Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.11-12 Pretoria Nov./Dec. 2016
http://dx.doi.org/10.17159/sajs.2016/20160202
RESEARCH LETTER
Cytotoxic activity of marine sponge extracts from the sub-Antarctic Islands and the Southern Ocean
Elisabeth K. OlsenI, II; Christopher K. de CerfI; Godwin A. DziwornuI; Eleonora PuccinelliIII, IV; Isabelle J. AnsorgeIV, V; Toufiek SamaaiV, VI; Laura M. K. DingleVII; Adrienne L. EdkinsVII; Suthananda N. SunasseeI, V, VIII
IDepartment of Chemistry, University of Cape Town, Cape Town, South Africa
IIMRC/NHLS Molecular Mycobacteriology Research Unit, University of Cape Town, Cape Town, South Africa
IIIDepartment of Zoology and Entomology, Rhodes University, Grahamstown, South Africa
IVDepartment of Oceanography, University of Cape Town, Cape Town, South Africa
VMarine Research (Ma-Re) Institute, University of Cape Town, Cape Town, South Africa
VIOceans and Coasts Research, Department of Environmental Affairs, Cape Town, South Africa
VIIBiomedical Biotechnology Research Unit, Department of Biochemistry and Microbiology, Rhodes University, Grahamstown, South Africa
VIIIMRC Drug Discovery and Development Research Unit, University of Cape Town, Cape Town, South Africa
ABSTRACT
Over the past 50 years, marine invertebrates, especially sponges, have proven to be a valuable source of new and/or bioactive natural products that have the potential to be further developed as lead compounds for pharmaceutical applications. Although marine benthic invertebrate communities occurring off the coast of South Africa have been explored for their biomedicinal potential, the natural product investigation of marine sponges from the sub-Antarctic Islands in the Southern Ocean for the presence of bioactive secondary metabolites has been relatively unexplored thus far. We report here the results for the biological screening of both aqueous and organic extracts prepared from nine specimens of eight species of marine sponges, collected from around Marion Island and the Prince Edward Islands in the Southern Ocean, for their cytotoxic activity against three cancer cell lines. The results obtained through this multidisciplinary collaborative research effort by exclusively South African institutions has provided an exciting opportunity to discover cytotoxic compounds from sub-Antarctic sponges, whilst contributing to our understanding of the biodiversity and geographic distributions of these cold-water invertebrates. Therefore, we acknowledge here the various contributions of the diverse scientific disciplines that played a pivotal role in providing the necessary platform for the future natural products chemistry investigation of these marine sponges from the sub-Antarctic Islands and the Southern Ocean.
SIGNIFICANCE:
• This study will contribute to understanding the biodiversity and geographic distributions of sponges in the Southern Ocean.
• This multidisciplinary project has enabled the investigation of marine sponges for the presence of cytotoxic compounds.
• Further investigation will lead to the isolation and identification of cytotoxic compounds present in the active sponge extracts.
Keywords: multidisciplinary; Prince Edward Islands; marine natural products; cancer cell lines; drug discovery
Introduction
Marine biodiscovery is the search for marine natural products with potential economic and societal benefits for use as agrochemicals, cosmetics, anti-fouling agents, nutraceuticals and pharmaceuticals.1,2 Generally, marine invertebrates utilise their secondary metabolites or natural products as a form of chemical defence against predators and as a competitive advantage in their perpetual battle for limited resources, such as nutrients and space.2,3 Over the past 50 years, marine invertebrates and their associated microorganisms have proved to be an important source of bioactive natural products for the development of new pharmaceuticals,4,5 especially anti-cancer agents, e.g. Adcetris®, Halaven® and Yondelis®, that were discovered by initially screening for cytotoxic compounds against cancer cell lines.6,7 Marine sponges (Phylum: Porifera) have arguably been one of the dominant sources of marine natural products that are utilised commercially for their beneficial pharmacological properties in biomedical research.6 There currently are seven FDA-approved marine natural products derived drugs, of which four are anti-cancer chemotherapeutic agents, and a further 13 marine-derived agents that are either in human clinical trials or in advanced pre-clinical status, including a synthetic derivative of the South African marine sponge natural product Hemiasterlin.6,7 The latest review by Newman and Cragg8 highlights the significant contribution of natural products research to the field of drug discovery, with an estimated 77% of all anti-cancer drugs approved between 1940 and 2014 being small molecule natural products and natural product-derived compounds.
Southern Africa's marine biota is home to a wealth of biodiversity along the approximately 3000-km coastline of South Africa and around the archipelagos of the Southern Ocean, including the Prince Edward Islands (PEIs) which consist of Marion Island and Prince Edward Island. The biomedicinal potential of southern African marine invertebrates was initially revealed through the marine biodiscovery efforts of Robert Pettit (Arizona State University) and Yoel Kashman (Tel Aviv University).9,10 The initial biological screening for cytotoxic compounds and subsequent natural products chemistry work by Pettit and his co-workers on their South African marine collections resulted in the isolation of two very important classes of bioactive compounds known as the cephalostatins and spongiostatins.9,10 The cephalostatins and spongiostatins were isolated through cytotoxic activity-directed purification of crude extracts and are well known to be some of the most cytotoxic secondary metabolites (about 1 nM activity) ever to be screened by the United States National Cancer Institute (NCI) against their 60 cancer cell line panel (NCI-60).9,10 Cephalostatin 1 and its closely related naturally occurring analogues
were isolated from aqueous and organic extracts of the South African marine tube worm Cephalodiscus gilchristi, while Spongiostatins 4 and 5 were isolated from the organic extract of the bright orange 'wall-sponge' Trachycladus spinispirulifer collected off the southern coasts of South Africa.9,10 The collaborative biodiscovery programme in Sodwana Bay between Kashman, the Oceanography Research Institute and the pharmaceutical company PharmaMar involved the primary biological screening of crude extracts for cytotoxic compounds against the P-388, A549, HT-29 and MEL-28 cancer cell lines. This screening resulted in the discovery of 33 new bioactive natural product compounds, mostly isolated from marine sponges, of which 9 compounds were patented for their anti-cancer properties.9,10 These compounds include two cytotoxic peptides - Hemiasterlin and Geodiamolide TA - isolated from the marine sponge Hemiasterella minor (Kirkpatrick); a synthetic analogue of Hemiasterlin was subsequently developed and has successfully entered phase one of human clinical trials as an anti-cancer drug.6,9
Between 1992 and 2012, the marine natural products research groups at Rhodes University, in collaboration with various other scientific research groups and institutes, led the search for, and provided important contributions to, the discovery of novel bioactive natural product compounds from southern African marine organisms.9-12 Davies-Coleman and colleagues13,14 have previously reported the screening of several extracts of southern African marine invertebrates for anti-oesophageal cancer activity, including five deep-water sponges collected from the PEIs that were tested against the WHCO1 cell line. The sponges were collected during the 2004 annual relief voyage by the SA Agulhas I.13To the best of our knowledge, this report was the first and only report of a multidisciplinary research consortium from exclusively South African institutions (Rhodes University, University of Cape Town and Council for Scientific and Industrial Research), searching for cytotoxic chemical compounds from marine sponges with potential anti-tumour properties from the Southern Ocean.13 The natural product investigation by Davies-Coleman et al.13 of the most promising extract was unfortunately hampered by the paucity of the sponge material collected (ca. 200 g), although the amount of crude organic extract derived was not given. As far as we know, a subsequent collection in 2005 has to date not yielded any new and/or bioactive marine natural product compounds.
The relatively unexplored natural product diversity and pharmaceutical potential of marine invertebrates from the sub-Antarctic Islands and Southern Ocean, therefore presents us with an exciting opportunity and we have thus initiated a new multidisciplinary effort by a concerted group of South African institutions to explore the cytotoxic potential of marine sponges from the sub-Antarctic Islands and the Southern Ocean. We report here the cytotoxic activity, against three different cancer cell lines, of both aqueous and organic crude extracts prepared from nine sponge specimens, collected from various depths around the PEIs in the Southern Ocean.
Materials and methods
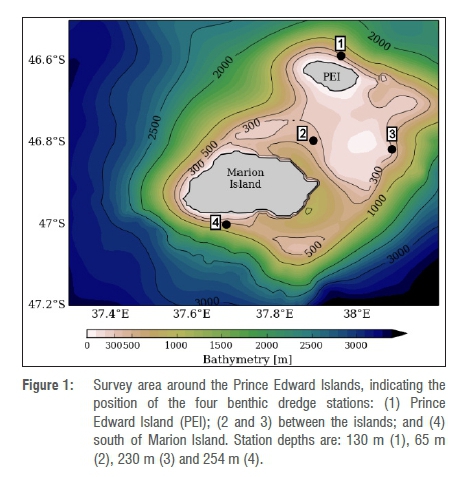
Researchers from the University of Cape Town, Rhodes University, the Council for Scientific and Industrial Research, the South African Environmental Observation Network and the Department of Environmental Affairs participated in the annual relief voyage to the PEIs on board the research vessel SA Agulhas II from 6 April to 16 May 2015. As part of the inter-island biodiversity survey between Prince Edward and Marion Islands (46.77°S, 37.85°E), benthic trawls were performed using a steel dredge (1.0 m x 0.3 m x 1.0 m) at various depths (60-254 m) along four pre-determined stations (Figure 1). At each station, the dredge was towed behind the vessel at 0.5 knots for approximately 15-20 min.
From the various benthic dredges, nine sponge specimens were collected, labelled and photographed and the relevant collection information, e.g. GPS coordinates and depths, was recorded. The sponge specimens were placed in sealed bags, immediately frozen on board the research vessel until the end of the journey and transferred after the expedition to a freezer in the Department of Chemistry at the University of Cape Town. Histological and genetic samples (~5-10 cm3) were taken from each specimen for identification and stored in 96% (v/v) ethanol. Vouchers of the sponges are housed in the collection of Dr Toufiek Samaai (Department of Environmental Affairs).
All sponge specimens were processed individually in the same manner. A frozen portion (up to 90% of the wet mass) of each sponge was cut into small pieces, frozen in liquid nitrogen and lyophilised until the material was dry. The mass of the dried sponges was recorded and the sponge materials were then subjected to solvent extraction. Both aqueous (100% deionised water) and organic (50:50 mixture of methanol and dichloromethane) crude extracts of the sponges were prepared by following, where possible, the protocol for marine specimens established by scientists at the Developmental Therapeutics Program of the NCI.15
The resulting 18 crude extracts, one organic (suffix and one aqueous (suffix '-2') for each sponge, were screened for their cytotoxic activity against the cervical (HeLa ATCC CCL-2), lung (A549 ATCC CCL-185) and breast (MCF7 ATCC HTB-22) cancer cell lines. Cells were cultured in Dulbecco's Modified Eagle's Medium supplemented with 10% (v/v) foetal bovine serum, 2 mM Glutamax and 100 U/mL penicillin/streptomycin/ amphotericin at 37 °C with 9% CO2. Sub-culturing was carried out by removing the media, washing with phosphate-buffered saline (10 mM Na2HPO4, 1.8 mM KH2PO4, 2.7 mM KCl, 140 mM NaCl, pH 7.4) and lifting the cells using a 1% (v/v) trypsin in 0.3% (w/v) ethylenediaminetetraacetic acid (EDTA) solution. Cells were confirmed to be mycoplasma free by microscopy after staining with Hoechst 33342. The chemosensitivity of the cell lines to the extracts was tested using the WST-1 assay. For each extract, 5 x 103 cells/well were seeded in a 96-well plate and allowed to sit overnight before being treated in triplicate with the extracts at a range of concentrations (0.1-100 μg/mL). The plates were incubated at 37 °C and 9% CO2 for 72 h. All the media were removed and 100 WST-1 reagent (0.5 mg/mL) was added to each well after the 72-h incubation. The plates were incubated at 37 °C in 9% CO2 and the absorbance at 450 nm read after 2 h, 4 h and 6 h. For all treatments the half maximum effective concentration (EC50) for data from the reading at 6 h was calculated by non-linear regression using GraphPad Prism version 4.0. Spectroscopic analyses of portions of the crude extracts were acquired using standard pulse sequences on a Bruker 600-MHz nuclear magnetic resonance (NMR) spectrometer, equipped with a 5-mm Prodigy cryoprobe.
Results
Taxonomic identification of the nine sponge specimens collected revealed eight sponge species (Table 1) as two sponge specimens (MAR15-005 and MAR15-012), collected from two distant geographical locations (Dredge Stations 3 and 1, respectively; Figure 1), were identified as the same Halichondria (Halichondria) cf. panicea sponge species. The preliminary biological screening results obtained for the crude extracts are summarised in Table 1 and indicate that only three (MAR15-001, MAR15-005, MAR15-012) of the nine sponge specimens exhibited cytotoxicity against the cancer cell lines tested.
The aqueous extract of the sponge Myxilla (Ectyomyxilla) kerguelensis (MAR15-001-2) displayed cytotoxic activity (EC50 11.7 μg/mL) against the HeLa cervical cancer cell line (Table 1). The organic extracts of the two sponges H. (H.) cf. panicea were similarly cytotoxic (MAR15-005-1; EC50 33.4 μg/mL and MAR15-012-1; EC50 35.2 μg/mL) against the A549 lung cancer cell line (Table 1). Interestingly, the aqueous extract of H. (H.) cf. panicea (MAR15-012-2) was similarly cytotoxic (EC50 ~16 μg/mL) to both the cervical (HeLa) and breast cancer (MCF7) cell lines, whilst the aqueous extract of the other H. (H.) cf. panicea specimen (MAR15-005-2) did not show any cytotoxic activity (Table 1) against any of the cancer cell lines. It is also interesting to note that a number of the samples, particularly the organic extracts, induced dose-dependent proliferation in the MCF7 and A549 cell lines (shown by P in Table 1).
Proton (1H) NMR spectra (Figure 2) were acquired for both organic and aqueous extracts of the two different sponge specimens H. (H.) cf. panicea to examine their secondary metabolite profile and assist us in the interpretation of the bioactivity results obtained. A comparative analysis of the 1H NMR spectra of the organic extracts for H. (H.) cf. panicea (MAR15-005-1 and MAR15-012-1) indicated a high degree of consistency in their spectroscopic profile (Figure 2a). A cursory examination of the 1H NMR spectra recorded for the MAR15-005-2 and MAR15-012-2 aqueous extracts revealed some degree of variation in their secondary metabolite spectroscopic profile (Figure 2b).
Conclusion
The results obtained from the biological screening of the nine sponge specimens (eight species) collected off the PEIs are exceptionally encouraging as the potential presence of cytotoxic secondary metabolites of three sponge specimens has been revealed. The similar cytotoxicity (EC50 ~34 μg/mL; Table 1) of the organic sponge extracts MAR15-005-1 and MAR15-012-1 (collected at two different locations; Figure 1) against the A549 lung cancer cell line, coupled with their significantly congruent 1H NMR profile (Figure 2a), is very interesting as it suggests the possible presence of the same active molecule(s) from allopatric specimens of H. (H.) cf. panicea, that is, different specimens of the same species from different locations. However, the aqueous extracts prepared from the same two H. (H.) cf. panicea sponge specimens exhibit different cytotoxic (Table 1) and spectroscopic profiles (Figure 2b), suggesting possible different polar secondary metabolite constituents in these extracts. This variation in the biological activity and secondary metabolite profile of different specimens of the same sponge species was also observed in three different H. (H.) cf. panicea specimens by Davies-Coleman and coworkers, which can be attributed to the production of different secondary metabolites as a result of different environmental pressures such as predation and competition with neighbouring species.13
Marine sponges belonging to the genus Halichondria are known as producers of a wide range of bioactive secondary metabolites, including the well-known polyether macrolide class of highly cytotoxic compounds, such as the marine natural product Halichondrin B that showed excellent initial cancer cell toxicity and subsequently led to the development of the approved anti-cancer drug Halaven®.6,7 Therefore, in our future work, we will aim to isolate and identify the cytotoxic compounds present in the two specimens of H. (H.) cf. panicea, as they could potentially be useful for further chemical and biological studies. The aqueous extract of the sponge Myxilla (Ectyomyxilla) kerguelensis (MAR15-001-2) displayed cytotoxic activity (EC50 11.7 μg/mL; Table 1) against the HeLa cervical cancer cell line. A literature search revealed that the Marion Island sponge Myxilla (Ectyomyxilla) kerguelensis has not been extensively studied for its natural products chemistry composition and therefore presents us with a unique opportunity to address this knowledge gap. We have also recently extended our primary biological screening of these sponge extracts to include antiplasmodial and anti-tubercular bioassays, in which none of the extracts exhibited any activity. The results obtained in this study will now guide the purification, isolation and characterisation of the bioactive molecules responsible for the observed cytotoxicity against our panel of three cancer cell lines. Additionally, to supplement the paucity in biomass for the sponges collected during the 2015 expedition, more samples were collected from the same location on the recent 2016 survey to Marion Island and will be investigated in the same manner.
We highlight and acknowledge the various contributions from diverse scientific disciplines that have played a pivotal role in providing the necessary platform to enable this newly established marine biodiscovery effort to explore the natural products chemistry and biomedicinal value of marine sponges from the PEIs and Southern Ocean. We hope that our future collections of sponges and other marine invertebrates from the Southern Ocean will lead to the isolation and identification of highly potent cytotoxic marine natural product compounds such as Halichondrin B6,7, Cephalostatin 1 and Spongiostatin 49,10, which have the potential to be further developed into anti-cancer agents. In our endeavour, we also hope to contribute to the understanding of the biodiversity and geographic distributions of these cold-water invertebrates.
Acknowledgements
Financial support from the Science Faculty Launching Grant and the South African Medical Research Council (MRC) Drug Discovery and Development Research Unit at the University of Cape Town (UCT) is gratefully acknowledged by S.N.S. E.K.O. was supported by a postdoctoral fellowship from the Molecular Mycobacteriology Research Unit, which constitutes the UCT node of the DST/NrF Centre of Excellence for Biomedical TB Research. C.K.C.D. and G.A.D. are grateful to UCT for financial support. Financial support from the MRC with funds from the National Treasury under its Economic Competitiveness and Support Package, the South African Research Chairs Initiative of the Department of Science and Technology (DST) and the National Research Foundation (NRF) of South Africa (grant no. 98566), the Cancer Association of South Africa (CANSA) and Rhodes University is gratefully acknowledged by A.L.E. L.M.K.D. was supported by an Innovations Postgraduate Fellowship from the NRF. We thank the DST, Department of Environmental Affairs - Antarctic Logistics and Support Division and the South African National Antarctic Programme (SANAP) for their financial and logistical support. We also thank Captains Gavin Syndercombe and Knowledge Bengu, and officers and crew of the research and supply vessel SA Agulhas II for their endless support. S.N.S., I.A. and E.P. thank Dr Charles von der Meden for the considerable help in samples collection. The views expressed are those of the authors and should not be attributed to the NRF, MRC, CANSA, Rhodes University or UCT.
Authors' contributions
E.K.O. was responsible for acquiring spectroscopic data on the sponge extracts; C.K.D.C. and G.A.D. were responsible for the preparation of the crude aqueous and organic sponge extracts; E.P. collected the sponge specimens; I.A. facilitated the annual relief voyage to Marion Island on board SA Agulhas II; T.S. identified the sponges collected from the 2015 survey; L.M.K.D. was responsible for designing and conducting the anti-tumour activity assays; A.L.E. was responsible for designing the anti-tumour activity assays and for analysis and interpretation of the data; S.N.S. was the overall project leader, oversaw the multidisciplinary process as a whole, especially the chemistry component of this project, and was responsible for conducting some of the experiments. S.N.S. wrote and edited the majority of the text and all authors provided input into the writing of the manuscript.
References
1. Fusetani N. Biotechnological potential of marine natural products. Pure Appl Chem. 2010;82(1):17-26. http://dx.doi.org/10.1351/pac-con-09-01-11 [ Links ]
2. Bolton JJ, Davies-Coleman MT, Coyne VE. Innovative processes and products involving marine organisms in South Africa. Afr J Mar Sci. 2013;35(3):449-464. http://dx.doi.org/10.2989/1814232X.2013.830990 [ Links ]
3. Simmons TL, Andrianasolo E, McPhail K, Flatt P Gerwick WH. Marine natural products as anticancer drugs. Mol Cancer Ther. 2005;4(2):333-342. [ Links ]
4. Blunt JW, Copp BR, Keyzers RA, Munro MHG, Prinsep MR. Marine natural products. Nat Prod Rep. 2016;33:382-431. http://dx.doi.org/10.1039/c5np00156k [ Links ]
5. Leal MC, Puga J, Serôdio J, Gomes NCM, Calado R. Trends in the discovery of new marine natural products from invertebrates over the last two decades - where and what are we bioprospecting? PLoS One. 2012;7(1):e30580. http://dx.doi.org/10.1371/journal.pone.0030580 [ Links ]
6. Gerwick WH, Moore BS. Lessons from the past and charting the future of marine natural products drug discovery and chemical biology. Chem Biol. 2012;19(1):85-98. http://dx.doi.org/10.1016/j.chembiol.2011.12.014 [ Links ]
7. Newman DJ, Cragg GM. Marine-sourced anti-cancer and cancer pain control agents in clinical and late preclinical development. Mar Drugs. 2014;12(1):255-278. http://dx.doi.org/10.3390/md12010255 [ Links ]
8. Newman DJ, Cragg GM. Natural products as sources of new drugs from 1981 to 2014. J Nat Prod. 2016;79(3):629-661. http://dx.doi.org/10.1021/acs.jnatprod.5b01055 [ Links ]
9. Davies-Coleman MT, Sunassee SN. Marine bioprospecting in southern Africa. In: Chibale K, Davies-Coleman MT, Masimirembwa C, editors. Drug discovery in Africa. Berlin, Heidelberg: SpringerVerlag; 2012. p. 193-209. http://dx.doi.org/10.1007/978-3-642-28175-4_8 [ Links ]
10. Davies-Coleman MT. Bioactive natural products from southern African marine invertebrates. In: Atta-ur-Rahman, editor. Studies in natural products chemistry. Amsterdam: Elsevier B.V.; 2005. p. 61-107. http://dx.doi.org/10.1016/S1572-5995(05)80054-7 [ Links ]
11. Davies-Coleman MT, Beukes DR. Ten years of marine natural products research at Rhodes University. S Afr J Sci. 2004;100:539-544. http://dx.doi.org/10.1002/chin.200537245 [ Links ]
12. Davies-Coleman MT. Natural products research in South Africa: End of an era on land or the beginning of an endless opportunity in the sea? S Afr J Chem. 2010;63:105-113. [ Links ]
13. Davies-Coleman MT, Froneman W, Keyzers RA, Whibley CE, Hendricks D, Samaai T, et al. Anti-oesophageal cancer activity in extracts of deep-water Marion Island sponges. S Afr J Sci. 2005;101(11-12):489-490. [ Links ]
14. Whibley CE, Keyzers RA, Soper AG, Davies-Coleman MT, Samaai T, Hendricks DT. Antiesophageal cancer activity from southern African marine organisms. Ann N Y Acad Sci. 2005;1056:405-412. http://dx.doi.org/10.1196/annals.1352.031 [ Links ]
15. McCloud TG. High throughput extraction of plant, marine and fungal specimens for preservation of biologically active molecules. Molecules. 2010;15(7):4526-1563. http://dx.doi.org/10.3390/molecules15074526 [ Links ]
 Correspondence:
Correspondence:
Suthananda Sunassee
sunny.sunassee@uct.ac.za
Received: 05 July 2016
Revised: 12 Sep. 2016
Accepted: 22 Oct. 2016
FUNDING: University of Cape Town; South African Medical Research Council; National Research Foundation (South Africa); CANSA; Rhodes University; Department of Science and Technology; Department of Environmental Affairs; SANAP














