Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.112 no.3-4 Pretoria mar./abr. 2016
http://dx.doi.org/10.17159/sajs.2016/20150393
RESEARCH LETTER
Dental microwear differences between eastern and southern African fossil bovids and hominins
Peter S. UngarI; Jessica R. ScottII; Christine M. SteiningerIII, IV
IDepartment of Anthropology, University of Arkansas, Fayetteville, Arkansas, USA
IIDepartment of Sociology and Anthropology, University of Arkansas, Little Rock, Arkansas, USA
IIIEvolutionary Studies Institute, School of Geosciences, University of the Witwatersrand, Johannesburg, South Africa
IVCentre of Excellence in Palaeosciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Dental microwear has proven to be a valuable tool for reconstructing diets of fossil vertebrates. However, recent studies have suggested that the pattern of microscopic scratches and pits on teeth may be more reflective of environmental grit than of food preferences. Could differences in dental microwear between early hominins, for example, therefore be a result of dust level rather than of diet? We investigated this possibility using a palaeocommunity approach. We compared microwear texture differences between eastern and southern African Hominini, along with Plio-Pleistocene specimens representing two tribes of bovids, Alcelaphini and Antilopini, from the same deposits as the early hominins. If exogenous grit swamps diet signals, we would expect community-wide microwear patterns separating samples by region. Results indicate that each of the three tribes shows a different pattern of variation of microwear textures between eastern and southern Africa. These results imply that differences in microwear reflect diet rather than grit load, and that microwear can provide valuable information not just about environmental dust level, but about food preferences of fossil vertebrates.
Keywords: grit; diet; habitat; fossil ruminants; tooth wear
Dental microwear is an important tool for reconstructing diets of fossil vertebrates, from Palaeozoic conodonts1 to Plio-Pleistocene hominins2. Microwear researchers have noted consistent and predictable relationships between pattern and behaviour in extant taxa from fishes3 to humans4; and these relationships have been used as a baseline to infer the diet of extinct species from their teeth. The basic assumption for mammalian cheek teeth has been that hard foods are crushed, causing pitting as opposing surfaces are pressed together, whereas tough foods are sheared, causing scratches as abrasives are drawn along opposing surfaces that slide past one another.5 For example, primates that eat hard nuts and palm fronds tend to have more microscopic dental pits than primates that eat tough leaves.6 This diet-microwear pattern association has been used to infer feeding behaviours of many fossil species, including early hominins.7
However, a recent study8 has called into question the efficacy of microwear as a proxy for diet, suggesting that experimental validation is needed to affirm relationships between pattern and foods eaten. For example, in an in vitro wear simulation study, Lucas and coauthors9 found that while quartz dust on foods can easily wear tooth enamel, phytoliths within them might not. This finding has led some to suggest that grit in the environment may be more important to wear pattern than factors intrinsic to items eaten.10 In fact, Strait et al.8 argued that microwear patterns for early hominins may reflect the dustiness of the environment and, 'say little about the nature of the foods themselves'8(p.348). The argument follows that the more striated and less pitted microwear seen for Plio-Pleistocene hominins from eastern Africa than those from southern Africa7 may have more to do with where they lived than what they ate. This possibility has important implications not only for studies of early hominins, but also for the countless other fossil vertebrates for which microwear has been documented and related to diet.11
Strait et al.8 suggested that quartz dust might cause heavy microwear pitting, and Williams12 opined that exogenous grit could lead to especially complex surface textures. These suggestions do not explain the lack of such pitting on Paranthropus boisei teeth, which have been suggested to evince extreme macrowear indicative of a gritty, abrasive environment.10 But they do raise the question: Is there a consistent relationship between environmental-grit level and microwear? While it is clear that soil quartz levels can play an important role in tooth wear13,14, studies of mammals living in different settings today have failed to find that grit obscures diet-related microwear signals15.
But what about the differences between Plio-Pleistocene hominins from eastern and southern Africa? If diet signals are 'swamped' by grit, it should be the case not only for early hominins, but also for other taxa. We predicted that, if environmental grit load explains the variation, other large-bodied, terrestrial mammals in the deposits with early hominins should show similar differences in microwear pattern between southern and eastern African samples.
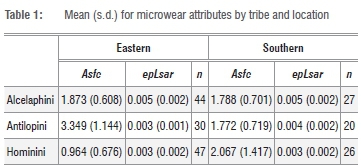
We compared dental microwear textures of alcelaphin and antilopin fossil bovids, along with published data for hominins16-20 found at the same sites. The bovid data were originally presented in Scott21 and Steininger22 |see Appendix 1 in the supplementary material). These tribes were selected because they are common at Plio-Pleistocene fossil sites in both eastern and southern Africa, and because extant representatives have very different dietary patterns.23,24 Extant alcelaphins are predominantly grazers, although some consume browse when grass is scare. Antilopins, in contrast, include the whole gamut from obligate grazers to obligate browsers; and these differences are clearly reflected in dental microwear texture patterns |Supplementary table 1).
Original specimens were cleaned with alcohol-soaked cotton swabs, and microwear impressions were taken on first or second molar teeth using President's Jet regular body polyvinyl-siloxane dental impression material (Coltène-Whaledent Inc, Cuyahoga Falls, OH, USA). Replicas were poured using Epotek 301 high-resolution epoxy and hardener (Epoxy Technologies, Billerica, MA, USA) and examined for post-mortem damage. Those specimens preserving ante-mortem microwear were scanned using the Sensofar plμ standard white-light confocal profilometer at the University of Arkansas to obtain point clouds representing four adjoining fields. The lateral (χ, y) sampling interval was 0.18 μm, vertical (z) resolution was 0.005 μm and field of view for each scan was 138x102 μm.
Observable artifacts, such as dust, were deleted electronically and the point clouds were imported into ToothFrax (Surfract Corp, www.surfract.com) to determine area-scale fractal complexity (Asfc) and length-scale anisotropy of relief (epLsar). Surfaces with pits of varying sizes tend to have high Asfc values, whereas those dominated by aligned scratches have higher epLsar values. These variables were chosen because previous studies have shown that browsing bovids have higher average Asfc values whereas grazers have higher epLsar values.25-27 In fact, these attributes together effectively parse extant bovids into Gagnon and Chew's28 fine-scale diet categories29: (1) obligate grazers (>90% monocots); (2) variable grazers (60-90% monocots); (3) browser-grazer intermediates (30-70% monocots and dicots, including some fruit); (4) generalists (>20% of each of the three food types); (5) browsers (>70% dicots only, part fruit); and (6) frugivores (>70% fruits).
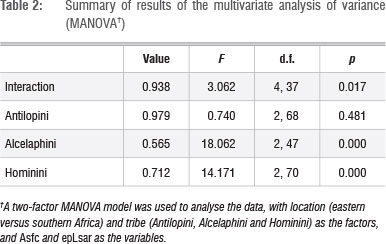
Median values of the four scans of each specimen were calculated, and the final data set was rank-transformed to mitigate violation of assumptions inherent to parametric study (see Scott et al.30 for details). A two-factor multivariate analysis of variance (MANOVA) model was used to analyse the data, with location (eastern versus southern Africa) and tribe (Antilopini, Alcelaphini and Hominini) as the factors, and Asfc and epLsar as the variables.
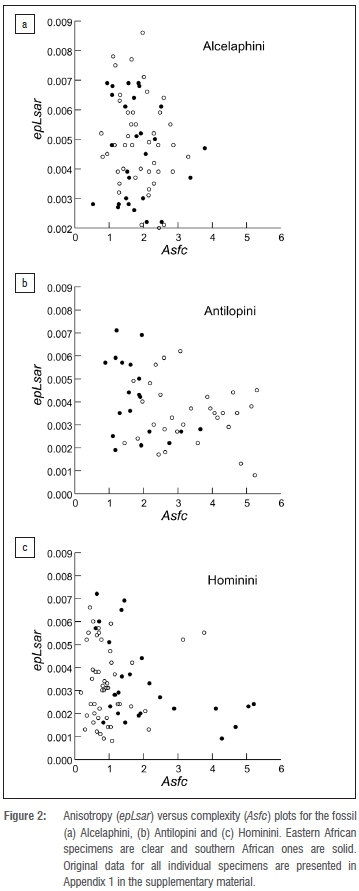
Results showed a significant interaction between location and tribe, indicating that the pattern of differences between eastern and southern African specimens varied between alcelaphins, antilopins and hominins. There was no significant difference in microwear texture between southern and eastern African alcelaphins (Tables 1 and 2; Figures 1 and 2). On the other hand, there was significant variation by location for both the antilopins and hominins considered. The differences were, however, in opposite directions: antilopins from eastern Africa had higher complexity values on average than those from southern Africa, whereas hominins from southern Africa had higher complexity averages than those from eastern Africa. The hominin pattern holds for both Australopithecus and Paranthropus samples.7
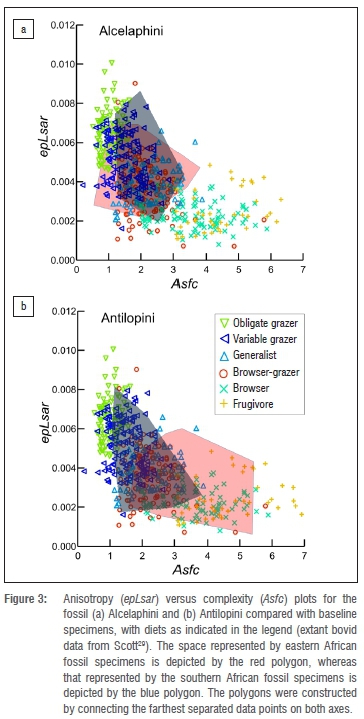
Further, when we superimpose the fossil bovid data on a microwear texture plot for extant species (Figure 3), the ranges of values for extinct alcelaphins and antilopins from southern Africa overlap primarily with variable grazers, whereas that for antilopins from eastern Africa covers much of the extant browser space.
These results indicate that fossil alcelaphins, antilopins and hominins from southern Africa and those from eastern Africa do not show similar differences in microwear textures. Therefore, assuming bovid and hominin foods were subject to the same abrasive environments at sites within these regions, dust or grit alone does not explain the microwear differences observed. Given the mix of grazers and browsers among the fossil bovids, and the combination of C3 and C4 isotope signatures of the hominins31, it seems likely that these taxa overlapped in feeding height and concentration of exogenous abrasives on food. Further, the fact that the distributions of microwear texture values for fossil and extant samples closely approximate one another for both the antilopin and alcelaphin tribes (compare Figure 2 to Supplementary figure 1) further supports the idea that differences between southern and eastern African hominins are not explained by grit or dust load. Finally, while it is possible that differences in masticatory biomechanics, mineralisation and enamel microstructure could complicate interpretations of differences in patterns seen between hominins and bovids, it is unlikely that these explain the differences between the antilopins and alcelaphins given that microwear differences so strongly mirror diet differences in extant species of these tribes (Supplementary figure 1).
In sum, while grit undoubtedly impacts tooth wear9,13,14, the lack of a consistent location signal among the tribes suggests that differences in microwear between eastern and southern African hominins are likely not a result of abrasive load alone. Diet remains the most plausible explanation for the variation in dental microwear among species. While Sanson et al.32 and Lucas et al.9 argued that exogenous grit is the operative wear agent for teeth because endogenous silicates within plant foods (phytoliths) are softer than enamel, it is clear that there is more to tooth wear than abrasive hardness. Because hydroxyapatite crystallites are attached to one another by a thin layer of protein 'glue', tissue removal requires only that contact pressure be sufficient to break the bonds holding enamel together. Indeed, tissue removal is achieved with particles much softer than enamel.33 In this light, it makes sense that primates known to consume phytolith-rich foods tend to have thicker tooth enamel34, that tell-tale siliceous plant opals have been found embedded in tooth enamel at the ends of microwear scratches35, and that experimental studies show cereals with different phytolith loads leave different microwear patterns36.
Acknowledgements
The hominin data were collected originally in collaboration with Robert Scott, Fred Grine, Mark Teaford, Kristin Krueger and Alejandro Pérez-Pérez. Funding for data collection came from the US National Science Foundation (PS.U. and J.R.S.) and the South African National Research Foundation, DST-NRF Centre of Excellence in Palaeosciences and Palaeontological Scientific Trust (C.M.S.). We thank three anonymous reviewers and Francis Thackeray for their helpful comments on an earlier version of this manuscript, and the curators at the various museums who allowed us to study specimens in their care.
Authors' contributions
P.S.U., J.R.S. and C.M.S. gathered the data, analysed the results and wrote the manuscript. PS.U. was the project leader.
References
1. Purnell MA. Microwear on conodont elements and macrophagy in the first vertebrates. Nature. 1994;374:798-800.http://dx.doi.org/10.1038/374798a0 [ Links ]
2. Grine FE. Trophic differences between 'gracile' and 'robust' australo-pithecines: A scanning electron microscope analysis of occlusal events. S Afr J Sci. 1981;77:203-230. [ Links ]
3. Purnell M, Seehausen O, Galis F. Quantitative three-dimensional microtextural analyses of tooth wear as a tool for dietary discrimination in fishes. J R Soc Interface. 2012;9:2225-2233. http://dx.doi.org/10.1098/rsif.2012.0140 [ Links ]
4. Krueger KL. Reconstructing diet and behavior in bioarchaeological groups using incisor microwear texture analysis. J Archaeol Sci Reports. 2015;1:29-37. http://dx.doi.org/10.1016/j.jasrep.2014.10.002 [ Links ]
5. Hua L-C, Brandt ET, Meullenet J-F, Zhou Z-R, Ungar PS. Technical note: An in vitro study of dental microwear formation using the BITE Master II chewing machine. Am J Phys Anthropol. 2015;158(4):769-775. http://dx.doi.org/10.1002/ajpa.22823 [ Links ]
6. Teaford MF, Walker A. Quantitative differences in dental microwear between primate species with different diets and a comment on the presumed diet of Sivapithecus. Am J Phys Anthropol. 1984;64:191-200. http://dx.doi.org/10.1002/ajpa.1330640213 [ Links ]
7. Ungar PS, Sponheimer M. The diets of early hominins. Science. 2011;334:190-193. http://dx.doi.org/10.1126/science.1207701 [ Links ]
8. Strait DS, Constantino P Lucas PW, Richmond BG, Spencer MA, Dechow PC, et al. Viewpoints: Diet and dietary adaptations in early hominins: the hard food perspective. Am J Phys Anthropol. 2013;151:339-355. http://dx.doi.org/10.1002/ajpa.22285 [ Links ]
9. Lucas PW, Omar R, Al-Fadhalah K, Almusallam AS, Henry AG, Michael S, et al. Mechanisms and causes of wear in tooth enamel: Implications for hominin diets. J R Soc Interface. 2013;10(80), Art. #20120923, 7 pages. http://dx.doi.org/10.1098/rsif.2012.0923 [ Links ]
10. Wood B. Palaeontology: Gritting their teeth. Nature. 2013;493:486-487. http://dx.doi.org/10.1038/493486a [ Links ]
11. Ungar PS. Mammalian dental function and wear: A review. Biosurf Biotribol. 2015;1:25-41. http://dx.doi.org/10.1016/j.bsbt.2014.12.001 [ Links ]
12. Williams FL. Dietary proclivities of Paranthropus robustus from Swartkrans, South Africa. Anthropol Rev. 2015;78:1-19. http://dx.doi.org/10.1515/anre-2015-0001 [ Links ]
13. Galbany J, Romero A, Mayo-Alesón M, Itsoma F, Gamarra B, Pérez-Pérez A, et al. Age-related tooth wear differs between forest and savanna primates. PLoS One. 2014;9(4), e94938, 7 pages. http://dx.doi.org/10.1371/journal.pone.0094938 [ Links ]
14. Madden R. Hypsodonty in mammals: Evolution, geomorphology, and the role of earth surface processes. Cambridge: Cambridge University Press; 2015. [ Links ]
15. Gomes Rodrigues H, Merceron G, Viriot L. Dental microwear patterns of extant and extinct Muridae (Rodentia, Mammalia): Ecological implications. Naturwissenschaften. 2009;96:537-542. http://dx.doi.org/10.1007/s00114-008-0501-x [ Links ]
16. Scott RS, Ungar PS, Bergstrom TS, Brown CA, Grine FE, Teaford MF, et al. Dental microwear texture analysis shows within-species diet variability in fossil hominins. Nature. 2005;436:693-695. http://dx.doi.org/10.1038/nature03822 [ Links ]
17. Ungar PS, Grine FE, Teaford MF. Dental microwear and diet of the Plio-Pleistocene hominin Paranthropus boisei. PLoS One. 2008;3(4), e2044, 6 pages. http://dx.doi.org/10.1371/journal.pone.0002044 [ Links ]
18. Ungar PS, Scott RS, Grine FE, Teaford MF. Molar microwear textures and the diets of Australopithecus anamensis and Australopithecus afarensis. Phil Trans R Soc Lond. B. 2010;465:3345-3354. http://dx.doi.org/10.1098/rstb.2010.0033 [ Links ]
19. Ungar PS, Krueger KL, Blumenschine RJ, Njau J, Scott RS. Dental microwear texture analysis of hominins recovered by Olduvai Landscape Paleoanthropology Project, 1995-2007. J Hum Evol. 2012;63:429-437. http://dx.doi.org/10.1016/j.jhevol.2011.04.006 [ Links ]
20. Ungar PS, Scott RS. Dental evidence for diets of early Homo. In: Grine FE, Leakey RE, Fleagle JG, editors. The first humans: Origins of the genus Homo. New York: Springer-Verlag; 2009. p. 121-134. http://dx.doi.org/10.1007/978-1-4020-9980-9_11 [ Links ]
21. Scott JR. Dental microwear texture analysis of Pliocene bovids from four early hominin fossil sites in eastern Africa: Implications for paleoenvironmental dynamics and human evolution [PhD dissertation]. Fayetteville, AR: University of Arkansas; 2011. [ Links ]
22. Steininger CM. Dietary behaviour of early Pleistocene bovids from Cooper's Cave and Swartkrans, South Africa [PhD thesis]. Johannesburg: University of the Witwatersrand; 2011. [ Links ]
23. Estes R. The behavior guide to African mammals: Including hoofed mammals, carnivores, primates. Berkeley and Los Angeles, CA: University of California Press; 1991. [ Links ]
24. Skinner J, Chimimba C. The mammals of the southern African subregion. Cambridge: Cambridge University Press; 2005. http://dx.doi.org/10.1017/CBO9781107340992.014 [ Links ]
25. Ungar PS, Merceron G, Scott RS. Dental microwear texture analysis of Varswater bovids and early Pliocene paleoenvironments of Langebaanweg, Western Cape Province, South Africa. J Mamm Evol. 2007;14:163-181. http://dx.doi.org/10.1007/s10914-007-9050-x [ Links ]
26. Merceron G, Escarguel G, Angibault J-M, Verheyden-Tixier H. Can dental microwear textures record inter-individual dietary variations? PLoS One. 2010;5(3), e9542, 9 pages. http://dx.doi.org/10.1371/journal.pone.0009542 [ Links ]
27. Schulz-Kornas E, Calandra I, Kaiser TM. Feeding ecology and chewing mechanics in hoofed mammals: 3D tribology of enamel wear. Wear. 2013;300:169-179. http://dx.doi.org/10.1016/j.wear.2013.01.115 [ Links ]
28. Gagnon M, Chew AE. Dietary preferences in extant African Bovidae. J Mammal. 2000;81(2):490-511. http://dx.doi.org/10.1644/1545-1542(2000)081<0490:DPIEAB>2.0.CO;2 [ Links ]
29. Scott JR. Dental microwear texture analysis of extant African Bovidae. Mammalia. 2012;76:157-174. http://dx.doi.org/10.1515/mammalia-2011-0083 [ Links ]
30. Scott RS, Ungar PS, Bergstrom TS, Brown CA, Childs BE, Teaford MF, et al. Dental microwear texture analysis: Technical considerations. J Hum Evol. 2006;51:339-349. http://dx.doi.org/10.1016/j.jhevol.2006.04.006 [ Links ]
31. Sponheimer M, Alemseged MZ, Cerling TE, Grine FE, Kimbel WH, Leakey MG, et al. Isotopic evidence of early hominin diets. Proc Natl Acad Sci USA. 2013;110(26):10513-10518. http://dx.doi.org/10.1073/pnas.1222579110 [ Links ]
32. Sanson GD, Kerr SA, Gross KA. Do silica phytoliths really wear mammalian teeth? J Archaeol Sci. 2007;34:526-531. http://dx.doi.org/10.1016/j.jas.2006.06.009 [ Links ]
33. Xia J, Zheng J, Huang D, Tian ZR, Chen L, Zhou Z, et al. New model to explain tooth wear with implications for microwear formation and diet reconstruction. Proc Natl Acad Sci USA. 2015;112:10669-10672. http://dx.doi.org/10.1073/pnas.1509491112 [ Links ]
34. Rabenold D, Pearson OM. Abrasive, silica phytoliths and the evolution of thick molar enamel in primates, with implications for the diet of Paranthropus boisei. PLoS One. 2011;6|12), e28379, 11 pages. http://dx.doi.org/10.1371/journal.pone.0028379 [ Links ]
35. Lalueza Fox C, Pérez-Pérez A. Dietary information through the examination of plant phytoliths on the enamel surface of human dentition. J Archaeol Sci. 1994;21:29-34. http://dx.doi.org/10.1006/jasc.1994.1005 [ Links ]
36. Gügel IL, Grupe G, Kunzelmann KH. Simulation of dental microwear: Characteristic traces by opal phytoliths give clues to ancient human dietary behavior. Am J Phys Anthropol. 2001;114:124-138. http://dx.doi.org/10.1002/1096-8644|200102)114:2<124::AID-AJPA1012>3.0.CO;2-S [ Links ]
 Correspondence:
Correspondence:
Peter Ungar
Department of Anthropology, University of Arkansas
Old Main 330, Fayetteville, Arkansas 72701, USA
pungar@uark.edu
Received: 11 Oct. 2015
Revised: 18 Dec. 2015
Accepted: 28 Dec. 2015














