Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.1-2 Pretoria Jan./Feb. 2016
http://dx.doi.org/10.17159/sajs.2016/20150058
RESEARCH ARTICLE
Responses of African elephants towards a bee threat: Its application in mitigating human-elephant conflict
Mduduzi NdlovuI, II; Emma DevereuxII; Melissa ChieffeII; Kendra AsklofII; Alicia RussoII
ISchool of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIOrganisation for Tropical Studies, Skukuza, South Africa
ABSTRACT
Human settlement expansion into elephant ranges, as well as increasing elephant populations within confined areas has led to heightened levels of human-elephant conflict in southern African communities living near protected areas. Several methods to mitigate this conflict have been suggested including the use of bees as an elephant deterrent. We investigated whether bee auditory and olfactory cues (as surrogates for live bees) could be used to effectively deter elephants. We evaluated the responses of elephants in the southern section of the Kruger National Park to five different treatments: (1) control noise, (2) buzzing bee noise, (3) control noise with honey scent, (4) honey scent, and (5) bee noise with honey scent. Elephants did not respond or displayed less heightened responses to the first four treatments. All elephants exposed to the bee noise with honey scent responded with defensive behaviours and 15 out of 21 individuals also fled. We concluded that buzzing bees or honey scent as isolated treatments (as may be the case with dormant beehives) were not effective elephant deterrents, but rather an active beehive emitting a combination of auditory and olfactory cues was a viable deterrent. However, mismatches in the timing of elephant raids and activity of bees may limit the use of bees in mitigating the prevailing human-elephant conflict.
Keywords: Loxodonta africana; South Africa; auditory cues; olfactory cues; behaviour
Introduction
The southern African region accounts for about 40% of the African elephant's (Loxodonta africana) total range area.1-3 Despite measures taken to manage elephant populations in protected areas over the course of the past century, elephant numbers in the region have increased from approximately 170 000 to about 268 000 between 1995 and 2012.1 As a result of growing African elephant populations within protected areas and increased land cultivation bordering these areas (resulting from human population growth and expansion), there have been numerous reported incidences of human-elephant encounters and conflict, particularly in poor rural farming communities.4,5 Human-elephant conflict typically refers to interactions between people and elephants that threaten the lives and livelihoods of both parties involved.6 Beyond growing human and elephant populations, the primary contributing factors to human-elephant conflict in recent years have been increased human settlement and land use change in established elephant migratory corridors. Such human interruptions have in turn affected elephant behaviour and socio-ecology.6
Elephant crop raiding is by far the most common cause of human-elephant conflict in South Africa.4,6 Increased strain on resource availability for growing elephant populations has forced many elephants to leave protected areas and forage on cultivated crops as a means of maximising nutrient intake and reproductive success.7 Consequently, elephant crop raiding has costs for both humans and elephants. The costs to humans include economic losses through destroyed crops, raided food stores, damaged infrastructure and water sources, and disturbed livestock.6 Although incidences of damage by elephant crop raiding are low overall, there have been some occurrences of complete crop devastation,5,6 which can have a substantial influence on the livelihood of the impacted farmer. In some rare cases, crop raiding has also caused injury and loss of human life.6 Costs to elephants are injury or death at the hands of humans.6,7
Considering the high cost of crop raiding for both humans and elephants, a number of deterrent methods have been explored.6,8,9 Some methods of deterring elephants have included the construction of barriers, translocation and the culling of problem elephants.8 Although these management strategies have proven effective in some cases, they are often very expensive, beyond the means of most rural communities and can be ethically controversial.8
Rural farmers have attempted to defend their crops against elephants using traditional methods, such as lighting fires, making loud noises, and throwing stones.8 Past research efforts (e.g. Graham and Ochieng10) investigated the use of warning alarms, loud noisemakers, watchtowers, spotlights, and African birds eye chillies (Capsicum frutescens) in an effort to find an effective deterrent strategy for the management of elephants. Unfortunately, problem elephants would avoid detection by raiding crops at night when people were asleep7 and hence most deterrent strategies were difficult to implement without constant vigilance. Furthermore, some of these methods have proven to be ineffective and only add costs to farmers, for example, the use of chillies.11
Currently, there is a need for an effective, inexpensive, and non-labour intensive method of elephant deterrence for rural communities.9 Based on evidence of elephants' acute hearing capabilities and sensitive olfactory systems, research has begun to focus on deterrents that target elephants' hearing and smell.12,13 Additionally, anecdotal evidence shows that despite their thick skin, elephants have sensitive soft regions (i.e. behind the ears, in the eyes, under the trunk and inner-trunk membranes) vulnerable to African honey bee (Apis mellifera scutellata) stings.9,14 There is a report of a bull elephant in Kenya that became permanently blind after being stung by bees multiple times in the eye.14 King et al.9 recently compared elephant responses to bee audio recordings and white noise recordings. They found that elephants retreated in response to bee noises and displayed defensive behavioural responses likely to prevent bee stings, including head shaking and dusting, but displayed no significant response to the other noise treatment. This suggests that bee presence could be used as a potential deterrent method for raiding elephants. However, its long-term effectiveness is still unknown as there is a possibility for animals to get habituated to the threat.
To our knowledge, this type of deterrent has never been explored and evaluated in the high elephant density regions of southern Africa. Therefore, we explored the efficacy of using African honey bee (the bee species found in Kruger National Park) presence to deter elephants. Using African honey bee sound and scent as surrogates for bee presence, we investigated whether bee auditory and olfactory cues could be used to effectively deter elephants. We evaluated the responses of elephants in the southern section of the Kruger National Park in South Africa to five different treatments that were a combination of sound and scent stimuli. Our present study tests the hypothesis that the presence of bees exhibiting a single cue, either olfactory or auditory, is sufficient to deter wild elephants.
Materials and methods
Study Site
Data were collected between November 2013 and February 2014 at the height of the summer rainy season in the southern region of the Kruger National Park (hereafter simply referred to as the Kruger) within a 50 km radius from Skukuza rest camp (S 24° 59' 43", E 31°35'34"). The southern section of Kruger is relatively flat and lies in the Lowveld region at altitudes between 200 m and 700 m above mean sea level.15 The region receives a mean annual rainfall of 500-700 mm,16 and is characterised by a savanna bushveld dominated by Acacia spp trees and high-bulk grasses such as buffalo grass (Panicum coloratum), red grass (Themedra triandra), and bushveld signal grass (Urochloa mosabicensis) species.16 Large herbivores such as elephant, white rhino (Ceratotherium simum), giraffe (Giraffa camelopardalis), greater kudu (Tragelaphus strepsiceros) and Burchell's zebra (Equus quagga burchellii) inhabit the landscape.17
Elephant numbers in the Kruger are rapidly increasing. By the end of 2011, there were approximately 14 273 individual elephants in the park.18 These high densities are reported to negatively alter vegetation structure and diversity in some parts of the park.19,20
Experimental design
We performed preliminary trials in a controlled environment to assess the risk associated with conducting this study in the field. These preliminary experiments involved the exposure of six captive elephants from an elephant sanctuary (S 25°01'39", E 31°07'30") to bee and waterfall audio recordings played from a speaker placed approximately 50 m from the animals. The waterfall and angry buzzing African honey bee noises used in the study were recorded in the Kruger using the high definition voice memo application on an iPhone 4 (Apple Inc, Cupertino, California, USA). Recorded sound treatments were played from an iPhone 4 connected to a 40 watt power Samson Expedition XP40iw rechargeable battery powered wireless PA - Channel 6 (Samson Technologies, Hauppauge, NY, USA) The speaker was placed on top of the research vehicle and sounds were played at maximum volume. Captive elephants (n=6) moved away from the bee noises and as expected, did not appear to respond significantly to our control treatment, the waterfall noise. We then shifted our focus to experimentation on wild elephants in the Kruger.
For trials in the Kruger, we drove on management roads around the southern region of the park and arbitrarily selected elephants for observation that were within a 50 m radius of the observation vehicle. Researchers were always accompanied by an armed research assistant. When elephants were located, we observed the animals' pre-stimulus behaviour for 10 min in order to establish a baseline from which we could judge changes in behaviour during the treatment and to ensure that the elephants were somewhat acclimatised to our presence. Most elephants in the Kruger appear to be habituated to the presence of researcher vehicles. We then conducted a behavioural response experiment where we randomly exposed different individuals and groups of wild elephants to one of the five treatments: namely (1) control noise (waterfall), (2) buzzing bee noise, (3) control noise with honey scent, (4) honey scent, and (5) buzzing bee noise with honey scent. The speaker was positioned on top of the research vehicle, a method similar to that used by McComb.22 Each treatment was presented for 2 min and the elephant's behavioural response was recorded throughout the duration of the treatment.
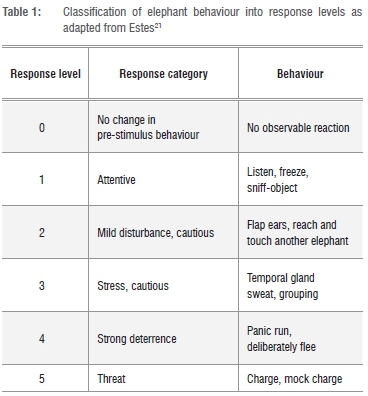
We used Estes'21 behavioural definitions to group and classify 11 possible responses that would indicate the efficacy of a deterrent on a scale of 0-5 (Table 1). Only the highest behavioural response exhibited by each elephant was eventually recorded as that individual's response level for a given trial (Table 1). For each trial, we also recorded total herd size, time of day, ambient temperature, age and sex of the individuals. Age of individual elephants was determined by our experienced game guards and confirmed using Estes.21 To minimise the chance of subjecting the same elephants to a second treatment on the same day, we only (1) searched each road once a day and (2) selected elephants for observation that were more than 2 km away from the previous herd or individual tested. In instances where we were certain about individual elephant identity, we never sampled those elephants again.
All sound treatments were kept constant by using the same speaker and volume level. For the honey scent treatment, 50 mL of honey was dissolved in 350 mL of boiling water and the resultant solution was dispersed in a fine mist using a 500 mL handheld plastic spray bottle pointed in the direction of the elephants. We used waterfall noise as our control, based on the assumption that it was a natural and non-threatening sound that would not significantly alter elephant behaviour. Researchers were aware of the treatment being given; however all response levels recorded were dictated by a predefined ethogram (Table 1) and agreed upon by the researchers.
Data Analysis
We used the non-parametric Kruskal-Wallis one-way analysis of variance by ranks test with a post-hoc multiple comparison to test for differences in behavioural response levels of elephants to each of the five treatments. A series of Mann-Whitney U tests were used to determine any age-related (adults vs juveniles) differences in behavioural responses for each experimental treatment. All statistical analyses were carried out using the STATISTICA 623 program and tested at the 5% level of significance.
Results
We encountered a total of 136 wild elephants during the study and sampled only 89 individuals, yielding an overall observation rate of 65%. We classed elephants into two age categories: Juveniles' (n=41) and 'Adults' (n=48). We exposed 11 elephants to treatment with control noise alone, 21 elephants to treatment with bee noise alone, 15 elephants to treatment with control noise and honey scent, 18 elephants to treatment with honey scent alone, and 24 elephants to treatment with bee noise and honey scent. The most frequently observed response across all five treatments was an attentive response (response level 1; n=27) where subjects would freeze and then raise their trunks to sniff towards the source of the treatment (Figure 1). Level 4 responses, where elephants ran away from the source of the treatment, was the highest behaviour recorded and it was elicited in 15 out of 21 elephants exposed to the bee noise with honey scent treatment (Figure 1). Interestingly, the honey scent alone treatment sometimes (5 out of 15) drew subjects towards the source of the treatment. Three elephants came within 2 m of the vehicle. We classed this behaviour as response level 1.
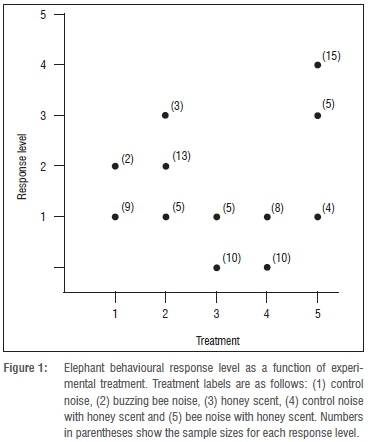
There was a significant difference in behavioural response levels of elephants to each of the five treatments (F=52.15, d.f.=4, p<0.001, n=89). The bee noise and honey scent treatment elicited the highest deterrent behavioural responses from elephants and these response levels were significantly higher than those of the control noise (p=0.004), control noise with honey scent (p<0.001) and honey scent (p<0.001) treatments (p<0.001). However, the response levels to the bee noise with honey scent treatment were not significantly different from the honey scent treatment (p=0.126; Table 2). There were no significant differences in the levels of response amongst the (1) control noise, (2) bee noise alone, (3) control noise with honey scent, and (4) honey scent alone treatments (Table 2).
There were no significant differences in behavioural response levels between adults and juveniles within each of the five treatments (control noise: U=12.5, p=0.788; bee noise; U=22, p=0.317; control noise with honey scent: U=21.5, p=0.679; honey scent: U=36, p=0.762; bee noise with honey scent; control noise with honey scent: U =71, p=1.000).
Elephants displayed different behavioural responses to each of the various bee threat surrogates and control stimuli presented (Figure 1). Adult and juvenile elephants exhibited a similar within-treatment behavioural response. Mild disturbance behaviour responses and in some instances no observable responses were recorded for all treatment trials except for elephants exposed to the bee noise with honey scent treatment. All elephants exposed to the bee noise with honey scent responded with a cautious behaviour and 15 out of 21 individuals also fled.
Discussion
The most frequently observed response across all five treatments was an attentive response and it is possible that these elephants were responding to our presence and the vehicle. Cautious responses of elephants to the buzzing bee noise with honey scent as compared with responses to other treatments can be attributed to (1) elephants' equal reliance on both sound and scent as cues for assessing their surroundings13 and (2) the varying degrees of perceived danger associated with each treatment. Elephants are sensitive to a wide range of sound frequencies aided by their large ear size12 and they also equally rely on their sense of smell to investigate the environment around them, as the olfactory system in elephants is the primary processing site for chemical stimuli.13 Another parsimonious explanation, linked to danger perception, is that elephants identified the treatment with the buzzing bee noise only as passing bees, whereas they probably associated the bee noise and honey scent treatment with an active beehive. Distinguishing between a passing swarm and an active beehive seems to have important implications for dictating elephant responses, presumably because of the greater inherent threat posed by encountering a hive as opposed to a passing swarm.14 Elephants are more at risk of being stung if they come in close contact with a beehive rather than just a passing swarm because African bees are notoriously territorial and have large defensive perimeters surrounding their hives.14,24 Therefore, elephants might be wary of encountering a large hive where the risk of defensive attack by bees protecting their territory is high.24
Contrary to our results that show a minimum response to bee sound alone, King et al.9 reported that bee sound alone was enough to elicit higher-level responses and also deter elephants in Samburu and Buffalo Springs National Reserves in Kenya. Our results raise new questions about what makes elephants in the Kruger different from elephants in other places. One possible explanation for our results is that the high density of elephants in the Kruger18 compared to elephant densities in Kenyan reserves1 increases the likelihood of bee encounters in the South African park. As a consequence of the increased probability of elephant exposure to bees in the Kruger, the subjects in our study were potentially familiarised with the sound and smell of live beehives, and therefore better equipped with cues that indicate a realistic bee threat.
We concluded that the observed cautious behavioural responses from elephants when exposed to our bee threat proxies provide strong support for our hypothesis that these same elephants would be deterred by a live bee threat. It therefore suggests that a treatment evoking both the olfactory and auditory cues of a 'bee threat' is required to deter wild elephants. The effectiveness of the mixed stimulus treatment can be explained by the fact that the bee noise and honey scent treatment better imitated the presence of an active beehive than the treatment with the bee noise alone. The combination of sound and scent was a more realistic representation of a bee threat, which elicited a greater response from the elephants because: (1) elephants likely associated this combined stimulus with the presence of an active beehive, which indicated a greater threat to elephants than the sound of a passing swarm; and (2) elephants rely on both auditory and olfactory cues to detect a threat.
Studies in Kenya have demonstrated the effectiveness of using beehive fences to deter elephants from raiding farms and damaging large trees.9,14 Our findings indicate that a similar innovation could also be used to mitigate the human-elephant conflict on farms and in settlements surrounding the Kruger (both in South Africa and Mozambique) and other parts of southern Africa. In addition to aiding in human-elephant conflict mitigation, apiculture (beekeeping) has potential benefits for sustainable community-based conservation, particularly because honey harvesting is a traditional practice in many African cultures.25 Aside from potentially reducing losses from elephant raids, apiculture can provide employment and income opportunities for communities through the production of marketable products such as honey and wax.25,26
However, our study also points out one key limitation to the use of bees to deter elephants. We know that elephant raids in most parts of southern Africa (1) occur at night when temperatures are low and (2) are prominent in winter when natural browse and graze opportunities are at their minima.6 Unfortunately, most African honey bees tend to be dormant (less active) at night and when temperatures are low. Our findings therefore imply that the use of active (buzzing and scent emitting) bees as recommended by King et al.9, may be seriously mismatched with the timing of elephant raids. Perhaps the development of some trigger mechanism to activate dormant bees when elephant raids occur will remedy the problem.
Authors' Contributions
M.N. designed the research. E.D., M.C., K.A., and A.R. collected and analysed the data. All authors contributed equally to the writing of the manuscript.
Acknowledgements
We thank the Organisation for Tropical Studies and South African National Parks (SANParks) for supporting this research. We are grateful to Ceinwin Smith, Karen Vickers, Dax Mackay, Philip Mhlava, Donovan Tye, Laurence Kruger, Alyssa Browning, Tyler Maddox, Cassandra Pestana and David Purdy for their assistance with data collection. Lastly, we wish to thank André Kotzé, the rest of the staff at Elephant Whispers Sanctuary and the SANParks research assistants. Their time and helpful insight into elephant behaviour is much appreciated.
References
1. International Union for the Conservation of Nature Species Survival Commission: African Elephant Specialist Group. Elephant Database: Regional totals for southern Africa for 2013 [document on the Internet]. [ Links ] c2013 [cited 2014 November 28]. Available from: http://www.elephantdatabase.org/preview_report/2013_africa/Loxodonta_africana/2012/Africa/Southern_Africa
2. Blanc J. Loxodonta africana. International Union for the Conservation of Nature (IUCN): IUCN red list of threatened species. Version 2013.1; 2008 [document on the Internet]. [ Links ] c2014 [cited 2014 November 25]. Available from: http://www.iucnredlist.org. http://dx.doi.org/10.2305/IUCN.UK.2008.RLTS.T12392A3339343.en
3. Carruthers J, Boshoff A, Slotow R, Biggs HC, Avery G, Matthews W. The elephant in South Africa: History and distribution. In: Scholes RJ, Mennel KG, editors. The 2007 scientific assessment of elephant management in South Africa. Johannesburg: Wits University Press; 2008. p. 1-40. [ Links ]
4. Lee PC, Graham MD. African elephants (Loxodonta africana) and human-elephant interactions: Implications for conservation. Int Zoo Yearb. 2006;40:9-19. http://dx.doi.org/10.1111/j.1748-1090.2006.00009.x [ Links ]
5. Graham MD, Notter B, Adams WM, Lee PC, Ochieng TN. Patterns of crop raiding by elephants, Loxodonta africana, in Laikipia, Kenya, and the management of human-elephant conflict. Syst Biodivers. 2009;8:435-445. http://dx.doi.org/10.1080/14772000.2010.533716 [ Links ]
6. Twine W, Magome H. Interactions between elephant and people. In: Scholes RJ, Mennel KG, editors. The 2007 scientific assessment of elephant management in South Africa. Johannesburg: Wits University Press; 2008. p. 148-168. [ Links ]
7. Chiyo PI, Moss CJ, Alberts CS. The influence of life history milestones and association networks on crop-raiding behavior in male African elephants. PLoS ONE. 2012;7:1-11. http://dx.doi.org/10.1371/journal.pone.0031382 [ Links ]
8. Graham MD, Nyumna TO, Kahiro G, Ngotho M, Adams WM. Trials of farm based deterrents to mitigate crop-raiding by elephants adjacent to the Rumuruti Forest in Laikipia, Kenya. Nanyuki: Laikipia Elephant Project; 2009. [ Links ]
9. King, LE, Soltis J, Douglas-Hamilton I, Savage A, Vollrath F. Bee threat elicits alarm call in African elephants. PLoS ONE. 2010;5:1-9. http://dx.doi.org/10.1371/journal.pone.0010346 [ Links ]
10. Graham MD, Ochieng, T. Uptake and performance of farm-based measures for reducing crop-raiding by elephants Loxodanta africana among smallholder farms in Laikipia District, Kenya. Oryx. 2008;42:76-82. http://dx.doi.org/10.1017/S0030605308000677 [ Links ]
11. Hedges S, Gunaryadi D. Reducing human-elephant conflict: Do chillies help deter elephants from entering crop fields. Oryx. 2009;44:139-146. http://dx.doi.org/10.1017/S0030605309990093 [ Links ]
12. Reuter T, Nummela S. Elephant hearing. J Acoust Soc Am. 1998;104:1122-1123 http://dx.doi.org/10.1121/L423341 [ Links ]
13. Ngwenya A, Patzke N, Ilhunwo AO, Manger PR. Organisation and chemical neuroanatomy of the African elephant (Loxodonta africana) olfactory bulb. Brain Struct Funct. 2011;216:403-416. http://dx.doi.org/10.1007/s00429-011-0316-y [ Links ]
14. Vollrath F, Douglas-Hamilton I. African bees to control African elephants. Naturwissenschaften. 2002;89:508-511. http://dx.doi.org/10.1007/s00114-002-0375-2 [ Links ]
15. Mabunda D, Pienaar DJ, Verhoef J. In: Du Toit JT, Rogers KH, Biggs HC, editors. The Kruger experience: Ecology and management of savanna heterogeneity. Washington, DC: Island Press; 2003. p. 3-21. [ Links ]
16. Venter FJ, Scholes RJ, Eckhardt HC. The abiotic template and its associated vegetation pattern. In: Du Toit JT, Rogers KH, Biggs HC, editors. The Kruger experience: Ecology and management of savanna heterogeneity. Washington, DC: Island Press; 2003. p. 83-129. [ Links ]
17. Owen-Smith N, Ogutu J. Rainfall influences on ungulate population dynamics. In: Du Toit JT, Rogers KH, Biggs HC, editors. The Kruger experience: Ecology and management of savanna heterogeneity. Washington, DC: Island Press; 2003. p. 310-331. [ Links ]
18. South African National Parks (SANParks). Estimates for animal abundances in parks in the northern region. South African National Parks annual report 2011/2012. Pretoria: SanParks. Available from: http://www.sanparks.co.za/assets/docs/general/annual-report-2012.pdf [ Links ]
19. Grainger M, Van Aarde RJ, Whyte I. Landscape heterogeneity and the use of space by elephants in the Kruger National Park, South Africa. Afr J Ecol. 2005;43:369-375. http://dx.doi.org/10.1111/j.1365-2028.2005.00592.x [ Links ]
20. Whyte I, Van Aarde R, Pimm SL. Managing the elephants of Kruger National Park. Anim Conserv. 1998;1:77-83. http://dx.doi.org/10.1111/j.1469-1795.1998.tb00014.x [ Links ]
21. Estes RD. Elephants: Order Proboscidea, Family Elephantidae. In: Estes RD, editor. The Behavior Guide to African Mammals. Johannesburg: Russel Friedman Books; 1991. p. 259-267. [ Links ]
22. McComb K, Shannon G, Durant SM, Sayialel S, Slotow R, Poole J, Moss C. Leadership in elephants: The adaptive value of age. Proc R Soc B. 2011;278:3270-3276. http://dx.doi.org/10.1098/rspb.2011.0168 [ Links ]
23. StatSoft, Inc. STATISTICA. Version 6.0. Tulsa, OK, USA; 2006. [ Links ]
24. Breed MD, Guzma-Novoa E, Hunt GJ. Defensive behavior of honey bees: Organization, genetics, and comparisons with other bees. Ann Rev Entomol. 2004;49:217-298. http://dx.doi.org/10.1146/annurev.ento.49.061802.123155 [ Links ]
25. King LE. The interaction between the African elephant (Loxodonta africana) and the African honey bee (Apis mellifera scutellata) and its potential applications as an elephant deterrent [MSc dissertation]. [ Links ] Oxford: University of Oxford; 2010.
26. Hill DB, Webster TC. Apiculture and forestry (bees and trees). Agroforest Syst. 1995;29:313-320. http://dx.doi.org/10.1007/BF00704877 [ Links ]
 Correspondence:
Correspondence:
Mduduzi Ndlovu
School of Animal, Plant and Environmental Sciences, University of the Witwatersrand
Private Bag 3, Wits 2050, South Africa
mdu.ndlovu@wits.ac.za
Received: 10 Feb. 2015
Revised: 22 May 2015
Accepted: 02 June 2015














