Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.1-2 Pretoria Jan./Feb. 2016
http://dx.doi.org/10.17159/sajs.2016/20150102
RESEARCH ARTICLE
Radioactive nuclides in phosphogypsum from the lowveld region of South Africa
Xolani MsilaI; Frans LabuschagneI; Werner BarnardII; David G. BillingIII
ISasol Dyno Nobel, Bronkhorstspruit, South Africa
IISasol Technology Research and Development, Sasolburg, South Africa
IIISchool of Chemistry, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
We evaluated the suitability of phosphogypsum from the Lowveld region of South Africa (LSA), for the manufacturing of building materials, with reference to (1) the National Nuclear Regulator Act 47 of 1999 and (2) the radioactivity associated risks as quantified in terms of the external and internal hazard indices, the activity concentration index and the radium equivalent. The distribution of radioactive nuclides in the LSA phosphogypsum was also examined. Analyses of 19 samples of the phosphogypsum show that phosphogypsum contains lower activity concentrations of naturally occurring radioactive nuclides of uranium and thorium and their progeny than the 500 Bg/kg limit set for regulation in South Africa. The potassium-40 (40K) activity concentration was below the minimum detectable amount of 100 Bq/kg. The values obtained for external and internal hazard indices and the activity concentration index were: 2.12 + 0.59, 3.44 + 0.64 and 2.65 + 0.76 respectively. The calculated radium equivalent Raeq was 513 + 76Bq/kg. The final decision regarding phosphogypsum's suitability for use as a building material should consider scenarios of use.
Keywords: apatite; radium equivalent; radiation hazard indices; Anderson-Darling; secular equilibrium
Introduction
A phosphoric acid production facility has been in operation in the Lowveld region of South Africa (LSA) since the 1960s. The process of producing phosphoric acid involves the digestion of fluoro-apatite ore (Ca10(PO4)6F2) with sulfhuric acid as shown in Equation 1:
Ca10(PO4)6F2 + 10H2SO4 + 20H2O → 10CaSO4.2H2O + 6H3PO4 + 2HF Equation 1
Each year, tons of calcium sulfate dihydrate (CaSO4.2H2O) or gypsum, or specifically phosphogypsum, is produced as a by-product. The LSA phosphogypsum is stored in waste stacks alongside the phosphoric acid factory. In a country like South Africa, that has a challenge to provide low cost or affordable housing,1 the use of phosphogypsum to manufacture building material is an attractive option. The manufacturing of low cost prefabricated building material from gypsum has in the recent past been demonstrated by Rajkovic and Toskovic2. But the solubility of uranium, thorium and their daughter products that exist in apatite ores3,4 result in these radioactive nuclides partitioning between the phosphoric acid and the phosphogypsum5,6 during the processing of the ore. Rajkovic and Toskovic2, Al-Jundi et al.6 and Hussein7 studied phosphogypsum from Serbia, Jordan and Egypt respectively. Hussein did not report on the activity concentrations of potassium-40 (40K), thorium-232 (232Th) and radium-226 (226Ra) but the results of radioactive nuclides activities from Serbia and Jordan are given in Table 1.
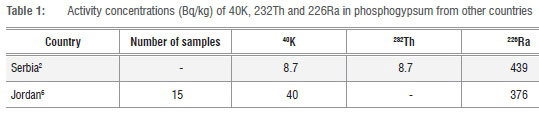
The worldwide average activities of 226Ra, 232Th and 40K on the earth's crust are about: 40; 40 and 400 Bq/kg, respectively.8 Humans are therefore exposed to this naturally occurring radioactive material (NORM). If the material used for the construction of human dwellings contributes additional radioactivity to the naturally occurring radioactive material, radiation exposure increases. The presence of these radioactive nuclides impurities in the LSA phosphogypsum can limit its use as a building material.
In this study we evaluated the suitability of the LSA phosphogypsum for the manufacturing of building materials, with reference to (1) the National Nuclear Regulator Act 47 of 19999 and (2) the radioactivity associated risks as quantified in terms of the external (Hex) and internal (Hi) hazard indices, the activity concentration index (Iy) and the radium equivalent (Raeq). The effect of ore processing on the distribution of the radioactive nuclides was also examined.
Materials and method
During the production of phosphoric acid for analysis, 19 grab samples of LSA phosphogypsum were taken.
Sample preparation
The phosphogypsum was dried overnight in an oven at 80 °C. About 100 g of the dried phosphogypsum was transferred to a grinding vessel (containing stainless steel grinding balls and block) with a swing mill. Milling was performed at 960 rpm. for one min to obtain a fine powder. The process was repeated until 0.5 kg of homogeneous fine powder was generated.
Gamma analysis
A standard glass container was filled with phosphogypsum and the mass recorded. The container was closed with a lid and sealed airtight with the aid of epoxy resin. The prepared sample was allowed to stand for 3 weeks so that radon-222 (and some of its progeny) reached radioactive equilibrium with radium-226. The γ-spectrum was acquired on a high purity germanium detector calibrated for the standard glass container geometry. The detector was housed in a lead shield to reduce background radiation. Gamma spectrum analysis was performed with Genie™ 2000 Model S501 Gamma Analysis software. The weighted average activity of bismuth-214 and lead-214 was calculated and reported for radium. Thorium-228 was calculated from lead-212 and thalium-208. Radium-228 was determined from actinium-228. Potassium-40 was measured directly.
Uranium and thorium analysis by activation analysis
Aliquots of the powder were transferred into irradiation capsules. Uranium and thorium standards were prepared by transferring known amounts of these elements from certified reference solutions into capsules and evaporated to dryness. Samples and standards were sequentially transferred with a pneumatic system into an in-core position of the SAFARI-1 reactor and irradiated with neutrons for a fixed time. After irradiation, the sample was transferred to a detector to measure neutrons emitted by products formed from fission of uranium-235.
The emission of neutrons between samples and standards was compared to calculate the uranium concentration in the sample. After a prolonged period, the irradiated samples and standard were measured on a gamma detector to determine neptunium-239 and protactinium-231. These nuclides formed from the neutron activation of uranium-238 and thorium-232 respectively. By comparing the activities of the nuclides in the standards and samples, the uranium-238 and thorium-232 sample activities were calculated.
Radiation indices
From the activity concentrations of the radioactive nuclides, Hex, Hi, Iy and Raeq were calculated using the formulae in Equations 2-5 respectively. The formulae and their applications are comprehensively defined by the European Commission for Radiation Protection8.
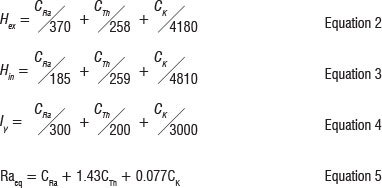
where CRa, CTh and CK are activities of 226Ra, 232Th and 40K in Bq/kg, respectively.10,11
Results and discussion
Radionuclide concentrations in phosphogypsum
The average activity concentrations of the radioactive nuclides: uranium-23 8 (238U), lead-210 (210Pb), uranium-235 (235U), radium-228 (228Ra), thorium-2 38 (238Th), 226Ra, 232Th and 40K in the LSA phosphogypsum are presented in Table 2. According to the National Nuclear Regulator Act, 47 of 1999,9 an operation or material is excluded from regulation if activity concentration of the naturally occurring radioactive nuclides of uranium, thorium and their progeny are each below 500 Bq/kg. The limit for 40K is 10 000 Bq/kg.
The results confirm that the activity concentrations measured in the 19 LSA phosphogypsum samples are all below the regulation limits set by the National Nuclear Regulator Act 47 of 19999for 238U, 235U, 210Pb, 228Ra, 226Ra, 228Th, and 232Th. The activity concentration of 40K in the LSA phosphogypsum was determined to be below the minimum detectable limit of 100 Bq/kg. Numerous studies have revealed that building materials contain appreciable activity concentrations of radioactive nuclides 226Ra, 232Th and 40K. There are limited data available on the radiological safety of phosphogypsum use as a building material beyond the study by Rajkovic and Toskovic2 and therefore to put the results obtained for LSA phosphogypsum into perspective, some of the results obtained for bricks from several countries are presented in Table 3.
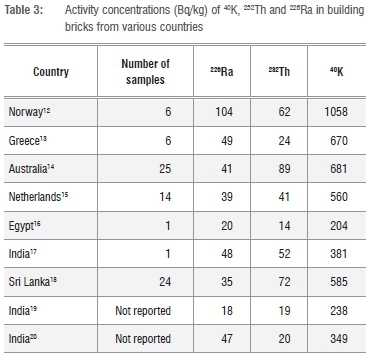
The average activity concentration of 226Ra in the bricks listed in Table 3 range from 18 Bq/kg to 104 Bq/kg. This is lower than the average activity concentration in the LSA phosphogypsum (109 Bq/kg ± 18 Bq/kg). Similarly, the average activity concentration of 232Th in the bricks ranges from 14 Bq/kg to 89 Bq/kg which is lower than the average in the LSA phosphogypsum, where 232Th is 253 Bq/kg ± 160 Bq/kg. The activity concentration of 40K (<100 Bq/kg) is lower in the LSA phosphogypsum compared to the range of activity concentrations reported for the bricks from other countries (204-1058 Bq/kg).
Radiation hazard indices
The LSA phosphogypsum is rich in 228Ra compared to 232Th and as a result the activity concentration of the former is used in the place of the activity of the latter to calculate the hazard indices in Equations 2-4. The calculated hazard indices Hex, Hin, Iy and the Ra are plotted in Figure 1.
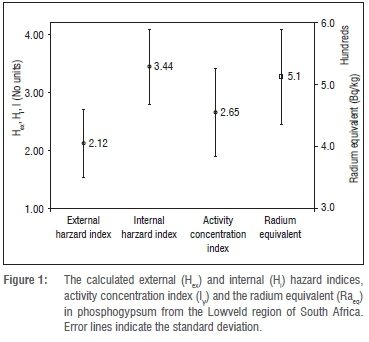
The error bars in Figure 1 represent the standard deviation of the results of the 19 LSA phosphogypsum samples. The values obtained for Hex, Hin and I are 2.12 ± 0.59, 3.44 ± 0.64 and 2.65 ± 0.76 respectively. The calculated Raeq is 513 ± 76 Bq/kg.
The assessment of a material's suitability for use as a building material should be based on scenarios where the material is used. The scenarios for use at different dose criteria8 are given in Table 4. The activity concentration index,Iy, should be evaluated against these criteria. If the Iv is 1 or less, the material can be used as building material, without restriction, as far as radioactivity is concerned, whereas if the Iv is above 1 and less or equal to 6, the material should be used superficially.
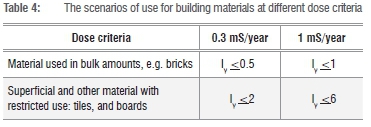
The Raeq as defined in Equation 5, is calculated from the activity concentrations of 226Ra, 232Th and 40K. Equation 2 is based on the estimation that 1 Bq/kg of 226Ra, 0.7 Bq/kg of 232Th and 13 Bq/kg of 40K generate the same γ-rays dose rate.10,21,22 According to Mondal et al.23 and supported by El-Taher and Makluf24, Iv=1 is equivalent to the Raeq of 370 Bq/kq. The calculated Raeq of 513 + 76 Bq/kg for LSA phosphogypsum is consistent with the activity concentration (1<Iy<6).
Radioactive nuclides distribution in LSA phosphogypsum
A simplified definition of secular equilibrium is found in Zhang et al.25 Activity of daughter radionuclides build up to that of the parent in about seven half-lives and thereafter, parent activity (A1) is the same as the activity of its progeny (A2). It follows therefore that at the state of secular equilibrium, the ratio of the activity of the parent to that of the daughter is 1 (A/A2= 1). The secular equilibrium observed in natural ores can be disturbed by mineral processing. Al-Jundi et al.7 showed that concentration of 238U and its decay products 210Pb and 226Ra originating from apatite ore are partitioned during processing in such a way that 238U accumulates in the phosphate fertiliser while 228Ra, 226Ra and 210Pb accumulate in the phosphogypsum. This partitioning behaviour can be attributed to the very low aqueous solubility of radium sulfate and lead sulfate relative to that of uranium sulfate as almost all the 228Ra and 226Ra reported by Van der Westhuizen26 as present in the phosphate rock is observed in the LSA phosphogypsum.
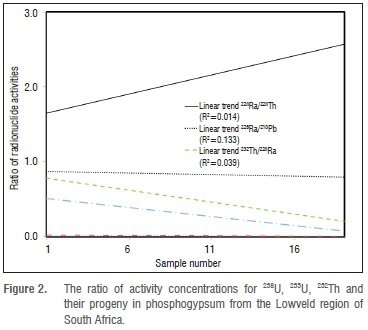
The ratios of activity concentrations in the LSA phosphogypsum for 238U, 235U, 232Th and their progeny in the natural decay series are plotted Figure 2. The 226Ra and 210Pb belong to the 238U natural decay series and are not in secular equilibrium in the LSA phosphogypsum. Although the linear trend of the ratio of these two radionuclides is close to unity, there is a poor correlation between their activities as shown by the regression coefficient (R2) of 0.133. The uranium radioactive nuclide 238U is also in disequilibrium with its progeny 226Ra. Similarly, 235U is in disequilibrium with 210Pb and disequilibrium is also observed between 232Th versus 228Ra and 228Ra versus 228Th.
The Anderson-Darling test has been shown to give appropriate empirical distribution function statistics for detecting departure from normality, even with small samples (n<25).27 The test was performed on a set of data to evaluate their fit to a chosen distribution pattern. For the Gaussian distribution test, the statistics are based on the squared difference between the normal and the empirical data. If the calculated p-value is less than a chosen alpha (one minus the confidence interval), the null hypothesis, that the data come from that distribution, is rejected. The Anderson-Darling (AD) test was performed on the radioactive nuclide activity concentration data using the Minitab® 16 statistical software to evaluate the distribution pattern of the activity concentration in the LSA phosphogypsum. The results obtained are presented in the probability plot in Figure 3.
At 95% confidence level, a p value of less than 0.05 indicates no deviation from the Gaussian distribution. This behaviour (normal distribution) is observed for the activity concentration of the radioactive nuclides 238U 235U, 228Th and 232Th in the LSA phosphogypsum. The contrary is observed for the 228Ra and 226Ra activity concentrations. The calculated p values are 0.318 and 0.434 respectively and the Anderson-Darling values are 0.406 and 0.350 respectively. This can be attributed to the low solubility of radium sulfate which results in accumulation in the phosphogypsum rather than the phosphoric acid.
Conclusion and recommendation
The LSA phosphogypsum contains appreciable amounts of the radioactive nuclides 238U; 210Pb; 235U; 228Ra; 228Th; 226Ra; 232Th and below-detectable levels of 40K. The average activities are below the limits set by the National Nuclear Regulator Act 47 of 1999.9The phosphogypsum is therefore excluded from regulatory control. The calculated results of hazard indices: Hex, Hin, IY and Raeq indicate that the LSA phosphogypsum can be utilised as building material if used superficially or with restriction. A final decision on the usability of the material can be made when the scenario of use is known and a more representative sample of the aged bulk material is analysed. The radioactive nuclides and their progenies are not in secular equilibrium in the LSA phosphogypsum and the distribution 238U, 235U, 232Th and 228Th deviates from Gaussian.
Authors' contributions
D.G.B. and W.B. were the project leaders, F.L. was responsible for sampling and arranging for analysis. X.M. handled the arrangement for sample analysis, performed the calculations and wrote the manuscript.
Acknowledgements
Many thanks to Deon Kotze (NECSA) for performing some of the radioactive nuclides analyses.
References
1. Ajayi JR. Strategies for sustainable housing cooperatives in South Africa. [ Links ] [PhD thesis]. Port Elizabeth: Nelson Mandela Metropolitan University; 2012.
2. Rajkovic MB, Toskovic DV. Investigation of the possibilities of phosphogypsum application for building partitioning walls - elements of a prefabricated house. APTEFF. 2002;33:71-92. http://dx.doi.org/10.2298/APT0233071R [ Links ]
3. Pereira F, Bilal E. Phosphoric acid extraction and rare earth recovery from apatites of the Brazilian phosphate ores. Romanian J Miner Dep. 2012;85(2):49-52. [ Links ]
4. Garcia-Talavera M, Leadermann JP Decombaz M, Daza MJ, Quintana B. Coincidence summing corrections for the natural decay series in Y-ray spectrometry. J Radiat Isotopes. 2001;54:769-776. http://dx.doi.org/10.1016/S0969-8043(00)00318-3 [ Links ]
5. Dias NMP Caires EF, Pires LF, Bacchi MA, Fernandes EAN. Radiological impact of phosphogypsum surface application in a no-till system in southern Brazil. Pesq Agropec bras Brasilia. 2010;45:1456-1464. http://dx.doi.org/10.1590/S0100-204X2010001200017 [ Links ]
6. Al-Jundi J, Al-Ahmad N, Shehadeh H, Afaneh F, Maghrabi M, Gerstmann U. Investigation on the activity concentrations of U-238, Ra-226, Pb-210 and K-40 in Jordan phosphogypsum and fertilizer. Radiat Prot Dosim. 2008;131(4):449-454. http://dx.doi.org/10.1093/rpd/ncn214 [ Links ]
7. Hussein EM. Radioactivity of phosphate ore, superphosphate and phosphogypsum in Abu Zaabal phosphate plant, Egypt. Health Phys. 1994;48:87-95. http://dx.doi.org/10.1097/00004032-199409000-00010 [ Links ]
8. European Commission. Radiation Protection 112: Radiological protection principles concerning the natural radioactivity of building materials. Brussels: Directorate-General: Environment, nuclear safety and civil production; 1999. [ Links ]
9. Department of Energy. National Nuclear Regulator Act 47 of 1999 [document on the Internet]. [ Links ] c1999 [cited 2015 December 04]. Available from: http://www.energy.gov.za/files/esources/nuclear/act47.pdf
10. Mehdizadeh S, Faghihi R, Sina S. Natural radioactivity in building materials in Iran. Nukleonika. 2011;56(4):363-368. [ Links ]
11. Radenkovic MB, Alshikh SM, Andric VB, Miljanic SS. Radioactivity of sand from several renowned public beaches and assessment of the corresponding environmental risks. J. Serb Chem Soc. 2009;74(4):461-470. http://dx.doi.org/10.2298/JSC0904461R [ Links ]
12. Papastefanou C, Manolopolou M, Charalambous S. Exposure from the radioactivity in the building materials. Health Phys. 1983;45:349-361. [ Links ]
13. Beretka J, Mathew PJ. Natural radioactivity of Australian building materials, industrial waste by-products. Health Phys. 1985;48(1):87-95. http://dx.doi.org/10.1097/00004032-198501000-00007 [ Links ]
14. Ackers JG, Den Boer JF, De Jong P Wolschrijin RA. Radioactivity and radon exhalation rates of building materials in the Netherlands. Sci Total Environ. 1985;45:151-156. http://dx.doi.org/10.1016/0048-9697(85)90215-3 [ Links ]
15. El-Thaway MS, Higgy RH. Natural radioactivity in different types of bricks fabricated and used in the Cairo region. Appl Radiat Isotopes. 1985;46(12):1401-1406. http://dx.doi.org/10.1016/0969-8043(95)00220-8 [ Links ]
16. Kumar V Ramachandra TV, Prasad R. Natural radioactivity of Indian building materials and by products. Appl Radiat Isotopes. 1999;51(1):93-96. http://dx.doi.org/10.1016/S0969-8043(98)00154-7 [ Links ]
17. Hewamanna R, Sumithrararachchi CS, Mahawatte P, Nanayakkara HLC, Ratnayakhe HC. Natural radioactivity and gamma dose from Sri Lankan clay bricks used in building construction. Appl Radiat Isotopes. 2001;54:365-369. http://dx.doi.org/10.1016/S0969-8043(00)00107-X [ Links ]
18. Ravisankar R, Vanasundari K, Chandrasekaran A, Suganya M, Eswaran P, Vijayagopal P. Measurement of natural radioactivity in brick samples of Namakkal, Tamilnadu, India using gamma-ray spectrometry. Arch Phys Res. 2011;2(2):95-99. [ Links ]
19. Viruthagiri G, Ponnarasi K. Measurement of natural radioactivity in bricks samples. Adv Appl Sci Res. 2011;2(2):103-108. [ Links ]
20. Dhanya B, Umadevi AG, Jose PA, Rajagopala M, Jojo PJ. a study on activity concentration of natural radionuclide of building materials in Kochi. Int J Fund Phy Sci. 2012;2(3):41-43. [ Links ]
21. Uosif MAM. Specific activity of 226Ra, 232Th, and 40K for assessment of radiation hazards from building materials commonly used in Upper Egypt. SDU J Sci. 2011;6(2):120-126. [ Links ]
22. Le Nhu S, Nguyen TB, Truong Y, Nguyen TN, Nguyen TL, Nguyen VP. Natural radioactivity in common building materials used in Vietnam 11255. WM2011 Conference; 2011 February 27-March 03; Phoenix, AZ; 2011. [ Links ]
23. Mondal T, Sengupta D, Mandal A. Natural radioactivity of ash and coal in major thermal power plants of West Bengal, India. Curr Sci. 2006;91 (10):1387-1393 [ Links ]
24. El-Taher A, Makhluf S. Natural radioactivity levels in phosphate fertilizer and its environmental implications in Assuit governorate, Upper Egypt. Indian J Pure Ap Phy. 2010;48:697-702. [ Links ]
25. Zhang Q, Burman C, Amols H. Transient and secular radioactive equilibrium revisited [article on the Internet]. [ Links ] c2014 [cited 2015 December 04]. Available from: http://arxiv.org/ftp/arxiv/papers (accessed: 4 December 2015)
26. Van der Westhuizen AJ. Rail transport of igneous phosphate rock. In: International Atomic Energy Agency (IAEA) conference on naturally occurring radioactive materials (NORM IV); 2004 May 17-21; Szczyrk, Poland. Vienna: IAEA; 2004. [ Links ]
27. Stephens MA. EDF Statistics for goodness of fit and some comparisons. J Am Stat Assoc. 1974;69:730-737 http://dx.doi.org/10.1080/01621459.1974.10480196 [ Links ]
 Correspondence:
Correspondence:
Xolani Msila
Sasol Dyno Nobel, 486 Brandbach Road, Ekandustria, Bronkhorstspruit 1021, South Africa
xolani.msila@sasol.com
Received: 11 Mar. 2015
Revised: 13 July 2015
Accepted: 01 Aug. 2015














