Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.7-8 Pretoria 2016
http://dx.doi.org/10.17159/sajs.2016/20160013
RESEARCH LETTER
Anaerobic digestion of donkey dung for biogas production
Patrick MukumbaI; Golden MakakaI; Sampson MamphweliII
IDepartment of Physics, University of Fort Hare, Alice, South Africa
IIFort Hare Institute of Technology, University of Fort Hare, Alice, South Africa
ABSTRACT
Biogas can provide a solution to some of South Africa's energy needs, especially in rural areas of the Eastern Cape province that have plentiful biogas substrates from donkeys, goats, sheep, cattle and chicken. We investigated the effectiveness of donkey dung for biogas production using a designed and constructed cylindrical field batch biogas digester. The donkey dung was collected from the University of Fort Hare's Honeydale Farm and was analysed for total solids, volatile solids, total alkalinity, calorific value, pH, chemical oxygen demand and ammonium nitrogen. The biogas composition was analysed using a gas analyser. We found that donkey dung produced biogas with an average methane yield of 55% without co-digesting it with other wastes. The results show that donkey dung is an effective substrate for biogas production.
Keywords: substrate; hydrolysis; fermentation; acetogenesis; methanogenesis
Introduction
Anaerobic digestion is a biological process that naturally occurs when bacteria decompose organic matter, producing mainly methane (CH4) and carbon dioxide (CO2) in an oxygen-free environment.1 The anaerobic digestion process is divided into four steps as follows: hydrolysis, fermentation (acidogenesis), anaerobic oxidation (acetogenesis) and methanisation.2-4
Hydrolysis
This is an enzyme-mediated stage, where insoluble organic compounds such as proteins, fats, lipids and carbohydrates are converted into soluble organic components, such as amino acids, fatty acids and monosac-charides.5
Fermentation
Acetate is the main end product of this step. Volatile fatty acids are also produced, as are carbon dioxide and hydrogen. Table 1 shows the major acids produced through fermentation processes in anaerobic digesters.6
Acetogenesis
In this step, the volatile acids are broken down into acetate and hydrogen.7 This process is represented by equations 1 to 3:
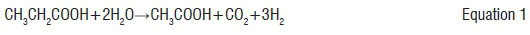
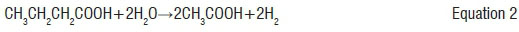

Methanogenesis
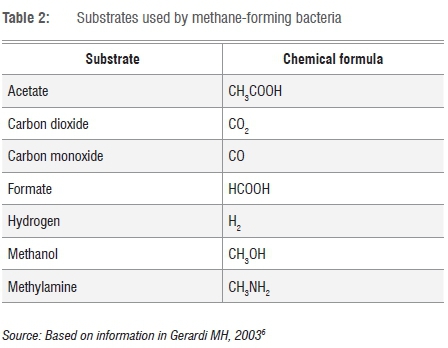
In this step, acetate, formaldehyde, hydrogen and carbon dioxide are converted to methane and water. Table 2 shows substrates used by methane-forming bacteria.6
Several parameters within an anaerobic digester play key roles in the physical environment and efficiency of digestion and biogas production potential. These parameters include pH-value, temperature, concentration of solids, hydraulic retention time, redox potential, volatile solids (VS) and loading rate, inocula, carbon-nitrogen ratio, toxicity, ammonium (NH4), particle size, water content, agitation frequency, organic loading rate and volatile fatty acids.
All metabolic processes in bacteria are brought about by enzymes. There is a temperature range within which these microbes thrive. When the temperature is not favourable, the enzymes may be denatured, which hampers their digestion process. In this regard, bacteria are classified according to their preferred temperature. Psychrophilic bacteria work best between 10 °C and 20 °C, mesophilic bacteria between 20 °C and 35°C, and thermophilic bacteria between 45 °C and 60 °C.2 Anaerobic digestion is very efficient in the thermophilic range. However, rural type digesters that lack external heating use mesophilic bacteria, as temperatures higher than 35 °C are very hard to obtain. For mesophilic bacteria, optimal digestion occurs at about 35 °C, whereas for thermophilic bacteria the optimum is 55 °C.8
Different substrates have different biogas yields. Swine manure has a better biogas output in terms of volume compared with cow dung, poultry manure, sheep manure, algae and night-soil. In addition, cow dung has a lower biogas yield than sheep manure.9 We were unable to find data on the biogas yield of donkey dung. The aim of our study was therefore to investigate the effectiveness and performance characteristics of anaerobic digestion of donkey dung for biogas production, using a cylindrical field batch biogas digester.
Methodology
Source of substrate
Donkey dung was collected from Honeydale Farm, which belongs to the University of Fort Hare. Before water was added to the substrates, total solids (TS) of the prepared sample was determined to find out the amount of water to be added to the substrates before feeding into the batch digester. The most favourable TS value for biogas production is 8%.10
Determination of total solids
Total solids is the weight of dry material remaining after drying; it is also called dry weight. This is the portion of a substrate remaining after the elimination of moisture. Different samples of substrates were weighed using digital weighing scales. The weighed samples were placed in an oven at 105 °C for 24 hours. After heating the samples were reweighed, and TS was calculated using equation 4.11
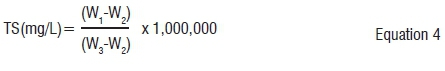
Where: W1 = weight of dried residue and dish (g)
W2 = weight of dish (g)
W3 = weight of wet sample and dish (g)
Determination of volatile solids
The dried residue was weighed and heated in a crucible inside a furnace for 2 hours at 550 °C to 600 °C. The residue was cooled in a desiccator and was then weighed. The ignition, cooling, desiccating and weighing steps were repeated until the weight change was 50 mg. The final weight was recorded. Volatile matter was determined using equation 5.11
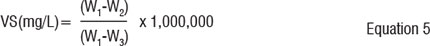
Where: W1 = weight of solids + weight of dish before ignition at 550°C (g)
W2 = weight of solids + weight of dish after ignition at 550°C (g)
W3 = weight of empty dish
Substrate parameters
The following parameters in substrate were determined: pH, chemical oxygen demand (COD), TS, VS, ammonia-nitrogen (NH4-N), total alkalinity (TA), and temperature (T). All the analytical determinations were performed according to the standard methods for examination of water and wastewater.11 The slurry temperature was measured using the type-K thermocouples, and the digital pH meter measured the feed, digester slurry and effluent pH. Figure 1 is a photo of a Crison pH meter that was used to measure pH values. Figure 2 shows the calorimeter (CaL2K-ECO) that was used to measure caloric values of donkey dung (with diagrammatic captions added).

Biogas analysis
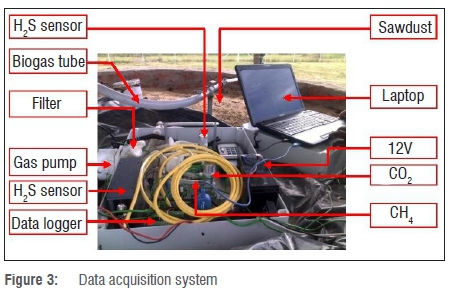
The data acquisition system consisted of a palladium-nickel (Pd/Ni) sensor and a non-dispersive infrared sensor. Biogas composition was analysed using a biogas analyser (a non-dispersive infrared sensor for sensing methane and carbon dioxide, and a palladium-nickel sensor for sensing hydrogen and hydrogen sulphide).
Data on biogas composition were recorded by a CR1000 data logger at intervals of 2 minutes. The biogas analyser and CR1000 data logger were powered by a 12 V DC battery that was connected to a 20 W photovoltaic module. The slurry and ambient temperatures were measured using type-K thermocouples connected to the same CR1000 data logger as the biogas sensors. The data logger was interfaced to a computer. Figure 3 shows the complete data acquisition system with biogas and temperature sensors.
Results and discussion
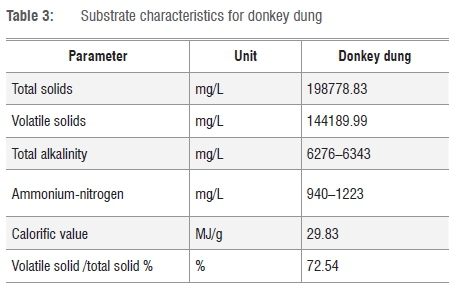
Table 3 shows characteristics of donkey dung. The higher concentrations of ammonium nitrogen between 940 mg/L and 1223 mg/L were beneficial to the anaerobic digestion micro-organisms, and this enhanced biogas production.
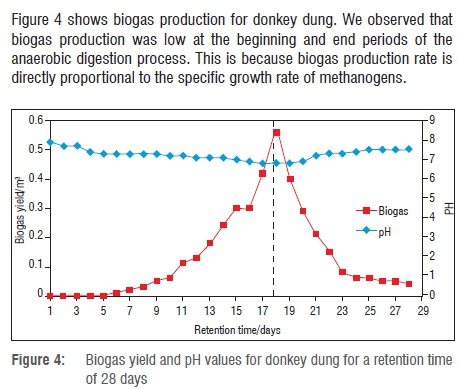
With reference to Figure 4, the donkey dung biogas production peaked at 0.56 m3 on Day 18. The total biogas yield of donkey dung was 3.8 m3 over a retention time of 28 days. The maximum biogas yield for donkey dung (63.4% or 2.41 m3) was attained in the first 18 days of the batch experiment, before the biogas yield declined. However, the biogas yield from Day 18 to Day 28 was 36.6% (1.39 m3) of the total biogas production. The biogas production (Y) of donkey dung from Day 4 to Day 18 is represented by the following equation:
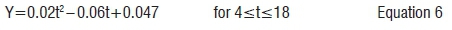
The biogas yield started to decline from Day 18 to Day 28. The decay equation is given by:

As shown in Figure 4, the initial pH of donkey dung was 7.9. The pH was observed to decline with time, attaining a minimum value of 6.8 where an optimum biogas production of 0.56 m3 was achieved. The decline is due to the conversion of substrate to acids during the stages of acidogenesis and acetogenesis of methane production. As from Day 18, the pH began to increase as the acids produced were converted to methane by methanogens. The pH (R) equation for the best fit is represented as follows:

Where R represents pH values and t represents retention time.
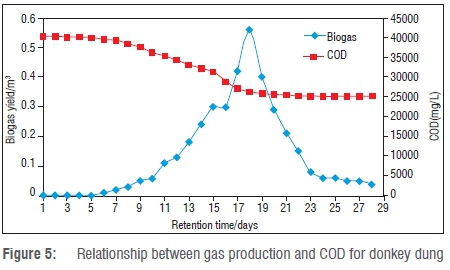
Figure 5 shows COD values during the anaerobic digestion of donkey dung. As shown in Figure 5, concentration COD of donkey dung entering the batch was 40 110 mg/L. The higher biogas yield from donkey dung was attributed to this higher concentration of COD. The concentration of COD decreased from 40 110 mg/L to 25 110 mg/L because of the conversion of substrate into biogas. Optimum biogas production occurred at a COD profile of 26 436 mg/L. The concentration of COD declined as of Day 20 and by Day 28 was very low, indicating a low biogas yield (a higher COD destruction means a high biogas yield). The COD (S) best fit can be approximated by the following equation:
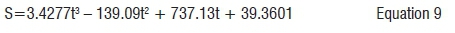
The relationship between biogas production and total alkalinity (TA) is shown in Figure 6. The effluent total alkalinity for donkey dung ranged from 6235 mg/L to 6343 mg/L. Therefore, alkalinity values were within the normal range, above the threshold alkalinity of 500 mg/L suggested for anaerobic digestion.
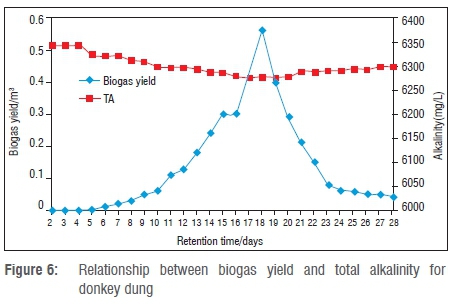
The high alkalinity levels imply that there was a high buffering capability that increased the biogas yield. The higher the alkalinity, the greater the buffering capacity in the anaerobic digestion process; this in turn promoted a stable pH value and resulted in an increased biogas yield.
The relationship between biogas yield and NH4-N for donkey dung is shown in Figure 7. The NH4-N concentrations for donkey dung ranged from 920 mg/L to 1223 mg/L. Therefore, donkey dung had appropriate NH4-N levels to maintain stable anaerobic digestion performance. The higher NH4-N final values corresponded with lower biogas production. The lowest NH4-N concentration was 920 mg/L. We observed that the lowest NH4-N concentrations corresponded to the highest biogas yield, and no inhibition occurred because the NH4-N concentrations fell within the desired optimum range (below 1500 mg/L) for better biogas production by the methanogens. Previous studies have reported that inhibiting concentrations of NH4-N are higher than 1500 mg/L.12-14

Table 4 shows the biogas composition for donkey dung. The biogas composition of donkey dung consisted of methane, carbon dioxide and hydrogen. The composition of hydrogen and other gases in the biogas was 5%, whereas the composition of carbon dioxide was 40%. In addition, the methane content in the biogas was 55%. We observed that biogas from donkey dung did not contain carbon monoxide.
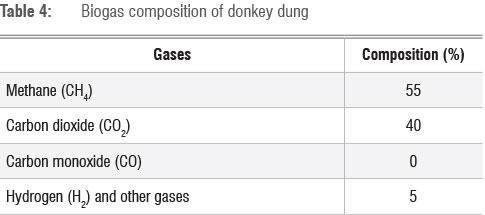
The concentration of COD entering the digester for donkey dung was high, and this contributed to a higher methane yield of 55% in donkey dung. Donkey dung had high values of CV, VS and COD and hence high methane yield.
Conclusion
We investigated the potency of donkey dung for biogas production. We analysed, and here present, the performance characteristics of anaerobic digestion in a cylindrical field batch biogas digester. The results showed that the biogas from donkey dung has an average methane yield of 55%. In addition, we observed that biogas production from donkey dung was low at the beginning and end of the retention period of the anaerobic digestion process, because biogas production rate is directly proportional to the specific growth rate of methanogens. From the results of the study, we conclude that donkey dung is a potential substrate in biogas digesters for rural digesters in the Eastern Cape Province of South Africa. This conclusion is based on the methane yield in donkey dung.
References
1. Arsova L. Anaerobic digestion of food waste: Current status, problems and an alternative product. [MSc thesis], USA: Columbia University; 2010. [ Links ]
2. Davidsson Ä. Increase of biogas production at waste water treatment plant: Addition of urban organic waste and pre-treatment of sludge. [PhD thesis]. Water and Environmental Engineering, Department of Chemical Engineering, Sweden: Lund University; 2007. [ Links ]
3. Leksell N. Present and future digestion capacity of Käppala waste treatment plant: A study in a laboratory scale. Sweden; 2005. [ Links ]
4. Polprasert C. Organic waste recycling - technology and management. London: IWA, Publishers; 2007. [ Links ]
5. Yadvika SS, Sreekrishman TR, Kohli S, Rana V. Enhancement of bio- gas production from solid substances using different techniques: A Review. Bioresour Technol. 2004;(95):1-10. http://dx.doi.org/10.1016/j.biortech.2004.02.010 [ Links ]
6. Gerardi MH. The microbiology of anaerobic digester. New Jersey: John Wiley & Sons; 2003. http://dx.doi.org/10.1002/0471468967 [ Links ]
7. Wiese J, König R. Monitoring of digesters in biogas plants: Laboratory analysis and process analysis: Biogas plant monitoring. Germany: EnerCess GmbH & Hach Lange GmbH; 2009. [ Links ]
8. Marchaim U. Biogas processes for sustainable development. FAO Agricultural Services. Bulletin 95; 1992. [ Links ]
9. Nijaguna BT. Biogas technology. New Delhi: New Age International (P) Limited, Publishers; 2002. [ Links ]
10. Ituen EE, John NM, Bassey BE. Biogas production from organic waste in Akwa Ibom State of Nigeria. In: Yanful EK, editor. Appropriate technology for environmental protection in the developing world. Selected papers from ERTEP 2007, July 17-19 2007, Ghana, Africa. [E-book publication] Springer Science & Business Media; 2009. p. 93-99 [ Links ]
11. American Public Health Association (APHA). Standard methods for examination of water and waste water. Washington DC: WEF; 2005. [ Links ]
12. Calli B, Mertoglu B, Inac B, Yenigun O. Effects of free ammonia concentrations on the performance of anaerobic bioreactors. Process Biochem. 2005;(40):1285-1292. http://dx.doi.org/10.1016/j.procbio.2004.05.008 [ Links ]
13. Chen Y Cheng JJ, Creamer KS. Inhibition of anaerobic digestion process: A review. Bioresour Technol. 2008;(9):4044-4064. [ Links ]
14. Fricke K, Santen H, Wallman R, Hütter A, Dichtl N. Operating problems in anaerobic digestion plants resulting from nitrogen in MSW. Waste Managt. 2007;(27):30-43. http://dx.doi.org/10.1016/j.wasman.2006.03.003 [ Links ]
 Correspondence:
Correspondence:
Patrick Mukumba
Department of Physics, University of Fort Hare
Private Bag X1314, Alice 5700
South Africa
pmukumba@ufh.ac.za
Received: 16 Jan. 2016
Revised:24 Apr. 2016
Accepted: 30 May 2016














