Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.11-12 Pretoria Nov./Dec. 2016
http://dx.doi.org/10.17159/sajs.2016/20150442
RESEARCH ARTICLES
Characterisation of smectite-rich clay soil: Implication for groundwater defluoridation
Rabelani MudzielwanaI; Mugera W. GitariI; Titus A.M. MsagatiII
IEnvironmental Remediation and Pollution Chemistry Research Group, Department of Ecology and Resource Management, University of Venda, Thohoyandou, South Africa
IICollege of Science Engineering and Technology, University of South Africa, Johannesburg, South Africa
ABSTRACT
Groundwater is a widely used and affordable source of drinking water in most of the rural areas of South Africa. Several studies have indicated that groundwater in some boreholes in South Africa has a fluoride concentration above the level recommended by the World Health Organization (1.5 mg/L). Fluoride concentrations above the permissible limit (>1.5 mg/L) lead to dental fluorosis, with even higher concentrations leading to skeletal fluorosis. In the present work, we evaluate the application of smectite-rich clay soil from Mukondeni (Limpopo Province, South Africa) in defluoridation of groundwater. The clay soil was characterised by mineralogy using X-ray diffraction, by elemental composition using X-ray fluorescence and by morphology using scanning electron microscopy. Surface area and pore volume was determined by the Brunauer-Emmett-Teller surface analysis method. Cation exchange capacity and pHpzc of the soil were also evaluated using standard laboratory methods. Batch experiments were conducted to evaluate and optimise various operational parameters such as contact time, adsorbent dose, pH and initial adsorbate concentration. It was observed that 0.8 g/100 mL of smectite-rich clay soil removed up to 92% of fluoride from the initial concentration of 3 mg/L at a pH of 2 with a contact time of 30 min. The experimental data fitted well to a Langmuir adsorption isotherm and followed pseudo second order reaction kinetics. Smectite-rich clay soil showed 52% fluoride removal from field groundwater with an initial fluoride concentration of 5.4 mg/L at an initial pH of 2 and 44% removal at a natural pH of 7.8. Therefore smectite-rich clay soil from Mukondeni has potential for application in defluoridation of groundwater. Chemical modification is recommended to improve the defluoridation capacity.
SIGNIFICANCE:
• Physicochemical and mineralogical characterisation of smectite-rich clay soil
• Defluoridation of groundwater using smectite-rich clay soil
• Adsorption modelling using adsorption isotherms and kinetic models
Keywords: fluoride; pH; adsorption isotherms; batch experiments
Introduction
Groundwater is the most affordable and widely used natural resource for many of the rural areas in South Africa. Its chemical nature is one of the most important criteria that determine its usefulness for a specific need and as such not all groundwater is fit for drinking.1 Although the presence of fluoride in drinking water, within permissible limits, is beneficial for the production and maintenance of healthy bones and teeth, excessive intake of fluoride can cause dental or even skeletal fluorosis.2 The permissible limit set by the World Health Organization (WHO) is 1.5 mg/L.3 The presence of fluoride in high concentration in drinking water is therefore a serious health concern.
Fluorosis is an incurable disease caused by drinking water with fluoride concentrations of greater than 1.5 mg/L for extended periods of time and therefore efforts must be made to prevent it by providing drinking water with fluoride concentrations below 1.5 mg/L. Ncube and Schutte4 indicated that groundwater which has fluoride concentrations beyond the recommended limits for drinking water will require defluoridation. Meenakshi and Maheshwari5 reported various methods for defluoridation, including adsorption methods, ion exchange, membrane techniques and precipitation methods. Among all the methods of defluoridation which have been reported, adsorption has been considered an effective method for defluoridation of groundwater in rural areas because it uses materials which are readily available and inexpensive. Different materials such as clay soils6, activated alumina7, activated carbon8 and other low-cost materials have been tested for defluoridation of groundwater. Among the tested adsorbents, activated alumina is widely used because it is inexpensive and it has a high defluoridation capacity.5 Recently, more studies have focused on the use of clay soils for defluoridation because of their good adsorptive properties such as a large specific surface area, chemical and mechanical stability, layered structure and high cation exchange capacity. Several studies that have reported the use of clay soils for defluoridation have been published, including a study on illite-goethite soil in China9, a study on selected South African clays6, the use of chemically modified bentonite clay10, magnesium incorporated bentonite clay11, Fe3+-modified bentonite in South Africa12 and Al3+-modified bentonite in South Africa13. Smectite-rich clay from Mukondeni (a village in the Limpopo Province of South Africa) has been used for a very long time to make ceramic water filters and has been found to be effective in improving water quality.14 Its use in the adsorption of inorganic pollutants has never been evaluated, although it is known to be rich in smectite.15 Smectite-rich clay soil is available naturally in large abundance at no cost.
We evaluated the physicochemical properties of the black Mukondeni smectite-rich clay soil and its potential application in defluoridation of groundwater using batch experiments. Optimum operating conditions such as contact time, adsorbent dosage, initial adsorbate concentration and initial pH were determined. The regeneration and re-usability potential of smectite-rich clay soil as well as the effects of co-existing ions on defluoridation efficiency was also evaluated. Lastly, the mechanism of fluoride adsorption on the clay soil was elucidated.
Smectite-rich clay soil and groundwater defluoridation
Material and methods
Sample collection and preparation
Smectite-rich clay soil was collected from Mukondeni Village, Vhembe District in the Limpopo Province of South Africa. Field water was collected from the community borehole in Siloam, in the Vhembe District. All reagents and total ionic strength adjustment buffer (TISAB-III) were obtained from Rochelle Chemicals & Lab Equipment CC, South Africa Ltd and were of analytical grade. A stock solution containing 1000 mg/L fluoride was prepared by dissolving 2.21 g NaF in 1 L of Milli-Q water (18.2 ΜΩ/cm) and fluoride solutions for batch experiments were prepared from fresh stock fluoride solution by appropriate dilution.
Preparation of clay soil
The soil was washed by mixing with Milli-Q water (18.2 ΜΩ/cm) at a ratio of 1:5 in a 1-L beaker; the mixture was stirred for 5 min, and the procedure was repeated twice. After stirring, mixtures were agitated for 15 min using a Stuart reciprocating shaker and then centrifuged for 10 min at 5000 rpm. Samples were then dried in an oven for 12 h at 105 °C. Soil samples were then milled to pass through a 250-μm sieve.
Physicochemical and mineralogical characterisation
Mineralogical and chemical composition of the clay soil were determined using X-ray diffraction (XRD) (PANalytical X'Pert pro power) and X-ray fluorescence (XRF) (Thermo Fisher ARL9400 XP+ sequential XRF with WinXRF software), respectively. Surface morphology was determined using scanning electron microscopy (SEM) (Leo1450 SEM, voltage 10 kV, working distance 14 mm). Surface area and pore volume were determined by the Brunauer-Emmett-Teller method using micrometrics TriStar II. Functional groups and surface chemistry were determined using Fourier transform infrared spectroscopy (FTIR). Cation exchange capacity (CEC) was determined using ammonium acetate buffers at pH 5.4 and 7.4. The concentration of exchangeable cations was determined using flame atomic absorption spectra (600 PerkinElmer). Point of zero charge (pHpzc) was determined using titration at 0.1 M, 0.01 M and 0.001 M KCl ionic strength.
Batch defluoridation experiments
Batch experiments were carried out to evaluate the effect of contact time, initial adsorbate concentration, adsorbent dosage and pH on fluoride adsorption. A 100-mL volume of a known fluoride concentration was pipetted into a 250-mL plastic bottle and a known mass of the smectite-rich clay was suspended in the mixtures and then agitated for 30 min in a table shaker to attain equilibrium. After agitation, samples were filtered using 0.45-μm pore membrane filters. Filtrates were analysed for residual fluoride using an ion selective electrode calibrated with four standards containing 1 mL of TISAB III per 10 mL of solution. The same ratio was maintained for the sample. TISAB III was added to decomplex fluoride ions (F-) from Al3+, Fe3+ and Si4+ complexes and to maintain pH at between 5.2 and 5.5, optimum for the F--selective electrode. To evaluate the effect of contact time, the contact time was varied from 5 min to 270 min. The effect of adsorbent dosage was evaluated by varying the adsorbent dosage from 0.1 g to 2 g. To evaluate the effect of adsorbate concentration, the initial concentration was varied from 3 mg/L to 15 mg/L and to evaluate the effect of initial pH, the pH of the solution was adjusted from 2 to 12 using 0.1 M NaOH and 0.1 M HCl. The effect of competing ions was investigated by spiking a 3 mg/L fluoride solution with 5 mg/L of SO42-, Cl-, CO32- and NO3-, separately; a blank experiment was set for control with initial fluoride concentration of 3 mg/L. Mixtures were agitated for 30 min. All experiments were carried out at room temperature. Equations 1 and 2 were used to calculate the percentage of removal and adsorption capacity Q, respectively.

where Cois the initial fluoride ion concentration and Ceis the equilibrium fluoride ion concentration in mg/L.

where Cois the initial fluoride concentration (mg/L), Ceis the fluoride concentration at equilibrium (mg/L), V is the volume of solution (L) and m is the mass of the adsorbent (g).
Regeneration of smectite-rich clay soil
Regeneration of the adsorbent was carried out as follows: 0.8 g of fluoride-loaded clay was agitated with 100 mL of 0.1 M NaOH for 30 min on a table shaker. After agitation, the adsorbent was filtered through a 0.45-μm pore membrane filter and the filtrate was diluted to 100 mL and then analysed for desorbed fluoride. The collected adsorbent on filter paper was washed with Milli-Q water and then dried at 110 °C for 3 h. Regenerated adsorbent was then re-used for defluoridation up to five times.
Method of analysis
The fluoride concentration in the treated water sample was measured using an ion selective electrode (9609 BNWP Orion, USA) attached to a Thermo Scientific Orion Star A215 ISE pH meter. A similar ion meter coupled with a pH electrode was used for measuring the pH of treated samples. All experiments were conducted in triplicate for better accuracy and the mean values were reported.
Results and discussion
Physicochemical characterisation
X-ray diffraction analysis
XRD analyses were carried out to identify the mineral phase of the adsorbent. As shown in Figure 1, the clay soil is mainly composed of montmorillonite, quartz, albite and anthophyllite. The quantitative results further confirm the presence of smectite (60.34%) as the major mineral and the presence of quartz (20.21%) and plagioclase (20.4%) as minor minerals.
X-ray fluorescence analysis
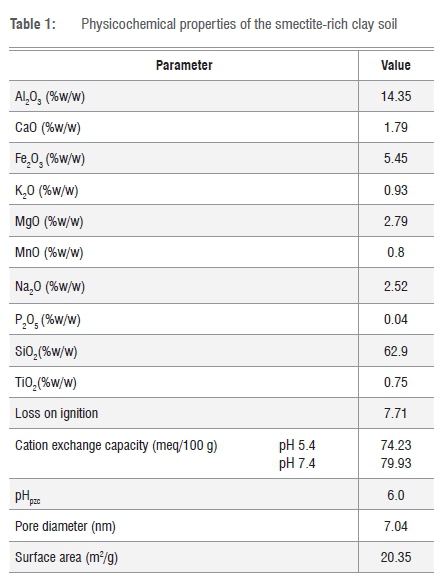
Table 1 presents the major elemental composition of smectite-rich clay soil. The analysis reveals that silica (SiO2) is the main component at 62.9%, followed by Al2O3 at 14.35%. High concentrations of SiO2 and Al2O3 reveal that the clay soil is an aluminosilicate material.
Scanning electron microscope analysis
Surface morphology of smectite-rich clay is shown in Figure 2. At lower magnifications, smectite-rich clay soil showed an irregular porous structure, whereas images at higher magnifications revealed that smectite-rich clay soil has a smooth surface.
Fourier transform infrared spectroscopy analysis
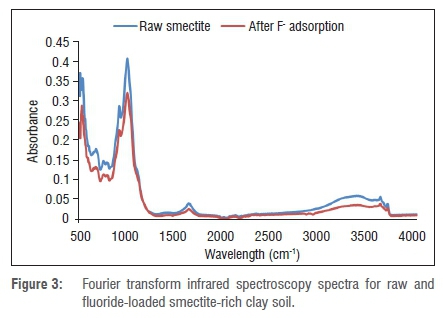
FTIR analysis was carried out in order to understand the surface chemist ry and functional groups of smectite-rich clay soil and also to help understand the adsorption mechanism. Figure 3 shows the FTIR spectra of raw and fluoride-loaded smectite-rich clay soils. The analysis shows three main absorption regions of smectite-rich clay soil, i.e. 3000-3800 cm-1, 1300-1800 cm-1 and 500-1200 cm-1. The same absorbance bands were observed by Toor et al.16 in montmorillonite clay. A notable difference was observed in those regions in raw smectite-rich clay soil and F--loaded smectite-rich clay soil. The absorption band at 3694.38 cm-1 may be a result of stretching and vibration of the structural OH- group and the broad band at 3620.51 cm-1 may be attributed to the stretching and vibration of structural hydroxyl groups and water. In the lower frequencies, a strong band at 996.06 was observed which may be caused by the stretching and vibration of Si-O groups in the clay mineral. The IR peak at 910 cm-1 may be attributed to stretching and vibration of Al-OH-Al. After fluoride sorption there was a decrease in the intensity of transmittance in all the peaks. This decrease confirms that the fluoride adsorption in smectite-rich clay soil was mainly through interaction with OH groups and also direct interaction with the metal surface.
CEC, surface area, pore volume and diameter and pHpzc
CEC refers to the total quantity of exchangeable cations that the soil can retain on their surfaces by electrostatic force at given pH. The CEC for smectite-rich clay was identified by measuring the concentrations of Na+, K+, Mg2+ and Ca2+ in ppm released in solution and the concentrations were then converted to milliequivalent per 100 g (meq/100 g). CEC values of smectite-rich clay soil at pH 5.4 and 7.4 are presented in Table 1. The CEC for smectite-rich clay soil was found to be 74.23 (meq/100 g) at pH 5.4 and it increased to 79.93 (meq/100 g) at pH 7.4. From the results it can be said that CEC is not dependent on pH as there was no significant difference in the concentration of cations at different pH. The results correlate with the results by Gitari et al.12 who also concluded that CEC is independent of pH. It can also be concluded that the smectite-rich clay soil has moderate CEC. The surface area and pore size of the clay are the significant properties that influence the sorption of ions by the clay. Surface area, pore volume and pore size of smectite-rich clay soil were determined using the Brunauer-Emmett-Teller method and the results are summarised in Table 1. The smectite clay has an average pore diameter of 7.04 nm, which suggests that it is mesoporous in nature. pHpzc of the smectite-rich clay soil evaluated in this study was found to be 6.0, which is lower than that for bentonite clay soil reported by Gitari et al.12 which was found to be 8.2. pHpzc refers to the suspension pH at which the clay soil has zero net charges on the surface17. Above the pHpzc the clay is negatively charged and below the pHpzc the clay is positively charged.18 Therefore, adsorption of anions is likely to be high at a pH below 6.
Batch adsorption experiments
Batch experiments were carried out to evaluate the effects of contact time, adsorbent dosage, initial concentration, pH and co-existing ions in the adsorption of fluoride onto smectite-rich clay soil.
Effect of contact time
The effect of contact time in adsorption of fluoride was evaluated at various initial concentrations ranging from 3 mg/L to 10 mg/L and adsorbent dosage of 0.3 g/100 mL. Contact time was varied from 5 min to 270 min at a 250-rpm shaking speed. Results are shown in Figure 4a which illustrates the plot of percentage fluoride removal with time. It was observed that percentage of fluoride removal increased gradually from 5 min up to 30 min, with a slight decrease at 60 min, after which the system seemed to approach equilibrium. This pattern was observed at all initial concentrations. The increase might be attributed to the availability of active sites for fluoride sorption which result in more exchange of ions and the decrease might be an indication that the active sites were getting saturated as the interaction continued, with the system finally reaching equilibrium. The percentage fluoride removal was reduced with an increase in initial fluoride concentration. Optimum fluoride uptake was observed at 30 min and this contact time was applied in subsequent experiments.
Effect of adsorbent dosage
The effect of adsorbent dosage was evaluated by varying the amount of clay from 0.1 g/100 mL to 2.0 g/100 mL. An initial fluoride concentration of 3 mg/L was used. The mixtures were agitated for 30 min at 250 rpm shaking speed. The results are reported as percentage fluoride removal in Figure 4b. It was observed that fluoride removal increased gradually from 46% at an adsorbent dosage of 0.1 g/100 mL to 62.2% at 0.8 g/100 mL. The increase is likely a result of an enhancement in the number of adsorption active sites available for adsorption of F- as the adsorbent mass increased.11 A slight change in the percentage F- removal was observed for an adsorbent dosage of 0.8 g/100 mL, which may be an indication that the system had reached equilibrium. Similar results have been reported by Kamble et al.10 with chemically modified bentonite for which they cited two reasons: (1) better utilisation of the available active sites at low adsorbent dose in comparison to high adsorbent dose at which too many sites are available for limited quantity of adsorbate and (2) reduced driving force for adsorption as high adsorbent dose causes lower equilibrium fluoride concentration. A dosage of 0.8 g/100 mL was then chosen as the optimum adsorbent dosage and was applied in subsequent experiments.
Effect of initial adsorbate concentration
The effect of adsorbate concentration was evaluated by varying the initial concentration from 3 mg/L to 15 mg/L at 30 min, 60 min and 120 min contact time at a shaking speed of 250 rpm and an adsorbent dosage of 0.8 mg/100 mL. Results are reported in terms of percentage removal in Figure 4c. It is observed that the percentage F- removal decreased with an increase in initial concentration. The same trend was observed for all three contact times. According to Thakre et al.11, the decrease in fluoride adsorption is because of the availability of more fluoride ions in solution at higher fluoride concentration, which also indicates that the fluoridebinding capacity of smectite-rich clay soil was approaching exhaustion. An initial adsorbate concentration of 3 mg/L was chosen as the optimum concentration for subsequent experiments.
Effect of initial pH
The effect of initial pH on percentage fluoride removal was evaluated by varying the initial pH of the solution from 2 to 12 using 0.1 M NaOH and 0.1 M HCl. The results are presented in Figure 4d. pH of the medium is one of the important parameters that influence fluoride removal efficiency significantly and help to understand the fluoride-uptake mechanism of the adsorbent. From the results presented in Figure 4d it is evident that smectite-rich clay showed significantly high fluoride removal efficiency over a wide range of pH, from 2 to 10. The optimal pH - with a fluoride removal of about 92% - was an acidic pH of 2. A decrease to 31.33% was observed at pH > 10. At pH 2 the surface of the clay is positively charged and F- would be electrostatically attracted to the clay surface, which explains the high F- adsorption at pH 2. The trend of change in pH during fluoride adsorption showed that at a pH below 6, the final pH was higher than the initial pH while at a pH above 6 the solution pH decreases. The increase may be attributed to the release of OH- ions from the adsorbent surface during ion exchange and the decrease in final pH at initial pH above the pHpzc may be attributed to the release of H+ ions from the adsorbent surface.
Effect of co-existing anion
Considering the complexity of natural water sources, many anions such as sulfates, chlorides, bicarbonates and nitrates might be present and might affect the efficiency of an adsorbent to adsorb fluoride. In order to evaluate the effects of co-existing anions on adsorption of fluoride by smectite-rich clay soil, defluoridation was conducted in the presence of each of 5 mg/L SO42- Cl-, CO32- and NO3-, separately, at an initial fluoride concentration of 3 mg/L; a blank experiment represented the control with an initial fluoride concentration of 3 mg/L. The results are presented in Figure 5.
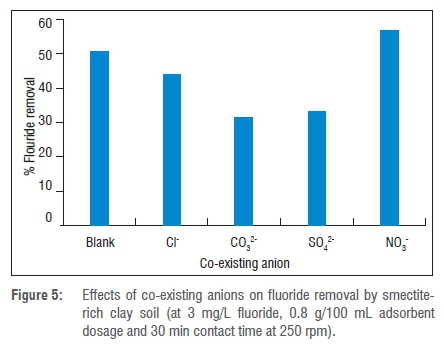
From the results in Figure 5 it is observed that the presence of NO3-increased the percentage of fluoride removal to 57%. This increase may be a result of an increase in the ionic strength of the solution or a weakening of lateral repulsion between adsorbed fluoride ions. However, the presence of Cl-, SO42- and CO32- decreased the fluoride adsorption to 44%, 33.33% and 31.66%, respectively, possibly because these ions compete with fluoride ions for the surface functional groups on the adsorbent surface, thereby decreasing the fluoride removal. Fluoride removal in the presence of anions increased in the order of CO32->SO42->Cl->NO3-.
Regeneration of adsorbent
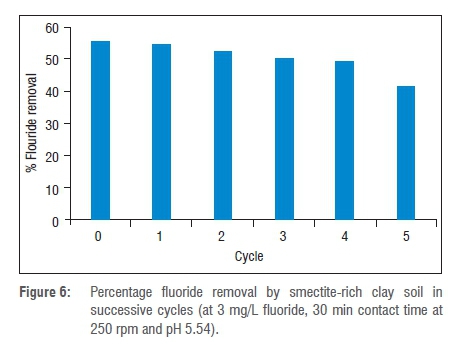
To evaluate the regeneration and recyclability of smectite-rich clay soil, five successive adsorption and desorption cycles were performed at an initial concentration of 3 mg/L fluoride, pH of 5.54 and contact time of 30 min. The results are presented in Figure 6. It is observed that the percentage fluoride removal after the first cycle decreased slightly from 56% to 54.6% and continued to decrease after each cycle. The same trend was reported in Zhang et al.19 and Jia et al.20 and it was attributed to inadequate regeneration of the adsorbent.
Defluoridation of field water
The efficiency of smectite-rich clay soil for fluoride removal was tested by using field groundwater collected from the Siloam borehole at the established optimum conditions of pH, contact time and adsorbent dosage. The adsorption of fluoride from groundwater by smectite-rich clay soil was conducted at optimum pH (pH 2) and natural pH (pH 7.8). Table 2 shows the physicochemical parameters of the field water before and after treatment.
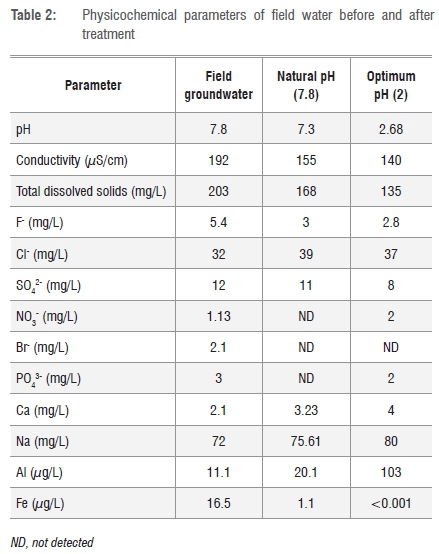
The percentage F- removal was found to be lower at natural pH (44%) than that at optimum pH (52%), as shown in Table 2, which may be a result of the abundance of OH- ions - which compete with F- ions - at natural pH (7.8). The percentage F- removal achieved from field groundwater is lower than the percentage F- removal achieved with synthetic fluoride solution (92%). This finding may be a result of the presence of competing anions such as Br-, PO43- and SO42- in the field water. From Table 2 it can be seen that the concentration of these coexisting ions decreased - indicating that they were adsorbed during the adsorption process. The concentration of Al and Fe chemical species in treated water were found to be within the WHO recommended limits of 0.2 mg/L and 0.3 mg/L, respectively, for potable water. The results show that smectite-rich clay soil from Mukondeni can be used for defluoridation of groundwater containing fluoride at concentrations below 3 mg/L in order to achieve the recommended limit of 1.5 mg/L. However, the fact that the soil removed a higher percentage at lower pH may be the limitation in its application, as the water will need to be acidified before treatment.
Adsorption modelling
Langmuir and Freundlich equations are generally used to describe the equilibrium relationship between adsorbate concentration and the amount of adsorbate adsorbed on the surface of the adsorbent. The Langmuir adsorption isotherm is the most applicable isotherm commonly applied in solid/liquid systems to describe saturated monolayers sorption.21 It assumes that the adsorbent surface is uniform, i.e. all the adsorption sites are equivalent and adsorption molecules do not interact.19
The linear equation of Langmuir adsorption is expressed by Equation 3 as follows:

where Ceis the equilibrium concentration (mg/L), Qeis the adsorption capacity (mg/g), Qmis the theoretical maximum adsorption capacity (mg/g) and b is the Langmuir constant related to enthalpy of adsorption (L/mg). Qm and b are determined from the slope and intercept of the plot of  . Figure 7a shows the plot of the Langmuir isotherm. The values of Langmuir parameters Qmand b are reported in Table 3. In order to predict the adsorption efficiency of the process, the dimensionless equilibrium parameter (RL) was calculated using Equation 4:
. Figure 7a shows the plot of the Langmuir isotherm. The values of Langmuir parameters Qmand b are reported in Table 3. In order to predict the adsorption efficiency of the process, the dimensionless equilibrium parameter (RL) was calculated using Equation 4:

where Co is the initial concentration and b is the Langmuir constant. A value of RL less than 1 generally indicates favourable adsorption and a value greater than 1 indicates unfavourable adsorption. Calculated RL values at various contact times in Figure 7b lie within the range 0-1, indicating that the adsorption process was favourable at room temperature for all the adsorbate concentrations tested.
The Freundlich adsorption model is an empirical model which is indicative of the surface heterogeneity of the adsorbent and considers multilayer adsorption.10 It is expressed by Equation 5:

where Ce is the equilibrium concentration (mg/L), Qeis the amount adsorbed at equilibrium (mg/g), Kf is the Freundlich constant related to adsorption capacity and represents the strength of adsorptive bonds and 1/n is the adsorption intensity. When 0<1/n<1, the adsorption is favourable; when 1/n_1, the adsorption is irreversible; and when 1/n>1, the adsorption is unfavourable. The values of Kfand 1/n are obtained from the slope and intercepts of a linear plot of log Qevs log Ce. Figure 7c shows the Freundlich isotherm. Values of Kfand 1/n are reported in Table 3. Based on the correlation coefficient values for both contact times, the adsorption data fit better to the Langmuir adsorption isotherm than the Freundlich adsorption isotherm. Better fit of the Langmuir isotherm model suggests monolayer uniform adsorption on the surface of the adsorbent. Calculated model constants for Langmuir and Freundlich isotherms are shown in Table 3. The value of RL, a dimensionless equilibrium parameter, and the value of 1/n - the Freundlich adsorption intensity - were both between 0 and 1, which indicates that the adsorption of F- onto smectite-rich clay soil was favourable.
Adsorption kinetics
Theoretically, the adsorption of fluoride onto a solid particle is controlled by different mechanisms. These mechanisms involve diffusion or transport of fluoride from bulk solution to the exterior surface of the adsorbent particle, adsorption of F- onto the particle surface, movement of the solute within the pores of the particle, attachment of the solute at sites on the interior surface of the adsorbent via sorption, complexation and intra-particle diffusion phenomena.22,23 Pseudo first and second order rate reactions and the intra-particle diffusion models are widely used to elucidate the adsorption mechanisms and the rate-limiting factors.
A pseudo first order equation is represented by Equation 6. The model is widely used to describe liquid-solid phase adsorption systems, and it is the earliest known kinetic model describing the adsorption rate based on the adsorption capacity.24

A pseudo second order equation is represented by linear Equation 7 and is used to describe chemisorption, as well as cation exchange reactions:

where qe and qt (both in mg/g) are the amounts adsorbed per unit mass at a time, t (in min). Kad (min-1) and K2ads (g/mg.min) are first and second order rate constants (g/mg.min). The value of Kad is determined from the slope and intercepts of log (qe-qt) vs t (min) and the value of K2ads is determined from the slope and intercepts of t/qt vs t. Figure 7d and 7e show plots of pseudo first and second order, respectively. The plots of pseudo second order yielded straight lines and higher correlation coefficients at both concentrations. The plots of pseudo first order did not give straight lines. Therefore adsorption of fluoride onto smectite-rich clay soil followed pseudo second order and occurred through chemisorption. Model constants for pseudo first and second order are presented in Table 3. In order to predict the rate-limiting step in the adsorption of F- onto smectite-rich clay soil, the possibility of intra-particle diffusion was evaluated using the Weber-Morris model of intra-particle diffusion expressed by Equation 823:

where qt is the amount adsorbed (mg/g) at a given time t (min) and Ki (mg/g.min) is the intra-particle diffusion rate constant determined from the slope of t0.5 vs qt. Figure 7f shows the plot of the amount of fluoride adsorbed and t0.5. The intercept did not pass through the origin at each of the evaluated concentrations and the data show a bilinear plot, with the initial portion (phase 1) indicating the boundary layer diffusion and the other represents the intra-particle diffusion (phase 2) and the values of Ki1 and Ki2 (intra-particle diffusion co-efficient rate constant for phase 1 and phase 2, respectively) obtained from the plot are shown in Table 3. At both initial concentrations, the Ki1 value is higher than the Ki2value, suggesting that the initial sorption step is more rapid than the final step (phase 2), which may be because of differences in the rate of mass transfer in the initial and final stages of adsorption.20 This observation is connected to a mechanism consisting of an external mass transfer followed by diffusion into micro- and mesoporous surfaces. This means adsorption of F- onto smectite-rich clay soil is a complex process involving both boundary and intra-particle diffusion adsorption processes.
Mechanistic aspects of adsorption of F- onto smectite-rich clay soil
The mechanism of fluoride adsorption onto smectite-rich clay was evaluated by comparing the FTIR spectra of smectite-rich clay soil before and after adsorption (Figure 3). FTIR spectra showed that the clay soil surface is mainly characterised by Si-OH and Al-OH groups which may be easily modified by changing the pH of the medium. pHpzc determination showed that at pH 5.8 the surface has neutral charge, below 5.8 the surface is positively charged and above 5.8 it is negatively charged. Figure 4d shows that the adsorption of F- onto smectite-rich clay soil was optimum at low pH and decreases as the pH increases, which may have been a result of the increase in OH- which competes with F- for adsorption sites. After F- adsorption, the FTIR spectra showed a decreased peak intensity of transmittance indicating that during adsorption there was ion exchange between OH- in the surface and F- in bulk solution, leading to the formation of new bonds such as SiF and AlF. Different mechanisms can be suggested at various pH levels. At pH below 5.8, the surface of the clay is positively charged (Equation 9) and therefore F- ions will be electrostatically adsorbed to the surface of the clay (Equation 10). Furthermore, low pH causes protonisation of surface -OH groups to -OH2+ which facilitates the ligand exchange mechanism because of stronger attractive forces between fluoride and the adsorbent surface and the presence of more hydroxylated sites for exchange of F- than at high pH.8 At moderate pH, fluoride adsorption occurs via ion exchange (Equation 11). At a pH above the pHpzc at which the surface of the clay is negatively charged, fluoride adsorption also occurs via ion exchange (Equation 12).
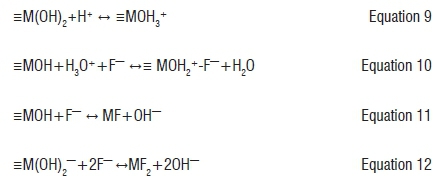
where ≡M represents Si and Al in the adsorbent surface.
Conclusions
In the assessment of the use of smectite-rich clay soil from Mukondeni for the defluoridation of groundwater, the following major conclusions were made:
• Optimum conditions were found to be 30 min contact time at a 250 rpm shaking speed on a table shaker, with an adsorbent dosage of 0.8 g/100 mL, a 3 mg/L adsorbate concentration and a pH of 2. Fluoride removal at optimum conditions was ≈92% for an initial concentration of 3 mg/L.
• Smectite-rich clay soil can be successfully regenerated using 0.1 M NaOH.
• Adsorption isotherms showed that the data fit better to the Langmuir isotherm than to the Freundlich isotherm, suggesting that adsorption of fluoride occurred on a monolayer surface.
• Adsorption kinetic studies showed that the data fit better to pseudo second order than to pseudo first order suggesting that the adsorption occurs via chemisorption.
• The data did not fit well to the intra-particle diffusion model of Weber-Morris and showed a bilinear plot which indicated that the adsorption of fluoride onto smectite-rich clay soil is a complex process.
• Ligand exchange and ion exchange mechanisms of F- adsorption are suggested to be the main mechanism taking place during the adsorption process.
• Field water defluoridation experimental results showed that smectite-rich clay soil is a potential candidate material for defluoridation.
• We recommend further research to improve the adsorptive capacity of the smectite-rich clay soils.
Acknowledgements
We acknowledge the following organisations for funding this work: Water Research Commission (project no. K5/2363/3), National Research Foundation (project no. CSUR13092849176, grant no. 90288 and THRIP project no. TP12082610644), and the Directorate of Research & Innovation, University of Venda.
Authors' contributions
M.W.G. was the project leader; M.W.G. and R.M. were responsible for the experimental and project design; R.M. carried out the experiments and performed the calculations; T.A.M.M. made conceptual contributions; and R.M. and M.W.G. wrote the manuscript.
References
1. Nagendra CR. Fluoride and environment: A review. In: Martin JB, Madha SV Vasantha KT, editors. Proceedings of the Third International Conference on Environment and Health; 2003 December 15-17; Chennai, India. Chennai / Toronto: Department of Geography, University of Madras / Faculty of Environmental Studies, York University; 2003. p. 386-399. [ Links ]
2. Susheela AK. Treatise on fluorosis. New Delhi: Fluorosis Research and Rural Development Foundation; 2001. [ Links ]
3. World Health Organization. Guideline for drinking water. Geneva: World Health Organization; 2011. [ Links ]
4. Ncube EJ, Schutte CF. The occurrence of fluoride in South African groundwater: A water quality and health problem. Water SA. 2005;31(1):35-40. http://dx.doi.org/10.4314/wsa.v31i1.5118 [ Links ]
5. Meenakshi SK, Maheshwari RC. Fluoride in drinking water and its removal. J Hazard Mater. 2006;137(1):456-463. http://dx.doi.org/10.1016/j.jhazmat.2006.02.024 [ Links ]
6. Coetzee PP, Coetzee LL, Mubenga S. Characterization of selected South African clays for defluoridation of natural water. Water SA. 2003;29(3):331-338. [ Links ]
7. Ghorai S, Pant KK. Investigations on the column performance of fluoride adsorption by activated alumina in a fixed-bed. Chem Eng J. 2004;98:165-173. http://dx.doi.org/10.1016/j.cej.2003.07.003 [ Links ]
8. Ma Y Wang SG, Fan M, Gong WX, Gao BY Characteristics and defluoridation performance of granular activated carbons coated with manganese oxides. J Hazard Mater. 2012;168:1140-1146. http://dx.doi.org/10.1016/j.jhazmat.2009.02.145 [ Links ]
9. Wang Y, Reardon EJ. Activation and regeneration of a soil sorbent for defluoridation of drinking water. Appl Geochem. 2001;16:531-539. http://dx.doi.org/10.1016/S0883-2927(00)00050-0 [ Links ]
10. Kamble S, Dixit P, Rayalu SS, Labhsetwar NK. Magnesium incorporated bentonite clay for defluoridation of drinking water. Desalination Water Treat. 2009;249:687-693. [ Links ]
11. Thakre D, Rayalu S, Kawade R, Meshram S, Subrt J, Labhsetwar N. Magnesium incorporated bentonite clay for defluoridation of drinking water. J Hazard Mater. 2012;180:122-130. http://dx.doi.org/10.1016/j.jhazmat.2010.04.001 [ Links ]
12. Gitari WM, Ngulube T, Masindi V, Gumbo JR. Defluoridation of groundwater using Fe3+-modified bentonite clay: Optimization of adsorption condition. Desalination Water Treat. 2015:53(6):1578-1590. http://dx.doi.org/10.1080/19443994.2013.855669 [ Links ]
13. Masindi V Gitari, MW, Ngulube T. Defluoridation of drinking water using Al3+-modified bentonite clay: Optimization of fluoride adsorption conditions. Toxicol Environ Chem. 2014;96(9):1294-1309. http://dx.doi.org/10.1080/02772248.2014.977289 [ Links ]
14. Tyeryar M, Hackett C, Harsh D, Hackett T. Establishing a ceramic water filter factory in Limpopo Province, South Africa. Jefferson Public Citizens. 2012;3:104-110. [ Links ]
15. Dacosta FA, Muzerengi C, Mhlongo SE, Mukwevho GF. Characterization of clays for making ceramic pots and water filters at Mukondeni Village, Limpopo Province, South Africa. J Eng Appl Sci. 2013;8(11):927-932. [ Links ]
16. Toor M, Jin B, Dai S, Vimonoses V. Activating natural bentonite as a cost effective adsorbent for removal of Congo red in waste water. J Ind Eng Chem. 2014;1979:1-9. [ Links ]
17. Spark DL. Environmental soil chemistry. 2nd ed. Amsterdam: Academic Press; 1997. [ Links ]
18. Bohn HL, Mcneal BL, O'Connor G. Soil chemistry. New York: John Wiley and Sons; 1975. [ Links ]
19. Zhang Z, Tan Y Zhong M. Defluoridation of wastewater by calcium chloride modified natural zeolite. Desalination. 2011;276:246-252. http://dx.doi.org/10.1016/j.desal.2011.03.057 [ Links ]
20. Jia Y Zhu BS, Zhang KS, Jin Z, Luo T, Yu XY, et al. Porous 2-line ferrihydrite/byerite composite (LFBC): Fluoride removal performance and mechanism. Chem Eng J. 2015;268:325-336. http://dx.doi.org/10.1016/j.cej.2015.01.080 [ Links ]
21. Sun Y Fang Q, Dong J, Cheng X, Xu J. Removal of fluoride from drinking water by natural stilbite zeolite modified with Fe(III). Desalination. 2011;277:121-127. http://dx.doi.org/10.1016/j.desal.2011.04.013 [ Links ]
22. Maliyekkal SM, Sharma AK, Philip L. Manganese-oxide-coated alumina: A promising sorbent for defluoridation of water. Water Res. 2006;40:3497-3506. http://dx.doi.org/10.1016/j.watres.2006.08.007 [ Links ]
23. Chen N, Zhang Z, Feng C, Li M, Zhu D, Sugiura N. Studies on fluoride adsorption of iron-impregnated granular ceramics from aqueous solution. Mater Chem Phys. 2011;125:293-298. http://dx.doi.org/10.1016/j.matchemphys.2010.09.037 [ Links ]
24. Oladoja NA, Helmreich B. Batch defluoridation appraisal of aluminium oxide infused diatomaceous earth. Chem Eng J. 2014;258:51-61. http://dx.doi.org/10.1016/j.cej.2014.07.070 [ Links ]
 Correspondence:
Correspondence:
Mugera Gitari
mugera.gitari@univen.ac.za
Received: 15 Nov. 2015
Revised: 03 Mar. 2016
Accepted: 02 July 2016
FUNDING: Water Research Commission; National Research Foundation (South Africa); University of Venda














