Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.7-8 Pretoria 2016
http://dx.doi.org/10.17159/sajs.2016/20160114
RESEARCH LETTER
Two dung beetle species that disperse mimetic seeds both feed on eland dung
Jeremy J. Midgley; Joseph D.M. White
Department of Biological Sciences, University of Cape Town, Cape Town, South Africa
ABSTRACT
Scarabaeus spretus zur Strassen was observed to roll and bury Ceratocaryum argenteum (Restionaceae) seeds in the sandplain fynbos of the Potberg area of the De Hoop Nature Reserve, South Africa. This species is the second dung beetle species found to be deceived by the faecal mimicry of C. argenteum seeds - the first species being Epirinus flagellatus. An isotopic analysis suggests that both these dung beetle species most likely feed on eland (Taurotragus oryx), not bontebok (Damaliscus pygargus pygargus), dung. Thus the model in this mimicry is eland dung; this interaction suggests large herbivores are an integral part of this fynbos.
Keywords: Scarabaeus spretus; Epirinus flagellatus; seed dispersal; mimicry; deception; eland; Ceratocaryum argenteum
Introduction
The dung beetle Epirinus flagellatus was observed to roll and bury seeds of the Cape plant Ceratocaryum argenteum (Restionaceae) at a site in the Potberg part of the De Hoop Nature Reserve in South Africa.1 This primary dispersal of seeds involves chemical and visual mimicry because neither the dung beetle nor its larvae can eat these hard seeds. Chemically the seeds have characteristics of the dung of both of the most common large herbivores in the reserve: the eland (Taurotragus oryx) and the bontebok (Damaliscus pygargus pygargus).1However, the seeds are more similar in shape and size to the smaller faeces of the bontebok, which is then the possible visual model that C. argenteum mimics. At the same Potberg site, during February 2016, we observed similar seed dispersal of C. argenteum seeds by another dung beetle, Scarabaeus spretus zur Strassen. The aim of this paper is to document this new burial behaviour and to investigate both dung beetle species to determine whether the faeces of the bontebok or the eland is the likely model of the mimic.
The bontebok is a short grass grazer whereas the eland is a mixed feeder.2,3 The two dominant grassland/ renosterveld grass species at Potberg are Cymbopogon popschilli (Andropogoneae) and Cynodon dactylon (Chloridoideae),3 to which can be added the relatively widespread Themeda triandra (Andropogoneae). All three species utilise the C4 photosynthetic pathway rather than the C3 pathway.4 This pathway is common in tropical grasses whereas the C3 system is more common in woody plants and temperate grasses. The enzymes of these two different photosynthetic pathways produce different carbon δ13C signatures in their photosynthetic products. The relatively rare stable isotope of carbon,13C, is slightly heavier than the more common 12C, which affects the ratios of these isotopes in different plants depending, for example, on enzyme preferences for the lighter isotopes. Fractionation is the process which reflects changes in relative proportions of isotopes, such as 13C:12C during C3 photosynthesis. Fractionation can also occur in 15N because, as it is heavier than 14N, it may increase in tissues depending on factors such as levels of metabolism, catabolism and excretion. Thus animals are typically enriched by +3-5‰ in δ15Ν compared to their diet, although typically they are less than +1‰ enriched in δ13C.5 During metamorphosis, larval tissue is broken down and then used to form new adult tissue and thus metamorphosis is also known to increase both 513C and δ15Ν in the adult tissue in much the same way as would happen in adult tissue with an increase in trophic level.6 Thus the hypothesis that bontebok dung is the likely model C. argenteum mimics can be tested using the isotopic method for diagnosing animal diets, including those of dung beetles.7
Methods
The study took place in the Potberg area of the De Hoop Nature Reserve (34.374420 S, 20.533060 E) in the sand plain vegetation type in which C. argenteum grows. During 3 days in early February 2016, we placed out 5 to 10 piles of C. argenteum seeds, with each pile comprising 10-20 seeds. Piles of seeds were 10 m apart on the edge of a 100-m stretch of a sand road through natural vegetation. We monitored the seed piles in the early morning for approximately 2 h (starting at about 08:00). This experiment took place after a 24-h rain event.
Dung samples were taken in various vegetation types in the Potberg reserve. These types were renosterveld (dominated by the shrub Elytopappus rhinocerotis (Asteraceae)), grassland (dominated by Cynodon dactylon), salt marshes (dominated by Chenopodiaceae), valley bottom fynbos (dominated by the Proteaceae shrubs Leucadendron linifolium/ L. coniferum), sand plain fynbos (dominated by Leucadendron laureolum, where C. argenteum occurs) and limestone fynbos (dominated by Leucadendron meridianum). Eland dung was found at all six sites whereas bontebok dung was found at all but the last two fynbos sites (sand plain fynbos and limestone fynbos). Previously, Radloff et al.3 noted that bontebok avoid fynbos whereas eland are found throughout fynbos, including limestone fynbos. To reduce chances of pseudoreplication, we sampled only a single pellet of bontebok or eland dung from a dung pile; only dung piles greater than 5 m apart were sampled and, as judged by colour, only relatively fresh samples were collected until a total of 10 pellets had been sampled from within each vegetation type. Dung pellets and dung beetle exoskeletons were dried and analysed for 513C and δ15Ν, in %o, using standard techniques at the Archaeometry Lab at the University of Cape Town. Dung beetle larvae have chewing teeth and are vigorous detritivores that depend on the plant remains that constitute the dung ball, rather than being dependent on microbiota associated with the ball.8 Although female beetles do select small fragments of plant remains from dung to constitute brood balls,8 this is not likely to significantly affect the isotopic signature of these balls nor the signature of the exoskeletons of adults that emerge from these balls.
Results
Dung beetles arrived at seed stations within a few minutes of placing seeds out; thus within 2 h each day, more than 10 beetles had arrived at seed piles along our short 100-m transect and had started burying seeds (Table 1 and Figure 1a-c). E. flagellatus crawled out of the vegetation towards seed piles, with only an occasional individual flying in, whereas all S. spretus individuals flew towards the seed piles. It was clear, based on the direct flight or crawling paths of both species to the seeds, that the attraction is strongly chemical. A S. spretus beetle even flew into a paper bag containing seeds. All cases of S. spretus burial involved limited movement of seeds (<0.25 m) from seed piles, whereas E. flagellatus moved seeds up to 2 m. S. spretus beetles were observed to frantically bury up to three seeds (n=2) and often five or more seeds (n=4) per excavated hole (see the video in the supplementary material online). E. flagellatus was observed to only bury seeds individually, similarly to observations by Midgley et al.1 Flies of the Sarcophagidae were frequently observed to settle on C. argenteum seeds (Figure 1d), indicating that they too are deceived by the scent of the seeds. These 'flesh flies' are typically attracted to dung, carrion or rotting vegetation.9 Lesser dung flies (Sphaeroceridae) were observed on S. spretus (Figure 1b).

Samples of the grasses Cynodon dactylon and Themeda triandra from Potberg have a typical C4 isotopic signal (n=2 for each species, mean (δ13C of -13.61V and -14.29V, respectively).
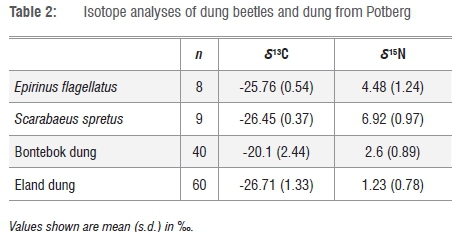
Dung of eland and bontebok are significantly different in both δ13C (U=52, p<0.001, Mann-Whitney test) and δ15Ν (U=309, p<0.0001, Mann-Whitney test) (Table 2). Bontebok graze a fairly equal mixture of C4 and C3 plants to create a mean δ13C value of -20.10V, whereas eland are mostly eating C3 plants (Figure 2 and Table 2).

Discussion
The increase in δ15N as a result of metamorphosis is in the range of + 3‰ and +5‰ for a selection of insects ranging from Diptera to Coleoptera to Lepidoptera6 and the increase in δ13C is about +1‰5. Dung beetle adults whose larvae fed on bontebok dung should thus have values of slightly more than -20‰ δ13C but up to 7.6‰ δ15Ν. The mean δ13C values of both E. flagellatus and S. spretus clearly indicate a C3 dung diet and are thus much closer to that of the eland dung (Table 2 and Figure 2). The δ15Ν values are 3.25‰ and 5.7‰ above eland dung but only 1.9‰ and 4.32‰ above bontebok dung. The evidence from δ15Ν of the beetles is less equivocal about the larval food source because of the small 1‰ difference in dung between the two herbivore species. Overall the isotope results are compatible with eland being the main larval source of dung. Also bontebok dung is rare in the lowland fynbos habitat of C. argenteum. As these two dung beetle species are from different genera and are both attracted to C. argenteum seeds, these results indicate that the seed chemistry and deception by C. argenteum is not dung beetle species-specific. The deception appears most likely to be modelled on the chemistry of eland, rather than bontebok, dung.
Not much is known of the feeding biology of Scarabaeus or Epirinus beetles.10 For both species, we observed diurnal activity and no pair formation at seed burial sites. C. argenteum seeds are about the same size as bontebok droppings and about half the size of eland droppings. That many seeds were buried per site for S. spretus suggests that several pellets of eland dung are typically used for feeding or egg laying, whereas E. flagellatus only buried a single seed per burial event. This distinctive burial behaviour of the two species likely results in differential recruitment patterns for C. argenteum seedlings. Single E. flagellatus burials would lead to lower intraspecific competition between seedlings compared with the multiple burials by S. spretus.
Many other dung beetles occur at Potberg, for example, the millipede-eating Sceliages adamastor.10The δ15Ν dung beetle values presented in Table 1 provide a framework to interpret those of S. adamastor to determine whether this beetle is an obligate insectivore. For example, δ15Ν values of an obligate millipede-eater should be a trophic level above herbivorous dung beetles such as S. spretus and E. flagellatus (i.e. they should have δ15Ν values greater than 7‰). The very large Addo flightless dung beetle (Circellium bacchus) also occurs at Potberg10; elsewhere it feeds on elephant dung10 but as there are no elephants at Potberg, its diet there is unknown and could too be clarified using the isotopic method. Being flightless, C. bacchus is often killed on roads and our analysis of nine roadkill individuals (mean δ13 of -27.70‰ and δ15Ν of 2.39‰) indicates eland dung is also its major larval food source.
The fact that Ceratocaryum argenteum is an element of deep sand fynbos,11 implies that sufficient quantities of large herbivore dung, such as that of eland, occurred in this vegetation. This would maintain the associated dung beetle species and the deceptive relationship between C. argenteum and these species. There is some debate as to whether large herbivores were once more common in fynbos and in this area of the Cape.3 Our observation that C. bacchus, E. flagellatus and S. spretus utilise eland dung suggests that the eland is, and has been, a key species
in this system and should be carefully managed as such. Finally, we suggest that there are now sufficient examples of seed dispersal by beetles for use of the term coleopterochory. This term would include primary dispersal such as that described above, as well as examples of beetle endozoochory12 in which small seeds are swallowed, as well as incidental or secondary dispersal in dung or with fruit1.
Acknowledgements
We thank the National Research Foundation for Incentive Funding to J.J.M. and a PhD bursary to J.D.M.W.
Authors' contributions
Both authors participated in field work, analysis and writing.
References
1. Midgley JJ, White JDM, Johnson SD, Bronner GN. Faecal mimicry by seeds ensures dispersal by dung beetles. Nat Plants. 2015;1, Art. #15141. http://dx.doi.org/10.1038/nplants.2015.141 [ Links ]
2. Skinner JD, Chimimba CT. Mammals of the southern African subregion. Cape Town: Cambridge University Press; 2005. http://dx.doi.org/10.1017/CBO9781107340992 [ Links ]
3. Radloff FG, Mucina L, Snyman D. The impact of native large herbivores and fire on the vegetation dynamics in the Cape renosterveld shrublands of South Africa: Insights from a six-yr field experiment. Appl Veg Sci. 2014;17:456-469. http://dx.doi.org/10.1111/avsc.12086 [ Links ]
4. Osborne CP Salomaa A, Kluyver TA, Visser V Kellogg EA , Morrone O, et al. A global database of C4 photosynthesis in grasses. New Phytol. 2014;204:441-446. http://dx.doi.org/10.1111/nph.12942 [ Links ]
5. Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Ann Rev Ecol Syst. 1987;18:293-320. http://dx.doi.org/10.1146/annurev.es.18.110187.001453 [ Links ]
6. Tibbets TM, Wheeless LA, Martinez del Rio C. Isotopic enrichment without change in diet: An ontogenetic shift in δ15N during insect metamorphosis. Funct Ecol. 2008;22:109-113. http://dx.doi.org/10.1111/j.1365-2435.2007.01342.x [ Links ]
7. Stavert JR, Gaskett AC, Scott DJ, Beggs JR. Dung beetles in an avian dominated island ecosystem: Feeding and trophic ecology. Oecologia. 2014;176:259-271. http://dx.doi.org/10.1007/s00442-014-3001-z [ Links ]
8. Byrne MJ, Watkins B, Bouwer G. Do dung beetle larvae need microbial symbionts from their parents to feed on dung? Ecol Entomol. 2013;38:250-257. http://dx.doi.org/10.1111/een.12011 [ Links ]
9. Picker M, Griffiths CL, Weaving A. A field guide to the insects of South Africa. Cape Town: Struik; 2004. [ Links ]
10. Davis ALV Frolov AV Scholtz CH. The African dung beetle genera. Pretoria: Protea Book House; 2008. p. 272. [ Links ]
11. Linder HP. Two new species of Ceratocaryum (Restionaceae). Kew Bull. 2001;56:465-477. http://dx.doi.org/10.2307/4110968 [ Links ]
12. De Vega C, Montserrat Arista M, Ortiz PL, Herrera CM, Talavera S. Endozoochory by beetles: A novel seed dispersal mechanism. Ann Bot. 2011;107:629-637. http://dx.doi.org/10.1093/aob/mcr013 [ Links ]
 Correspondence:
Correspondence:
Jeremy Midgley
Department of Biological Sciences
University of Cape Town
Private Bag X3, Rondebosch 7701
South Africa
jeremy.midgley@uct.ac.za
Received: 11 Apr. 2016
Revised:26 May 2016
Accepted: 28 May 2016














