaththi-Arachchige and I. Lindstroem for valuable feedback. We also thank the Norwegian Centre for Imported and Tropical Diseases, Oslo University Hospital Ullevaal (VIRUUS), Shipowner Tom Wilhelmsen Foundation, S.G. Sonneland Foundation and General Travel Scholarships for funding. The funders had no role in data collection, study design and analysis, decision to publish, or preparation of the manuscript.
Authors' contributions
M.M. and E.H. worked on the original report, analysed the data and conceptualised the paper together with E.F.K. and B.J.V.; S.G.Z. and E.F.K. contributed to data collection. All authors contributed to the editing of the manuscript.
References
1. World Health Organization. Helminth control in school-age children. A guide for managers of control programmes. Geneva: Preventive Chemotherapy and Transmission Control (PCT), Department of Control of Neglected Tropical Diseases (NTD); 2011. Available from: http://apps.who.int/iris/bitstream/10665/44671/1/9789241548267_eng.pdf [ Links ]
2. Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368(9541):1106-1118. http://dx.doi.org/10.1016/S0140-6736(06)69440-3 [ Links ]
3. World Health Organization. Neglected tropical diseases: PCT databank: Schistosomiasis [database on the Internet]. c2014 [cited 2014 Nov 03]. [ Links ] Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/sch/en/
4. World Health Organization. Neglected tropical diseases: PCT databank: Soil- transmitted helmithiases [database on the Internet]. [ Links ] c2014 [cited 2014 Nov 11]. Available from: http://www.who.int/neglected_diseases/preventive_chemotherapy/sth/en/
5. Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121:23-38. http://dx.doi.org/10.1017/S0031182000006491 [ Links ]
6. King CH, Dangerfield-Cha M. The unacknowledged impact of chronic schistosomiasis. Chronic Illn. 2008;4(1):65-79. http://dx.doi.org/10.1177/1742395307084407 [ Links ]
7. Ezeamama AE, Friedman JF, Olveda RM, Acosta LP Kurtis JD, Mor V et al. Functional significance of low-intensity polyparasite helminth infections in anemia. J Infect Dis. 2005;192(12):2160-2170. http://dx.doi.org/10.1086/498219 [ Links ]
8. Vennervald BJ, Polman K. Helminths and malignancy. Parasite Immunol. 2009;31(11):686-696. http://dx.doi.org/10.1111/j.1365-3024.2009.01163.x [ Links ]
9. Kjetland EF, Ndhlovu PD, Gomo E, Mduluza T, Midzi N, Gwanzura L, et al. Association between genital schistosomiasis and HIV in rural Zimbabwean women. AIDS. 2006;20(4):593-600. http://dx.doi.org/10.1097/01.aids.0000210614.45212.0a [ Links ]
10. Downs JA, Mguta C, Kaatano GM, Mitchell KB, Bang H, Simplice H, et al. Urogenital schistosomiasis in women of reproductive age in Tanzania's Lake Victoria region. Am J Trop Med Hyg. 2011;84(3):364-369. http://dx.doi.org/10.4269/ajtmh.2011.10-0585 [ Links ]
11. World Health Organization. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: A manual for health professionals and programme managers. Geneva: Preventive Chemotherapy and Transmission Control (PCT), Department of Control of Neglected Tropical Diseases (NTD); 2006. Available from: http://apps.who.int/iris/bitstream/10665/43545/1/9241547103_eng.pdf [ Links ]
12. Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: Systematic review of randomised trials. BMJ. 2000;320(7251):1697-1701. http://dx.doi.org/10.1136/bmj.320.7251.1697 [ Links ]
13. Yajima A, Gabrielli AF, Montresor A, Engels D. Moderate and high endemicity of schistosomiasis is a predictor of the endemicity of soil-transmitted helminthiasis: A systematic review. Trans R Soc Trop Med Hyg. 2011;105(2):68-73. http://dx.doi.org/10.1016/j.trstmh.2010.11.003 [ Links ]
14. Campbell SJ, Savage GB, Gray DJ, Atkinson JA, Soares Magalhaes RJ, Nery SV, et al. Water, sanitation, and hygiene (WASH): A critical component for sustainable soil-transmitted helminth and schistosomiasis control. PLoS Negl Trop Dis. 2014;8(4), Art. #e2651, 5 pages. http://dx.doi.org/10.1371/journal.pntd.0002651 [ Links ]
15. South African Human Rights Commission (SAHRC). Report on the right to access sufficient water and decent sanitation in South Africa. Johannesburg: SAHRC; 2014. Available from: https://www.sahrc.org.za/home/21/files/FINAL%204th%20Proof%204%20March%20-%20Water%20%20Sanitation%20low%20res%20(2).pdf [ Links ]
16. Ugu District Municipality. Water and sanitation services: Ugu District [homepage on the Internet]. c2014 [cited 2014 Nov 04]. [ Links ] Available from: http://www.ugu.gov.za/Water_Services.aspx.
17. Savioli L, Dixon H, Kisumku UM, Mott KE. Control of morbidity due to Schistosoma haematobium on Pemba Island; selective population chemotherapy of schoolchildren with haematuria to identify high-risk localities. Trans R Soc Trop Med Hyg. 1989;83(6):805-810. http://dx.doi.org/10.1016/0035-9203(89)90336-2 [ Links ]
18. Houmsou RS, Kela SL, Suleiman MM. Performance of microhaematuria and proteinuria as measured by urine reagent strips in estimating intensity and prevalence of Schistosoma haematobium infection in Nigeria. Asian Pac J Trop Med. 2011;4(12):997-1000. http://dx.doi.org/10.1016/S1995-7645(11)60233-2 [ Links ]
19. Brooker S, Kabatereine NB, Gyapong JO, Stothard JR, Utzinger J. Rapid mapping of schistosomiasis and other neglected tropical diseases in the context of integrated control programmes in Africa. Parasitology. 2009;136(13):1707-1718. http://dx.doi.org/10.1017/S0031182009005940 [ Links ]
20. Gundersen SG, Kjetland EF, Poggensee G, Helling-Giese G, Richter J, Chitsulo L, et al. Urine reagent strips for diagnosis of schistosomiasis haematobium in women of fertile age. Acta Trop. 1996;62(4):281-287. http://dx.doi.org/10.1016/S0001-706X(96)00029-0 [ Links ]
21. Department of Basic Education (DBE) and Department of Health (DoH). Integrated school health policy 2012. Pretoria: DBE/DoH; 2012. Available from: http://www.education.gov.za/Portals/0/Documents/Policies/INTEGRATED%20SCHOOL%20HEALTH%20POLICYB-W_1.pdf?ver=2014-06-14-172322-000 [ Links ]
22. Mkhize-Kwitshana ZL, Mabaso MLH. The neglected triple disease burden and interaction of helminths, HIV and tuberculosis: An opportunity for integrated action in South Africa. S Afr Med J. 2014;104(4):258-259. http://dx.doi.org/10.7196/SAMJ.7947 [ Links ]
23. Appleton CC, Kvalsvig JD. A school-based helminth control programme successfully implemented in KwaZulu-Natal. South Afr J Epidemiol Infect. 2006;21(2):55-67. [ Links ]
24. Appleton CC, Maurihungirire M, Gouws E. The distribution of helminth infections along the coastal plain of Kwazulu-Natal province, South Africa. Ann Trop Med Parasitol. 1999;93(8):859-868. [ Links ]
25. Hegertun IEA, Sulheim Gundersen KM, Kleppa E, Zulu SG, Gundersen SG, Taylor M, et al. S. haematobium as a common cause of genital morbidity in girls: A cross-sectional study of children in South Africa. PLoS Negl Trop Dis. 2013;7(3), Art. #e2104, 8 pages. http://dx.doi.org/10.1371/journal.pntd.0002104 [ Links ]
26. Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. Epidemiology of helminth infections: Implications for parasite control programmes, a South African perspective. Public Health Nutr. 2001;4(6):1211-1219. http://dx.doi.org/10.1079/PHN2001180 [ Links ]
27. Ugu District Municipality. Ugu District Municipality integrated development plan review 2013/2014. Port Shepstone: Ugu District Municipality; 2013. [ Links ]
28. Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick- smear technique in schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397-400. [ Links ]
29. World Health Organization (WHO). Basic laboratory methods in medical parasitology. Geneva: WHO; 1991. Available from: http://apps.who.int/iris/bitstream/10665/40793/1/9241544104_%28part1%29.pdf [ Links ]
30. Stothard JR, French MD, Khamis IS, Basanez MG, Rollinson D. The epidemiology and control of urinary schistosomiasis and soil-transmitted helminthiasis in schoolchildren on Unguja Island, Zanzibar. Trans R Soc Trop Med Hyg. 2009;103(10):1031-1044. http://dx.doi.org/10.1016/j.trstmh.2009.03.024 [ Links ]
31. Jinabhai CC, Taylor M, Coutsoudis A, Coovadia HM, Tomkins AM, Sullivan KR. A health and nutritional profile of rural school children in KwaZulu-Natal, South Africa. Ann Trop Paediatr. 2001;21(1):50-58. http://dx.doi.org/10.1080/02724930020028920 [ Links ]
32. Hotez PJ, Bundy DAP Beegle K, Brooker S, Drake L, De Silva N, et al. Helminth infections: Soil-transmitted helminth infections and schistosomiasis. In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al, editors. Disease control priorities in developing countries. 2nd ed. Washington DC: World Bank; 2006. [ Links ]
33. Montresor A. Arithmetic or geometric means of eggs per gram are not appropriate indicators to estimate the impact of control measures in helminth infections. Trans R Soc Trop Med Hyg. 2007;101(8):773-776. http://dx.doi.org/10.1016/j.trstmh.2007.04.008 [ Links ]
34. Brooker S, Miguel EA, Moulin S, Luoba AI, Bundy DA, Kremer M. Epidemiology of single and multiple species of helminth infections among school children in Busia District, Kenya. East Afr Med J. 2000;77(3):157-161. http://dx.doi.org/10.4314/eamj.v77i3.46613 [ Links ]
35. Bundy DA, Cooper ES, Thompson DE, Didier JM, Anderson RM, Simmons I. Predisposition to Trichuris trichiura infection in humans. Epidemiol Infect. 1987;98(1):65-71. http://dx.doi.org/10.1017/S0950268800061719 [ Links ]
36. Saathoff E, Olsen A, Magnussen P Kvalsvig JD, Becker W, Appleton CC. Patterns of Schistosoma haematobium infection, impact of praziquantel treatment and re-infection after treatment in a cohort of schoolchildren from rural KwaZulu-Natal/South Africa. BMC Infect Dis. 2004;4:40. http://dx.doi.org/10.1186/1471-2334-4-40 [ Links ]
37. Balen J, Raso G, Li YS, Zhao ZY Yuan LP Williams GM, et al. Risk factors for helminth infections in a rural and a peri-urban setting of the Dongting Lake area, People's Republic of China. Int J Parasitol. 2011;41(11):1165-1173. http://dx.doi.org/10.1016/j.ijpara.2011.07.006 [ Links ]
38. De Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil- transmitted helminth infections: Updating the global picture. Trends Parasitol. 2003;19(12):547-551. http://dx.doi.org/10.1016/j.pt.2003.10.002 [ Links ]
39. Bock J. Evolutionary demography and intrahousehold time allocation: School attendance and child labor among the Okavango Delta Peoples of Botswana. Am J Hum Biol. 2002;14(2):206-221. http://dx.doi.org/10.1002/ajhb.10040 [ Links ]
40. The World Bank. Net enrolment rate, primary, both sexes (%) [homepage on the Internet]. [ Links ] c2012 [cited 2014 Dec 16]. Available from: http://data.worldbank.org/indicator/SE.PRM.NENR/countries/ZA-ZF-XT?display=graph
41. Rudge JW, Stothard JR, Basanez MG, Mgeni AF, Khamis IS, Khamis AN, et al. Micro-epidemiology of urinary schistosomiasis in Zanzibar: Local risk factors associated with distribution of infections among schoolchildren and relevance for control. Acta Trop. 2008;105(1):45-54. http://dx.doi.org/10.1016/j.actatropica.2007.09.006 [ Links ]
42. Knopp S, Mgeni AF, Khamis IS, Steinmann P Stothard JR, Rollinson D, et al. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: Effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2(11), Art. #e331, 8 pages. http://dx.doi.org/10.1371/journal.pntd.0000331 [ Links ]
43. Mkhize-Kwitshana ZL, Mabaso MH. Status of medical parasitology in South Africa: New challenges and missed opportunities. Trends Parasitol. 2012;28(6):217-219. http://dx.doi.org/10.1016/j.pt.2012.03.005 [ Links ]
44. Kvalsvig JD, Appleton CC, Archer C, Mthethwa P Memela C, Mpanza JT et al. The KwaZulu-Natal Parasite Control Programme 1998-2000. Durban: Child Development Programme, School of Psychology and School of Life and Environmental Sciences, UKZN; 2001. [ Links ]
45. Kjetland EF, Leutscher PD, Ndhlovu PD. A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58-65. http://dx.doi.org/10.1016/j.pt.2011.10.008 [ Links ]
 Correspondence:
Correspondence:
Mari Molvik
marimolvik@hotmail.com
Received: 25 Aug. 2016
Revised: 17 Oct. 2016
Accepted: 19 Oct. 2016
FUNDING: Norwegian Centre for Imported and Tropical Diseases, Oslo University Hospital Ullevaal (VIRUUS); S.G. S0nneland Foundation; Shipowner Tom Wilhelmsen Foundation














