Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.7-8 Pretoria 2016
http://dx.doi.org/10.17159/sajs.2016/20150302
RESEARCH ARTICLE
Estimated abundance and diversity of heterotrophic protists in South African biocrusts
Kenneth DumackI; Robert KollerI, II; Bettina WeberIII; Michael BonkowskiI
IBiocenter Cologne, Faculty of Zoology, Department of Terrestrial Ecology, University of Cologne, Cologne, Germany
IIInstitute of Bio- and Geosciences, IBG-2: Plant Sciences, Forschungszentrum Jülich GmbH, Jülich, 52425, Germany
IIIMultiphase Chemistry Department, Max Planck Institut for Chemistry, Max-PlanckGesellschaft, Mainz, Germany
ABSTRACT
Biological soil crusts (biocrusts) occur widely in the uppermost millimeters of the soil in arid and semi-arid systems. Worldwide they cover large terrestrial areas and play a major role in the global terrestrial carbon and nitrogen cycles. However, knowledge of the microbial decomposer foodwebs within biocrusts is particularly scarce. Heterotrophic protists in soil are predominantly bacterivores, and because of their high biomass compared with other soil fauna and fast turnover rates, protists are considered an important factor for soil nutrient cycling and energy fluxes. Thus, knowledge of their biodiversity, abundance and functional roles is important to understand soil ecosystem functions. We investigated the diversity and abundance of heterotrophic soil protists in different types of biocrusts from the Succulent Karoo, South Africa. With an overall diversity of 23 distinct morphotypes, soil protist biodiversity was shown to be high. The most abundant groups were Spumella-like chrysomonads, gliding bodonids, glissomonads and heteroloboseans. Protist abundance was highly variable among samples. The abundance and diversity did not differ significantly among different types of biocrusts, indicating that microscale differences, but not macroscopic soil crust builders (e.g. cyanobacteria, lichens and bryophytes), have a major impact on the protist community.
Keywords: biological soil crusts; biodiversity; protozoa; cryptogams; cyanobacteria
Introduction
Biological soil crusts (biocrusts) are microscopic ecosystems. They comprise primary producers such as cyanobacteria, algae, lichens, and bryophytes together with decomposers such as fungi, bacteria and archaea. The microscopic consumers in biocrust foodwebs are adapted to arid soil conditions and taxonomically diverse, including protists, nematodes, rotifers, tardigrades, and microarthropods in variable proportions. Biocrusts grow within the uppermost millimeters of the soil in arid and semiarid regions throughout the world,1 where they fulfil several highly relevant ecosystem services. They limit soil erosion by both wind and water2-4; influence water runoff, infiltration and retention within the uppermost soil layer5-7; and fertilise impoverished desert soils8. On a global scale, biocrusts cover over 10% of the terrestrial surface area, also influencing global nutrient cycling and climate processes.9,10 Biocrusts form one subgroup within cryptogamic covers, with the latter also comprising cryptogamic communities on rock and epiphytically on plants. These cryptogamic covers have been estimated to account for 7% of the net primary production fulfilled by plants, and fix about 49 Tg N per year, corresponding to about half the maximum value estimated for the total terrestrial biological nitrogen fixation.11
Detailed information is available on the diversity and species composition of the primary producers in biocrusts. According to the dominant photoautotrophic organism group (i.e. the dominant soil crust builder), they have been coarsely defined as cyanobacteria-dominated, lichen-dominated and bryophyte-dominated soil crusts.12However, data on the microbial decomposer foodwebs, especially heterotrophic protists, are scarce. Nematodes, tardigrades, rotifers, mites, collembolans, heterotrophic protists, and even larger arthropods and molluscs have been observed to utilise biocrusts as a habitat.13-16 However, their diversity, frequency, geographical distribution and feeding behaviour have been investigated in only a few local studies.13-16
Protists - with their high abundance, turnover and diversity - have been increasingly studied by soil ecologists in recent years.17,18 Based on their general morphology and means of locomotion, soil-inhabiting protists comprise amoebae, flagellates and ciliates. Most heterotrophic protists in soil are bacterivores. As a result of their high numbers and turnover rates, protists are considered to play a major ecological role in soil foodwebs by the release of nutrients from consumed microbial biomass.13,18
Heterotrophic protists have been determined in arid soils in a number of habitats among different continents, namely southwestern USA, the Negev Desert of Israel, arid Australia, China and Antarctica.13,19-23 However, data on biocrusts from Africa are lacking. Based on 73 soil samples from the Etosha region and the Namib Desert of Namibia, Foissner et al.24 identified 365 ciliate species, of which 35% had been undescribed, including a new order and suborder, three new families, and 34 new genera and subgenera of soil ciliates. These findings suggest that biocrusts in South Africa can harbour a substantial undiscovered diversity of protists.
We investigated the heterotrophic protist community of biocrusts in the Succulent Karoo, South Africa. Besides determination of the diversity and abundance of amoeboid and flagellated protists, we evaluated whether they are affected by the identity of the major primary producers in primary producers in biocrusts that are dominated by cyanobacteria, chlorolichens and bryophytes.
Material and methods
The study site was situated in the Succulent Karoo biome, South Africa, in the vicinity of the village Soebatsfontein, about 60 km south of Springbok. The Succulent Karoo biome is a unique dryland system hosting a biodiversity hotspot with an extraordinarily high plant diversity and a unique flora of succulent plants.25 Samples were collected next to the BIOTA observatory in Soebatsfontein (observatory number S22 at 30.19° S, 17.54° E, altitude 392 m). The hilly region comprises soils of sandy texture, some granite inselbergs and a dense pattern of fossil termite mounds.26 The semi-arid climate of the region is characterised by mild winter and hot summer conditions, with air temperatures below 2 °C in July and sometimes above 44 °C in February.27 The study site is located in the winter rainfall area, with a mean of 129 mm rainfall which falls mainly during the cool winter months (July to August) and a second smaller peak in autumn (April to May). The study area is densely covered by diverse communities of biocrusts, reaching an overall surface coverage above 25% in regions without inselbergs and roads.28
Biocrust communities and samp/ing
Biocrust communities within southern Africa have been divided into seven main biocrust types,12 of which we investigated three. First we analysed well-established cyanobacteria-dominated biocrusts, which are characterised by a more-or-less uniform dark surface coloration caused by cyanobacteria growing close to the surface. When being removed, the biocrust forms relatively large flakes of up to 3.9 mm thick. Dominating cyanobacterial genera are Nostoc, Phormidium, Scytonema, Microco/eus and Lepto/yngbya, with the latter two already occurring in early, initially formed biocrusts. As a second type we investigated chlorolichen-dominated biocrusts, which can only form on stabilised surfaces, normally developing from well-established cyanobacteria-dominated biocrusts12. The dominating chlorolichen species was Psora decipiens, which has a particularly wide geographical distribution and occurs frequently within the study area11. Bryophyte-dominated biocrusts were the third type we analysed. This biocrust type is a late-successional stage that develops from previously well-established biocrusts, and because bryophytes require somewhat more water than the other biocrust components, they frequently occur in the shade or vicinity of small shrubs. The dominating moss species in these samples was Ceratodon purpureus, a small species with an almost global distribution29.
The three different types of biocrusts were collected in 10-cm Petri dishes, with five replicates each. For each sample, the lower lid of the Petri dish was pressed approximately 1 cm deep into the soil, then a trowel was pushed below the lid and together with the sample was lifted from the surrounding soil. The Petri dish was carefully turned around, surplus soil was removed, and the dish was covered with the upper lid, which was subsequently sealed with parafilm and taping band. As the biocrusts were completely dry during sampling, no additional drying of the samples was necessary. The samples were transported to Germany and stored at 4 °C in the dark for 6 weeks.
Microbial determination of taxonomic units
Protist abundance and community composition were assessed by a liquid aliquot method according to Butler and Rogerson30. Briefly, 1 g of a homogenised surface soil (uppermost 2 mm) was suspended in 350 ml of sterile distilled water and shaken for 20 min. For incubation, the suspension was diluted by a factor of 4, and 20 μΙ. of the suspension was added to 180 μΙ. of wheat grass medium (WG). The WG was made by adding 0.15% dried wheat grass powder (Weizengras, Sanatur GmbH, D-78224 Singen) to PJ medium31. In total 144 wells per sample were stored at 15 °C in the dark. The plates were inspected for protists after 7 and 21 days using an inverted microscope (Nikon Eclipse TS100) at 100x and 400x magnification. Protist morphotypes were determined according to Jeuck and Arndt32, Bass et al.33, Smirnov34 and Smirnov and Brown35.
Protist diversity was determined by the Shannon-Weaver-Index36:

where pi is the proportion of the morphotype.
Furthermore, the evenness was calculated by the following formula:

where S is the total number of morphotypes.
Change in protist abundance between treatments was analysed by analysis of variance (ANOVA) followed by Tukey tests, using R software (Version 3.1.0; package: agricolae).
Results
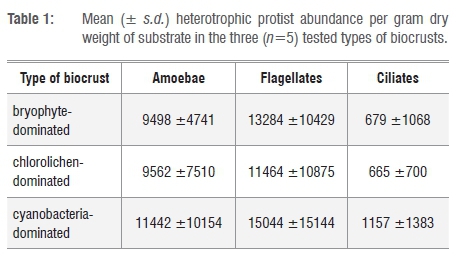
The investigation of biocrust samples revealed abundances between 21 χ 103 and 27 χ 103 individuals per gram dry weight for the cultivable heterotrophic protist communities. However, numbers varied widely among the samples, from 7.0 χ 103 to 56 χ 103 individuals per gram dry weight. We observed similar abundances for flagellates and amoebae, followed by ciliates (Table 1).
The investigation of the biocrusts revealed high protist diversity in soil crusts, with 23 different morphotypes from various morphologies such as testate amoebae, naked amoebae, flagellates, amoeboflagellates and ciliates (Figure 1).
Independent of sampling sites, Spume//a-like chrysomonads, gliding bodonids (Neobodo- and Parabodo-like), glissomonads and heterolo-boseans were the most abundant groups of protists. Among the less abundant protists, we found high diversities enabling a high taxonomic resolution of amoeboid organisms (Figure 1). Diversity and evenness values were similar among all types of biocrusts, with an average Shannon-Weaver index of H=2.1 ± 0.1 (F=0.36, p=0.71) and evenness of 0.83 ± 0.5 (F=0.74, p=0.50). There was no significant difference in morphotype composition of protists among different biocrust types (data not shown).
Discussion
Protist abundance
Protist abundance ranged between 7.0 χ 103 and 56 χ 103 individuals per gram dry weight, with amoebae and flagellates sharing similar abundances (12.5 χ 103 and 16 χ 103 individuals per gram dry weight, respectively). These values were more than 10 times higher than protist abundances reported from biocrusts of the Colorado Plateau and the Chihuahua Desert in the Western USA13, and up to more than 3 to 30 times higher than Bamforth37 reported (ciliates and flagellates) for arid desert soils and litters of northern Arizona. However, total protist abundances were low compared with those in studies of soils in temperate regions. For example, Domonell et al.38 reported protist abundances in the range of 17 χ 103 to 127 χ 103 individuals per gram dry weight for grassland and forest soils in Germany, whereas Finlay et al.39 reported soil heterotrophic (flagellated) protist abundances of up to 539 χ 103 individuals per gram dry weight for grassland soils, and up to 417 χ 103 individuals per gram dry weight in forest soils, respectively.
Although we homogenised our sampled soil to minimise the effect of microscale differences, variation in protist abundances (by a factor of 8) was nevertheless very high in biocrusts. This indicates large differences in the small-scale distribution patterns of soil protists in these mostly dry soil systems. It is known that protist abundance tends to vary with a number of environmental parameters such as local humidity, distance to vascular plants, disturbance and distribution of bacterial food.17,18,40,41
In the Succulent Karroo, short periods of moisture (soils are wet for roughly 45 days per year42) and large temperature extremes between day and night27 mean that the protist community is likely to be influenced more by an accumulation of morning dew during the night than by periodic rainfall events43. Small protists with a rapid life cycle will have an adaptive advantage under these conditions.
All the protists we identified were cyst-forming bacterivores. Cysts enable protists to tolerate frequent wetting-drying cycles17 and must be considered a major functional adaptation in desert protists37,44. Non-encysting protists that feed on cyanobacterial filaments identified in cyanobacterial crusts of Australia are likely an exception.20 Cysts of some protist taxa are viable over decades45,46 and will gradually accumulate under conditions where microbial production and environmental conditions are favourable. Cultivation-based methods therefore estimate the abundance of cysts together with active stages of protists, which can be regarded as an integrated measure of past microbial production in these soils.
Protist diversity
The diversity of heterotrophic protists in biocrusts comprised 'typical' soil protists such as acanthamoebae, vermamoebae and cercozoans (especially glissomonads and cercomonads), as well as less-frequently found and likely rarer taxa such as apusomonads.39 A high abundance of glissomonads and heteroloboseans is commonly observed in soils, but the general lack of thaumatomonads and vannellids was quite surprising. It has recently been shown that protist diversity responds to changes in soil dryness, especially with regard to larger protists, which quickly disappear with decreasing soil moisture content.47 Prolonged dryness therefore might have far-reaching negative effects on some protist groups, but more detailed studies are necessary to confirm this possibility.
Knowledge on the functional roles of protists is particularly scarce. Generally, chrysomonads, bicosoecids and some bodonids are considered as interception feeders in biofilms.48,49 They create a water flow with their flagella to capture bacteria in water films.48 Organisms that depend on a water current for feeding may be considered more strongly moisture-dependent than (for example) amoebae that graze within biofilms.49 Therefore it was not surprising that bicosoecids and the mostly swimming or sessile bodonids, such as Bodo saltans, were missing from our samples. However, Spumella-like chrysomonads, which usually show high abundances in soil systems,38,39 could be confirmed for soil crusts.
Most amoebae and amoeboflagellates (Cercomonads, i.e. Cercomonas-like and Paracercomonas-like amoeboflagellates) can attach to particles and feed on bacteria in biofilms and in tiny soil pores,49 resulting in relatively high abundances in drier soil. In addition, Darby et al.50found amoebae (rather than nematodes and other protists such as ciliates and flagellates) to be highly tolerant to extreme environmental conditions, including increased temperatures and altered precipitation. In line with these results, our findings also showed that amoebae and amoeboflagellates were the most abundant functional groups in our study.
The comparison of protist diversity among the three different biocrust types revealed no statistically significant differences. This finding indicates that the different macroscopic soil crust builders might have no major effect on bacterivorous protist community composition. However, data on protists in soil are too scarce for us to propose a general conclusion. To our knowledge, ours is the first study on protist communities in biocrusts on the African continent, and is one of few studies that give detailed quantitative estimates on the cultivable amoeboid and flagellated protists.13,38,39,51 Future studies need to include molecular approaches in order to estimate the non-cultivable taxa to the protist community.
Conclusion
This is the first detailed study on protist abundance and their morphotypes in biocrusts on the African continent. The diversity of protists was high, comprising solely cyst-forming protists. The abundance of heterotrophic protists in biocrusts was found to be more than 10 times higher than in comparative studies on desert biocrusts in the south-western USA. Protist diversity and abundance varied substantially among samples, and there was no significant difference between types of biocrusts. This indicates that protist abundance and diversity are regulated at much smaller spatial scales (e.g. within single pores or on single leaves), and that the macroscopically visible biocrust components are likely of little relevance for protist occurrence. Further studies, especially on the combination of taxonomic and next-generation sequencing techniques, are necessary to characterise the protist communities of arid soil systems.
Acknowledgements
This study was supported by the German Research Foundation (project numbers WE 2393/2-1, WE 2393/2-2) and the Max Planck Society (Nobel Laureate Fellowship). Research in South Africa was conducted with South African research (No. 86/2013) and export permits (No. 87/2013).
Authors' contributions
K.D. performed the experimental analyses and wrote the article, R.K. and M.B. guided the experimental analyses and gave support in writing the article, and B.W. collected the samples and provided substantial assistance with writing the article. B.W. and M.B. contributed to an equal extent in expertise, time and resources for this article.
References
1. Belnap J, Büdel B, Lange OL. Biological soil crusts: Characteristics and distribution. In: Belnap J, Lange OL, editors. Ecological studies 150: Biological soil crusts: Structure, function, and management. Berlin, Heidelberg, New York: Springer; 2003. p. 3-30. http://dx.doi.org/10.1007/978-3-642-56475-8 [ Links ]
2. Belnap J, Gillette DA. Disturbance of biological soil crusts: Impacts on potential wind erodibility of sandy desert soils in Southeastern Utah. Land Degrad Dev. 1997;8(4):355-362. http://dx.doi.org/10.1002/(SICI)1099-145X(199712)8:4<355::AID-LDR266>3.0.CO;2-H [ Links ]
3. Gaskin S, Gardner R. The role of cryptogams in runoff and erosion control on bariland in the Nepal Middle Hills of the Southern Himalaya. Earth Surf Proc Land. 2001;6(12):1303-1315. http://dx.doi.org/10.1002/esp.277 [ Links ]
4. Zhao Y Qin N, Weber B, Xu M. Response of biological soil crusts to raindrop erosivity and underlying influences in the hilly Loess Plateau region, China. Biodivers Conserv. 2014;23(7):1669-1686. http://dx.doi.org/10.1007/s10531-014-0680-z [ Links ]
5. Belnap J. The potential roles of biological soil crusts in dryland hydrologic cycles. Hydrol Process. 2006;20(15):3159-3178. http://dx.doi.org/10.1002/hyp.6325 [ Links ]
6. Chamizo S, Cantón Y Domingo F, Belnap J. Evaporative losses from soils covered by physical and different types of biological soil crusts. Hydrol Process. 2013;27(3):324-332. http://dx.doi.org/10.1002/hyp.8421 [ Links ]
7. Eldridge DJ, Zaady E, Shachak M. Infiltration through three contrasting biological soil crusts in patterned landscapes in the Negev, Israel. Catena. 2000;40(3):323-336. http://dx.doi.org/10.1016/S0341-8162(00)00082-5 [ Links ]
8. Evans RD, Lange OL. Biological soil crusts and ecosystem nitrogen and carbon dynamics. In: Belnap J, Lange OL editors. Ecological studies 150: Biological soil crusts: Structure, function, and management. Berlin, Heidelberg, New York: Springer; 2003. p. 263-279. [ Links ]
9. Porada P Weber B, Elbert W, Pöschl U, Kleidon A. Estimating global carbon uptake by lichens and bryophytes with a process-based model. Biogeosc. 2013;10(11):6989-7033. http://dx.doi.org/10.5194/bg-10-6989-2013 [ Links ]
10. Porada P, Weber B, Elbert W, Pöschl U, Kleidon A. Estimating impacts of lichens and bryophytes on global biogeochemical cycles. Global Biogeochem Cy. 2014;28(2):71-85. http://dx.doi.org/10.1002/2013GB004705 [ Links ]
11. Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, et al. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nat Geosc. 2012;5:459-462. http://dx.doi.org/10.1038/ngeo1486 [ Links ]
12. Büdel B, Darienko T, Deutschewitz K, Dojani S, Friedl T, Mohr K, et al. Southern African biological soil crusts are ubiquitous and highly diverse in drylands, being restricted by rainfall frequency. Microbial Ecol. 2009;57(2):229-247. http://dx.doi.org/10.1007/s00248-008-9449-9 [ Links ]
13. Darby BJ, Housman DC, Zaki AM, Shamout Y Adl,SM Belnap J, et al. Effects of altered temperature and precipitation on desert protozoa associated with biological soil crusts. J Eukaryot Microbiol. 2006;53(6):507-514. http://dx.doi.org/10.1111/j.1550-7408.2006.00134.x [ Links ]
14. Darby BJ, Neher DA. Stable isotope composition of microfauna supports the occurrence of biologically fixed nitrogen from cyanobacteria in desert soil food webs. J Arid Environ. 2012;85(1):76-78. http://dx.doi.org/10.1016/j.jaridenv.2012.06.006 [ Links ]
15. Shachak M, Steinberger YAn algae - desert snail food chain: Energy flow and soil turnover. Oecologia. 1980;46(3):402-411. http://dx.doi.org/10.1007/BF00346271 [ Links ]
16. Shepherd UL, Brantley SL, Tarleton CA. Species richness and abundance patterns of microarthropods on cryptobiotic crusts in a pinon-juniper habitat: A call for greater knowledge. J Arid Environ. 2012;52(3):349-360. http://dx.doi.org/10.1006/jare.2002.1003 [ Links ]
17. Adl MS, Gupta V. Protists in soil ecology and forest nutrient cycling. Can J Forest Res. 2016;36(7):1805-1817. http://dx.doi.org/10.1139/x06-056 [ Links ]
18. Bonkowski M. Protozoa and plant growth: The microbial loop in soil revisited. New Phytol. 2014;162(3):617-631. http://dx.doi.org/10.1111/j.1469-8137.2004.01066.x [ Links ]
19. Rodriguez-Zaragoza S, Mayzlish E, Steinberger Y. Vertical distribution of the free-living amoeba population in soil under desert shrubs in the Negev Desert, Israel. Appl Environ Microb. 2005;71(4):2053-2060. http://dx.doi.org/10.1128/AEM.71.4.2053-2060.2005 [ Links ]
20. Robinson BS, Bamforth SS, Dobson PJ. Density and diversity of protozoa in some arid australian soils. J Eukaryot Microbiol. 2002;49(6):449-153. http://dx.doi.org/10.1111/j.1550-7408.2002.tb00227.x [ Links ]
21. Colesie C, Green TGA, Turk, R, Hogg ID, Sancho LG, Budel B. Terrestrial biodiversity along the Ross Sea coastline, Antarctica: Lack of a latitudinal gradient and potential limits of bioclimatic modeling. Polar Biol. 2014;37(8):1197-1208. http://dx.doi.org/10.1007/s00300-014-1513-y [ Links ]
22. Bamforth SS, Wall DH, Virginia RA. Distribution and diversity of soil protozoa in the McMurdo Dry Valleys of Antarctica. Polar Biol. 2005;28(10):756-762. http://dx.doi.org/10.1007/s00300-005-0006-4 [ Links ]
23. Liu Y, Li X, Jia R, Huang L, Zhou Y, Gao Y. Effects of biological soil crusts on soil nematode communities following dune stabilization in the Tengger Desert, Northern China. Appl Soil Eco. 2011;49(1):118-124. http://dx.doi.org/10.1016/j.apsoil.2011.06.007 [ Links ]
24. Foissner W, Agatha S, Berger H. Soil ciliates (Protozoa, Ciliophora) from Namibia (Southwest Africa), with emphasis on two contrasting environments, the Etosha Region and the Namib Desert. Denisia. 2002;5:1-1459. [ Links ]
25. Brooks TM, Mittermeier RA, Mittermeier CG, Da Fonseca GAB, Rylands AB, Konstant WR, et al. Habitat loss and extinction in the hotspots of biodiversity. Conserv Biol. 2002;16(4):909-923. http://dx.doi.org/10.1046/j.1523-1739.2002.00530.x [ Links ]
26. Weber B, Graf T, Bass M. Ecophysiological analysis of moss-dominated biological soil crusts and their separate components from the Succulent Karoo, South Africa. Planta. 2012;236(1):129-139. http://dx.doi.org/10.1007/s00425-012-1595-0 [ Links ]
27. BIOTA Southern Africa, weather data station: Soebatsfontein, as at 10 March 2011. Available at: http://www.biota-africa.org [ Links ]
28. Weber B, Olehowski C, Knerr T, Hill J, Deutschwitz K, Wessels DCJ, et al. A new approach for mapping of biological soil crusts in semidesert areas with hyperspectral imagery. Remote Sens Environ. 2008;112(5):2187-2201. http://dx.doi.org/10.1016/j.rse.2007.09.014 [ Links ]
29. Ireland RR. Moss flora of the Maritime provinces. Publications in Botany, No. 13. National Museums of Canada. Ottawa 1982 [ Links ]
30. Butler H, Rogerson A. Temporal and spatial abundance of naked amoebae (Gymnamoebae) in marine benthic sediments of the Clyde Sea Area, Scotland. J Eukaryot Microbiol. 1995;42(6):724-730. http://dx.doi.org/10.1111/j.1550-7408.1995.tb01624.x [ Links ]
31. Prescott DM, James TW. Culturing of Amoeba proteus on Tetrahymena. Exp Cell Res. 1955;8(1):256-258. http://dx.doi.org/10.1016/0014-4827(55)90067-7 [ Links ]
32. Jeuck A, Arndt H. A short guide to common heterotrophic flagellates of freshwater habitats based on the morphology of living organisms. Protist. 2013;164(6):842-860. http://dx.doi.org/10.1016/j.protis.2013.08.003 [ Links ]
33. Bass D, Howe AT, Mylnikov AP Vickerman K, Chao EE, Smallbone JE, et al. Phylogeny and classification of Cercomonadida (Protozoa, Cercozoa): Cercomonas, Eocercomonas, Paracercomonas, and Cavernomonas gen. nov. Protist. 2009;160(4):483-521. http://dx.doi.org/10.1016/j.protis.2009.01.004 [ Links ]
34. Smirnov A. A revised classification of naked lobose amoebae (Amoebozoa: Lobosa). Protist. 2011;162(4):545-570. http://dx.doi.org/10.1016/j.protis.2011O4.004 [ Links ]
35. Smirnov A, Brown S. Guide to the methods of study and identification of soil gymnamoebae. Protistology. 2004;3(3):148-190. [ Links ]
36. Shannon CE, Weaver W. The mathematical theory of communication. Illinois: University of Illinois Press; 1949. [ Links ]
37. Bamforth SS. Microbial distributions in Arizona deserts and woodlands. Soil Biol & Biochem. 1984;16(2):133-137. http://dx.doi.org/10.1016/0038-0717(84)90103-2 [ Links ]
38. Domonell A, Brabender M, Nitsche F, Bonkowski M, Arndt H. Community structure of cultivable protists in different grassland and forest soils of Thuringia. Pedobiologia. 2013;56(1):1-7. http://dx.doi.org/10.1016/j.pedobi.2012.07.001 [ Links ]
39. Finlay BJ, Black HIJ, Brown S, Clarke KJ, Esteban GF, Hindle RM, et al. Estimating the growth potential of the soil protozoan community. Protist. 2000;151(1):69-80. http://dx.doi.org/10.1078/1434-4610-00008 [ Links ]
40. Adl MS, Coleman DC. Dynamics of soil protozoa using a direct count method. Biol Fert Soils. 2005;42(2):168-171. http://dx.doi.org/10.1007/s00374-005-0009-x [ Links ]
41. Clarholm M, Popovic B, Rosswall T, Soderstrom B, Sohlenius B, Staaf H, et al. Biological aspects of nitrogen mineralization in humus from a pine forest podsol incubated under different moisture and temperature conditions. Oikos. 1981;37(2):137-145. http://dx.doi.org/10.2307/3544457 [ Links ]
42. Weber, personal communication.
43. Mueller JA, Mueller WPP Colpoda cucullus: A terrestrial aquatic. Am Midl Nat. 1970;84(1):1- 12. http://dx.doi.org/10.2307/2423721 [ Links ]
44. Bamforth SS. Water film fauna of microbiotic crusts of a warm desert. J Arid Environ. 2004;56(3):413-423. http://dx.doi.org/10.1016/S0140-1963(03)00065-X [ Links ]
45. Goodey T. Note on the remarkable retention of vitality by protozoa from old stored soils. Ann Appl Biol. 1915;1(3-4):395-399. http://dx.doi.org/10.1111/j.1744-7348.1915.tb08008.x [ Links ]
46. Moon-van der Staay SY Tzeneva VA, Van Der Staay GWM, De Vos WM, Smidt H, Hackstein JHP. Eukaryotic diversity in historical soil samples. FEMS Microbiol Ecol. 2006;57(1):420-428. http://dx.doi.org/10.1111/j.1574-6941.2006.00130.x [ Links ]
47. Geisen S, Bandow C, Römbke J, Bonkowski M. Soil water availability strongly alters the community composition of soil protists. Pedobiologia. 2014;57(4-6):205-213. http://dx.doi.org/10.1016/j.pedobi.2014.10.001 [ Links ]
48. Boenigk J, Arndt H. Bacterivory by heterotrophic flagellates: Community structure and feeding strategies. Anton Leeuw J Microb. 2002;81(1):465-480. http://dx.doi.org/10.1023/A:1020509305868 [ Links ]
49. Parry JD. Protozoan grazing of freshwater biofilms. Adv Appl Microbiol. 2004;54(1):167-195. http://dx.doi.org/10.1016/S0065-2164(04)54007-8 [ Links ]
50. Darby BJ, Neher DA, Housman DC, Belnap J. Few apparent short-term effects of elevated soil temperature and increased frequency of summer precipitation on the abundance and taxonomic diversity of desert soil micro-and meso-fauna. Soil Biol & Biochem. 2001;43(7):1474-1481. http://dx.doi.org/10.1016/j.soilbio.2011.03.020 [ Links ]
51. Ekelund F, R0nn R, Griffiths BS. Quantitative estimation of flagellate community structure and diversity in soil samples. Protist. 2001;152(4):301-314. http://dx.doi.org/10.1078/1434-4610-00069 [ Links ]
 Correspondence:
Correspondence:
Kenneth Dumack
University of Cologne
Zülpicher Strasse 47B, Cologne NRW 50674
Germany
kenneth.dumack@uni-koeln.de
Received: 13 Aug. 2015
Revised:01 Feb. 2016
Accepted: 23 Feb. 2016














