Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.9-10 Pretoria Sep./Oct. 2016
http://dx.doi.org/10.17159/sajs.2016/20160028
RESEARCH ARTICLES
http://dx.doi.org/10.17159/sajs.2016/20160028
Differential involvement of ascorbate and guaiacol peroxidases in soybean drought resistance
Makoena J. MoloiI; Obed J. MwenyeII; Rouxlene van der MerweI
IDepartment of Plant Sciences, University of the Free State, Bloemfontein, South Africa
IIBvumbwe Research Station, Limbe, Malawi
ABSTRACT
Soybean (Glycine max L.) is a small but growing component of the agricultural economy of South Africa and is predicted to become a major crop in Africa because of its high protein content. Drought induction at flowering or early stages of pod development has detrimental effects on soybean yield. As antioxidative enzymes play a protective role in plants during various abiotic stress conditions, this study was conducted to investigate how ascorbate (Enzyme Commission (EC) number 1.11.1.1) and guaiacol (EC: 1.11.1.7) peroxidases are involved in soybean drought resistance at different maturity stages (flowering and pod development). We also investigated whether the levels of these enzymes decline with plant maturity. Three tolerant soybean genotypes (G1, G2, G3) and a susceptible genotype (G4*) were used. These cultivars were categorised according to their sensitivity to drought stress in previous studies. The activity of ascorbate peroxidase was significantly induced by drought stress at both growth stages with higher activity in the resistant than susceptible plants, strongly supporting the protective role of this enzyme against drought stress at both developmental stages. The guaiacol peroxidase activity was induced to higher levels in the resistant than in the susceptible plants at flowering only, with no significant increase observed at pod development stage, indicating its selective protective involvement against drought stress. Interestingly, the levels of these enzyme activities were induced in all cultivars at both developmental stages, irrespective of drought stress, indicating that their activities increased with maturity.
SIGNIFICANCE:
• Guaiacol peroxidase is selectively involved in soybean drought resistance at flowering stage.
• The upregulation of ascorbate peroxidase activity at both growth stages in drought-resistant cultivars suggests that this enzyme could be used as a biochemical marker of drought resistance in soybeans.
• In contrast to the literature, activities of both enzymes increased with maturity irrespective of whether the plant is drought susceptible or resistant.
Keywords: antioxidative enzymes; drought stress; maturity stage; drought tolerance
Introduction
Soybean (Glycine max L.) is a small but growing component of the agricultural economy of South Africa1 and is predicted to become a major crop in Africa because of its high protein content2. Among important abiotic factors that influence soybean growth and yields are temperatures above 30 °C and lower than 13 °C for long periods during the flowering stage (which inhibits flower and seed development) as well as drought stress imposed during flowering and pod formation stages.3 Drought stress occurs when available water in the soil is reduced and atmospheric conditions cause continuous loss of water through transpiration or evaporation. In most crops, inhibition of growth and yield are mainly associated with altered metabolic functions such as reduced photosynthesis.4
Plants respond to drought stress with a cascade of biochemical reactions such as production of abscisic acid, which is aimed at facilitating stomatal closure thereby reducing water loss through transpiration. Although this action reduces water loss, it limits carbon dioxide fixation and reduces regeneration of NADP+ by Calvin cycle, which results in increased formation of oxygen radicals or reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide anion (O2-) and hydroxyl radicals (OH-) through enhanced leakage of electrons to molecular oxygen.5 Studies show that ROS such as H2O2 can act as signal molecules for induction of defence responses in plants when moderately produced.6,7 However, excessive production of these molecules may lead to oxidative stress, which in turn may have damaging effects on the photosynthetic pigments, membrane lipids, proteins and nucleic acids.8 To avoid oxidative stress and to thrive, plants need to keep ROS production in the cells to a minimum,9 which can be achieved through employment of different antioxidative mechanisms that may be enzymatic or non-enzymatic in nature. The major ROS scavenging mechanisms include enzymes of the ascorbate-glutathione pathway (e.g. ascorbate peroxidase (APX) and glutathione reductase), guaiacol peroxidase (POD) and catalase.10 These enzymes may act independently or in conjunction to catalyse conversion of H2O2 to water and O2,11,12 thereby minimising damage within the plant.
However, it is important to note that ROS production in plants depends on the stress intensity, plant species and genotype as well as the developmental stage.13 Drought tolerance was found to be correlated with the ROS scavenging capability.14 However, the degree to which the activities of antioxidative enzymes are elevated or inhibited under drought stress is variable among plant species and even between cultivars of the same species.15 As a consequence of the increase in intensity of drought spells in South Africa, we undertook this study to investigate whether APX and POD are differentially or similarly involved in soybean drought resistance at the two maturity stages (flowering and pod development). We also sought to clarify whether the levels of enzyme activities were affected by the different growth stages, as numerous studies have reported decreases or unchanged enzyme activities with maturity. We therefore report, for the first time, on the involvement of APX and POD when drought stress is applied at flowering and pod development. Results will further indicate whether these enzymes may be used as biochemical markers of drought resistance in soybeans.
Materials and methods
Drought tolerant (G1, G2, G3) and susceptible (G4*) soybean cultivars were planted in lysimeter units (1.40 m long), each filled with air-dried A (0.25 m, top) and B (1.05 m, bottom) horizon of Bainsvlei Amalia 2300 soil with filter sand at the bottom of the cylinders and a mulch of styrofoam at the top of the cylinders. The choice of cultivars was based on previous glasshouse and field trials, which showed that G1, G2 and G3 were less sensitive whereas G4* was very sensitive to drought stress. Although the G1, G2 and G3 cultivars were all tolerant, there were differences in their mode of drought resistance (results not shown).
The experiment was conducted in replicates of three per cultivar per treatment, in the glasshouse under controlled temperature (25 °C day and 18 °C night) in a randomised complete block design. Drought stress was applied according to literature16,17 at the beginning of flowering and pod development stages by withdrawing water supply to the plants for 14 days, followed by optimal watering (optimal watering refers to lysimeters being watered every day based on the amount of depleted soil water and irrigated back to field capacity). Other sets of tolerant and susceptible plants, representing the controls, received optimal water supply throughout the experimental period. At the end of each stress treatment, the top three fully expanded leaves were harvested on the stressed as well as the control plants and immediately frozen in liquid nitrogen before storage at -20 °C for the antioxidative enzyme analysis. Two plants per treatment were sampled. The obtained data were subjected to analysis of variance using GenStat Release 17.1 software to separate the sources of variation.
Enzyme extract preparation and assay
Enzyme extracts were prepared in accordance with Pukacka and Ratajczak18. Pre-weighed leaves (0.5 g) of each treatment were homogenised to a fine paste on ice using a mortar and pestle in 5 mL 50 mM potassium phosphate buffer (pH 7.0) containing 1 mM EDTA, 2% PVPP 0.1% Triton X-100 and 1 mM ascorbate. The homogenate was centrifuged at 15 000 x g for 20 min at 4 °C. The resulting aliquot was used as the enzyme extract. Three replicates per assay were used.
The APX assay was performed according to a method described by Mishra et al.19 with modifications. The reaction mixture (1 mL) consisted of 500 μL 50 mM phosphate buffer (pH 7.0), 200 μL 0.1 mM H2O2, 150 μL 0.5 mM sodium ascorbate, 50 μL 0.1 mM EDTA and 100 μΙ_ enzyme. A decrease in absorbance as a result of ascorbate oxidation was measured at 290 nm for 5 min at 20 °C against a blank in which the enzyme was replaced with phosphate buffer. An extinction coefficient of 2.8 mM-1cm-1 was used to calculate enzyme activity.
A modified method described by Zieslin and Ben-Zaken20 was used for determination of POD. The reaction mixture contained 50 μL 0.2 M H2O2, 100 μL 50 mM guaiacol, 340 μL distilled H2O, 500 μL 80 mM phosphate buffer (pH 5.5) and 10 enzyme. An increase in absorbance as a result of tetraguaiacol formation was measured at 470 nm for 3 min at 30 °C. The blank contained all reagents except for the enzyme, which was replaced with phosphate buffer. An extinction coefficient of 26.6 mM-1cm-1 was used to calculate enzyme activity. Protein concentration determination was done according to Bradford21.
Results
Highly significant differences in APX (p<0.001) were observed between drought-stressed and well-watered soybean genotypes at different growth stages (flowering and pod development). In contrast, a significant effect of drought stress on POD (p<0.01) was observed only at flowering and not at pod development stage. Activities of both APX and POD were highly significant for all cultivars, which indicated that there were large differences in antioxidative enzyme levels across the four genotypes. This finding was applicable for both flowering and pod development stages. Water treatment by genotype interaction was highly significant at flowering stage (p<0.001) for both enzymes. However, at pod development stage of all studied cultivars, drought stress had a selective significant effect (p<0.01) on APX but not on POD (Table 1).
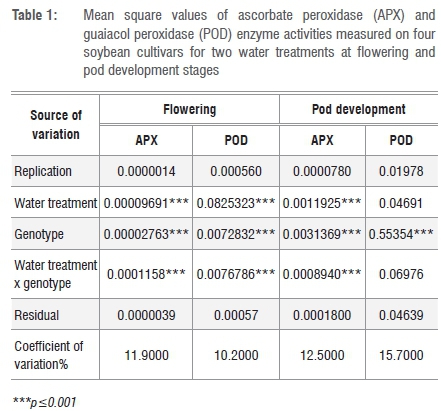
At flowering, drought stress induced significant increases in APX activities of G1 (2.5-fold), G2 (1.4-fold) and G3 (1.2-fold) cultivars, whereas in the susceptible cultivar (G4*), drought stress led to a substantial decrease (43%) in APX activity. Similarly at pod developmental stage, drought stress led to substantial increases in APX activities of G1 (1.3-fold), G2 (1.4-fold) and G3 (1.1-fold) cultivars and a decrease (15%) in the susceptible cultivar (Figure 1).
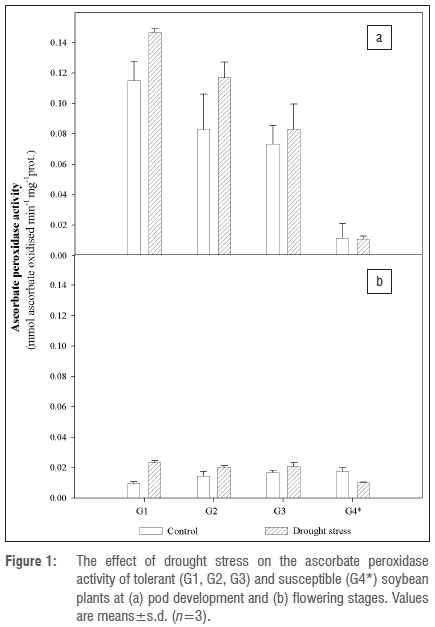
At flowering, resistant cultivars (G1, G2, G3) responded to drought stress with a significant increase in POD activity of 2.2-fold, 2.0-fold and 1.4fold, respectively. In the susceptible cultivar at the same developmental stage, no significant increase in POD activity post drought stress application was observed. In contrast to the APX activity results and flowering stage above, there were no significant changes in POD activity of the various cultivars at pod development stage (Figure 2).
Discussion
Drought at flowering or early pod development stages significantly increases the rate of pod abortion and consequently decreases seed yield.22 Prolonged drought stress leads to overproduction of ROS23; therefore plants need to respond with a battery of antioxidative enzymes in order to thrive9. APX was significantly affected when drought stress was applied at flowering and pod development stages (Table 1), confirming that this enzyme is highly sensitive to drought stress because of its high affinity for H2O2.24 Further evidence showed that APX was significantly different among the cultivars used in this study (Table 1), showing that sensitivity to drought stress for this enzyme cannot be generalised. This finding further confirmed earlier sensitivity trials (results not available) that showed that the mode of tolerance for these cultivars was different. The measure of various antioxidative enzyme activities and/or expression may be used as an approach to assess the involvement of a scavenging system during drought stress.15 Numerous studies that have been conducted in various plants are in support of these findings. Drought stress also induced increases in many antioxidative enzymes including APX in sunflowers.25 Likewise in soybean (cultivar Enrei), drought stress led to a substantial increase in APX activity, measured using Western blot, enzyme activity assay and biophoton emission.26 To show that the response of plants to drought stress is species or cultivar specific, a different response was observed in mycorrhizal soybeans in which APX activity declined significantly after drought stress introduction.27 Additionally, drought stress had no effect on APX transcript levels in spinach28, whereas it led to a significant decrease in this enzyme's activity in tomato29.
Although APX activity was significantly increased by drought stress at both developmental stages in resistant cultivars, evidence also showed that the levels of activity at these stages were not the same.
Activity levels at pod development were higher than at flowering (Figure 1), suggesting that activity of this enzyme intensified with maturity, in contrast to numerous studies that have reported a decrease. Antioxidative enzyme activity of barley under cadmium stress remained the same in two maturity stages.30 Mature leaves of neem and pigeon pea had lower APX activity than younger leaves.31 Antioxidative enzyme activities declined significantly with progressive growth stages in drought-stressed maize.32 Results further indicated that APX activity in all tolerant plants (G1, G2, G3) was significantly upregulated by drought stress irrespective of the growth stage. In the susceptible cultivar (G4*), however, the enzyme was downregulated (Figure 1). As APX is one of the enzymes responsible for scavenging H2O2 which may be produced during water deficits,23 significant increases in activity in the tolerant cultivars and a substantial decline in the susceptible cultivar suggest that tolerance to drought stress for these cultivars was somehow associated with this enzyme. These results indicate that APX could be used as a biochemical marker for drought resistance in soybean, as did those of Zarei et al.33 who showed that APX activity induction during drought stress was correlated with drought tolerance in tobacco.
During stress conditions, PODs are often the first antioxidative enzyme activities to be altered.34 Similarly to APX, lower POD activity at flowering than at pod development showed that induction of antioxidative enzymes increased with maturity in these cultivars. Significantly higher increases in POD activity of tolerant than of susceptible cultivars after drought stress induction at flowering (Figure 2) further showed that this enzyme was part of the drought resistance mechanisms when stress was introduced at flowering. Lack of significant interaction between water treatment (i.e. drought stress) and genotypes at pod development stage for POD (Table 1) showed that POD was an essential part of the ROS detoxification system at this stage for the majority of cultivars. However, this may not mean that these cultivars were not tolerant to drought stress; other studies have shown that if any of the antioxidative enzymes are upregulated under water-stress conditions, the genotype is still likely to be tolerant.34 Studies in Brassica napus (rapeseed), Helianthus annuus (sunflowers) and Triticum aestivum (wheat), support the finding that upregulation of POD is associated with drought tolerance.13,25,34,35
In conclusion, the upregulation of APX and POD activities in tolerant soybean cultivars suggests their involvement in drought stress resistance. However, APX and POD were found to be differentially involved, with APX being part of the drought resistance mechanisms at both maturity stages whereas POD was involved at flowering only. The presented evidence shows that APX could be used as a biochemical marker for drought resistance in soybeans. Interestingly, the levels of these enzyme activities were induced in all cultivars at both developmental stages, irrespective of drought stress, indicating that their activities increased with maturity.
Acknowledgement
We acknowledge financial support from the National Research Foundation (South Africa).
Authors' contributions
M.J.M. wrote the manuscript, performed the experiments and analysed the data; O.J.M. performed the glasshouse trials; and R.v.d.M. was the leader of the project and acquired the funding.
References
1. De Beer A, Prinsloo T. The national soybean cultivar trials in South Africa - 34 years experiences and progress. Potchefstroom: ARC-GCI; 2013. Available from: http://www.proteinresearch.net/html_images/wsrc2013/18-february-session-2/134_debeer-as.pdf [ Links ]
2. Sinclair TR, Marrou H, Soltani A, Vadez V, Chandolu KC. Soybean production potential in Africa. Glob Food Sec. 2014;3:31-40. http://dx.doi.org/10.1016/j.gfs.2013.12.001 [ Links ]
3. Pannar Seed (Pty) Ltd. Soybeans production guide. Production guide series: Soybeans. Greytown: Pannar Seed; 2006. p. 1-15. [ Links ]
4. Jaleel CA, Manivannan P, Wahid A, Farooq M, Al-Juburi HJ, Somasundaram R, et al. Drought stress in plants: A review on morphological characteristics and pigments composition. Int J Agric Biol. 2009;11:100-105. [ Links ]
5. Arora A, Sairam RK, Sriuastava GC. Oxidative stress and antioxidative system in plants. Curr Sci. 2002;82:1227-1238. [ Links ]
6. Chamnongpol S, Willekens H, Moeder W, Langebartels C, Sandermann H, Van Montagu M, et al. Defence activation and enhance pathogen tolerance induced by H2O2 in transgenic plants. Proc Natl Acad Sci USA. 1998;58:5818-5823. [ Links ]
7. Moloi MJ, Van der Westhuizen AJ. The reactive oxygen species are involved in the resistance responses of wheat to the Russian wheat aphid. J Plant Physiol. 2006;163:1118-1125. http://dx.doi.org/10.1016/j.jplph.2005.07.014 [ Links ]
8. Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405-410. http://dx.doi.org/10.1016/S1360-1385(02)02312-9 [ Links ]
9. Del Rio LA, Corpas CJ, Sandalio LM, Palma JM, Gomez M, Barroso JB. Reactive oxygen species, antioxidant systems, and nitric oxide in peroxisomes. J Exp Bot. 2002;53:1255-1272. http://dx.doi.org/10.1093/jexbot/53.372.1255 [ Links ]
10. Xu PL, Guo YK, Bai JG, Shang L, Wang XJ. Effects of long-term chilling on ultrastructure and antioxidative activity in leaves of two cucumber cultivars under low light. Physiol Plant. 2008;132:467-478. http://dx.doi.org/10.1111/j.1399-3054.2007.01036.x [ Links ]
11. Noctor G, Foyer CH. Ascorbate and glutathione: Keeping active oxygen under control. Annu Rev Plant Phys. 1998;49:249-279. http://dx.doi.org/10.1146/annurev.arplant.49.1.249 [ Links ]
12. Gratao PL, Polle A, Lea PJ, Azevedo RA. Making the life of heavy metal- stressed plants a little easier. Funct Plant Biol. 2005;32:481-494. http://dx.doi.org/10.1071/FP05016 [ Links ]
13. Abedi T, Pakniyat H. Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed. 2010;46:27-34. [ Links ]
14. Selote DS, Khanna-Chopra. Drought-induced spikelet sterility is associated with an inefficient antioxidant defense in rice panicles. Plant Physiol. 2004;121:462-471. http://dx.doi.org/10.1111/j.1399-3054.2004.00341.x [ Links ]
15. Cruz de Carvalho MH. Drought stress and reactive oxygen species. Plant Signal Behav. 2008;3:156-165. http://dx.doi.org/10.4161/psb.3.3.5536 [ Links ]
16. Karam F, Masaad R, Sfeir T, Mouzer O, Rouphael Y Evapotranspiration and seed yield of field grown soybean under deficit irrigation conditions. Agric Water Manage. 2005;75:226-244. http://dx.doi.org/10.1016/j.agwat.2004.12.015 [ Links ]
17. Dogan E, Kirnak H, Copur O. Deficit irrigations during soybean reproductive stages and CROPGRO-soybean simulations under semi-arid climatic conditions. Field Crop Res. 2007;103:154-159. http://dx.doi.org/10.1016/j.fcr.2007.05.009 [ Links ]
18. Pukacka S, Ratajczak E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J Plant Physiol. 2005;162:873-885. http://dx.doi.org/10.1016/j.jplph.2004.10.012 [ Links ]
19. Mishra NP, Mishra RK, Singhal GS. Changes in the activities of antioxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993;102:903-910. [ Links ]
20. Zieslin N, Ben-Zaken R. Peroxidase, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol Biochem. 1991;29:147-151. [ Links ]
21. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. http://dx.doi.org/10.1016/0003-2697(76)90527-3 [ Links ]
22. Westgate ME, Peterson CM. Flower and pod development in water-deficient soybean (Glycine max L. Merr.). J Exp Bot. 1993;258:109-117. http://dx.doi.org/10.1093/jxb/44.1.109 [ Links ]
23. Smirnoff W. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytol. 1993;125:27-58. http://dx.doi.org/10.1111/j.1469-8137.1993.tb03863.x [ Links ]
24. Mittler R, Zilinkas BA. Regulation of pea cytosolic ascorbate peroxidase and other antioxidant enzymes during the progression of drought stress and following recovery from drought. Plant J. 1994;5:397-405. http://dx.doi.org/10.1111/j.1365-313X.1994.00397.x [ Links ]
25. Nazarli H, Zardashti MR, Darvishzadeh R, Mohammadi M. Change in activity of antioxidative enzymes in young leaves of sunflower (Helianthus annus L.) by application of super absorbent synthetic polymers under drought stress condition. Aust J Crop Sci. 2011;5:1334-1338. [ Links ]
26. Kausar R, Hossain Z, Makino T, Komatsu S. Characterization of ascorbate peroxidase in soybean under flooding and drought stresses. Mol Biol Rep. 2012;39(12):10573-10579. http://dx.doi.org/10.1007/s11033-012-1945-9 [ Links ]
27. Porcel R, Barea JM, Ruiz-Lozano JM. Antioxidant activities in mycorrhizal soybean plants under drought stress and their possible relationship to the process of nodule senescence. New Phytol. 2003;157:135-143. http://dx.doi.org/10.1046/j.1469-8137.2003.00658.x [ Links ]
28. Yoshimura K, Yabuta Y Ishikawa T, Shigeoka S. Expression of spinach ascorbate peroxidase isozymes in response to oxidative stress. Plant Physiol. 2000;123(1):223-234. http://dx.doi.org/10.1104/pp.123.1.223 [ Links ]
29. Ünyayar S, Keles Y Cekic FÖ. The antioxidative response of two tomato species with different drought tolerances as a result of drought and cadmium stress combinations. Plant Soil Environ. 2005;51:57-64. [ Links ]
30. Hegedüs A, Erdei S, Horvath G. Comparative studies of H2O2 detoxifying enzymes in green and greening barley seedlings under cadmium stress. Plant Sci. 2001;160:1085-1093. http://dx.doi.org/10.1016/S0168-9452(01)00330-2 [ Links ]
31. Goud PB, Kachole MS. Antioxidant enzyme changes in neem, pigeon pea and mulberry leaves in two stages of maturity. Plant Signal Behav. 2012;7:1258-1262. http://dx.doi.org/10.4161/psb.21584 [ Links ]
32. Bai L-P Sui F-G, Ge T-D, Sun Z-H, Lu Y-Y Zhou G-S. Effect of drought stress on leaf water status, membrane permeability and enzymatic antioxidant system of maize. Pedosphere. 2006;16:326-332. http://dx.doi.org/10.1016/S1002-0160(06)60059-3 [ Links ]
33. Zarei S, Ehsanpour AA, Abbaspour J. The role of overexpression of P5CS gene on proline, ascorbate peroxidase activity and lipid peroxidation of transgenic tobacco (Nicotiana tabbacum L.) plant under in vitro drought stress. J Cell Mol Res. 2012;4:43-49. [ Links ]
34. Devi R, Kaur N, Gupta AK. Potential of antioxidant enzymes in depicting drought tolerance of wheat (Triticum aestivum L.). Indian J Biochem Biophys. 2012;49:257-265. [ Links ]
35. Sheoran S, Thakur V, Narwal S, Turan R, Mamrutha HM, Singh V, et al. Differential activity and expression profile of antioxidant enzymes and physiological changes in wheat (Triticum aestivum L.) under drought stress. Appl Biochem Biotech. 2015;177:1282-1298. http://dx.doi.org/10.1007/s12010-015-1813-x [ Links ]
 Correspondence:
Correspondence:
Makoena Moloi
makoena.moloi@gmail.com
Received: 26 Jan. 2016
Revised: 30 Mar. 2016
Accepted: 23 May 2016
FUNDING: National Research Foundation (South Africa)














