Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.112 no.1-2 Pretoria Jan./Fev. 2016
http://dx.doi.org/10.17159/sajs.2016/20150126
RESEARCH ARTICLE
Physicochemical characteristics of Bambara groundnut dietary fibres extracted using wet milling
Yvonne Maphosa; Victoria. A. Jideani
Department of Food Technology, Cape Peninsula University of Technology, Cape Town, South Africa
ABSTRACT
The objectives of this study were to extract soluble and insoluble dietary fibres from four Bambara groundnut (BGN) varieties (black-eye, brown-eye, brown and red) using the wet milling method and evaluate their physicochemical properties. The swelling capacities of brown-eye (6.5 g/mL) and black-eye (6.2 g/mL) fibres were higher (p<0.05) than those of red (6.0 g/mL) and brown (5.5 g/mL) fibres while the water holding capacities of black-eye and brown-eye fibres (2.84 g and 2.83 g water/g sample) were higher (p<0.05) than those of brown and red fibres. The bulk densities of insoluble dietary fibres (IDFs) and soluble dietary fibres (SDFs) ranged between 0.57 g/mL (red) to 0.67 g/mL (brown-eye) and 0.46 g/mL (brown-eye) to 0.57 g/mL (black-eye), respectively. The oil binding capacities (OBCs) of SDFs ranged between 2.78 g oil/g sample (brown) and 4.03 g oil/g sample (brown-eye) while the OBC of all IDFs did not differ (p>0.05), ranging between 1.52 g oil/g sample (brown) and 1.40 g oil/g sample (brown-eye and black-eye). Black-eye and brown-eye dietary fibres had higher phenolic and total sugar content. The findings of this study indicate the potential of BGN fibres in food systems as fat replacers, emulsion stabilisers, water binders, bulking agents, thickeners and nutritional additives.
Keywords: swelling capacity; bulk density; water holding capacity; soluble dietary fibre; insoluble dietary fibre
Introduction
Bambara groundnut (BGN) is an underutilised crop predominantly grown in African countries.1,2 Legume seeds such as BGN are good sources of dietary fibre3 and BGN fibre has potential for both food and non-food applications.4 An increase in consumer awareness of the health benefits of dietary fibre (DF) has led to the investigation of alternative sources by a number of researchers.5-7 These health benefits include reduced risk of diseases of lifestyle, such as obesity, diabetes, coronary heart disease, some cancers and haemorrhoids.7-9
Legumes that have been researched for DF extraction include cowpeas, lentils and chickpeas.5,10 The basis of DF extraction methods is similar, however, the approach differs depending on the desired end product, source of fibre and availability of equipment. All DF extraction methods involve fractionation as this allows for the separation of constituents to obtain the desired concentrates and isolates.11 Some methods of extracting DF include microbiological retting, chemical, enzymatic, dry processing and wet processing.12
The modified wet milling method as reported by Dalgetty and Baik5 is more efficient than the conventional wet methods that rely solely on the differences in swelling capacity to separate starch and fibres, as it makes use of the enzyme α-amylase to digest any remaining starch, thus purifying the fibre concentrate. Extracted DF from BGN using the wet milling method is not documented. Furthermore, the properties and applications of BGN DFs are not largely documented. An understanding of the physicochemical properties of BGN DF will highlight the behaviour in different food and non-food systems including in the human gastro-intestinal tract.13,14 Diedericks15 applied an enzymatic-gravimetric method of extracting DF from BGN. The method proved to be very costly and time consuming costing approximately ZAR26 388.57/kg DF. The wet milling method is cheaper and easy to handle.5 Therefore, the objectives of this study were to extract soluble and insoluble fibres from whole seeds of BGN varieties using the wet milling method as an alternative to the enzymatic-gravimetric method and evaluate their physicochemical properties.
Materials and Methods
Materials
BGN seeds were purchased from Triotrade in Johannesburg, South Africa, and sorted into four varieties according to the 'eye' colour, namely, the black-eye, brown-eye, brown and red varieties. Chemicals used in this study were of analytical grade (Sigma-Aldrich, Johannesburg, South Africa). Equipment used was obtained from the Departments of Food Technology and Oxidative Stress of the Cape Peninsula University of Technology.
Milling of Bambara groundnut seeds
BGN seeds were washed and then dried at 50 °C for 48 h (Cabinet drier, Model: 1069616, Geiger & Klotzbucher, Cape Town, South Africa). The seeds were then milled using a hammer mill (Bauermeister, Bauermeister Inc., Vernon Hills, IL, USA) with a sieve size of 250 μm.
Wet fractionation of BGN flour into individual constituents
The method of Dalgetty and Baik5 was adopted in this study. BGN flour (200 g) was mixed with 500 mL distilled water and blended for 3 min at the highest setting. The slurry was centrifuged (15 min, 25 °C, 1500 x g). The residue was used for the isolation of insoluble dietary fibre (IDF) and the supernatant was used in the isolation of soluble dietary fibre (SDF).
Isolation of BGN insoluble dietary fibre
The residue (26 g) was wet screened in 2 L of water through a 53 μm sieve. The supernatant was collected as starch concentrate. To purify IDF, the collected sediment was digested using 13 units/mg 10 MU heat-stable o-amylase in 400 mL of water at pH 6 for 30 min, in a shaking water bath (100 °C). After digestion, the tubes were left to cool down to room temperature and centrifuged (10 min, 25 °C, 1500 x g). The residue was collected and dried at 50 °C (Cabinet drier, Model: 1069616) for 48 h and then vacuum dried in an air oven at 100 °C for 2.5 h.
Isolation of soluble dietary fibre
Soluble dietary fibre was isolated from the supernatant collected after wet fractionation. Firstly, proteins were precipitated by adjusting the pH of the soluble fraction from pH 3 to pH 9 using 1 N NaOH and 1 N HCl. Following precipitation, the soluble fraction was centrifuged (10 min, 25 °C, 1500 x g). The sediment was collected as protein concentrate. The supernatant was subjected to a tangential flow filtration system (Spectrum Laboratories Inc., Rancho Dominguez, CA, USA) and each fibre solution was washed with four diafiltration volumes to remove any contaminants. Waste was removed through a hollow fibre filtration outlet with a molecular weight cut-off of 10 kD.
Assessment of the physicochemical properties of BGN fibres
Hydration properties
The swelling capacities of BGN IDFs were determined using the method of Wang and Toews.16 Dry, purified IDF (0.2 g) was hydrated with 10 mL of distilled water in a graduated cylinder and left to stand at 24 °C for 18 h. The swelling capacity of the fibres was then calculated as the bed volume occupied by fibres per gram of dry sample.
The method described by Dalgetty and Baik5 was applied with modifications in the determination of the water holding capacity (WHC) of BGN IDFs. In a 50 mL centrifuge tube, 1 g of fibre and 30 mL of distilled water were added and the tubes were held for 18 h at 24 °C to allow sufficient hydration of the fibre. The tubes were then centrifuged (3000 x g, 20 min, 23 °C). The supernatant was decanted and the tubes carefully inverted for 10 min to drain any remaining free water. The weight of the residue was then recorded and the difference between the original volume of water and the volume of the supernatant was calculated to determine the WHC. The WHC was expressed as mL/g.
Density of BGN fibres
The method of Parrott and Thrall17 was followed in determining both bulk and direct densities. Bulk density was determined by adding 2 g of each BGN fibre into a graduated syringe and manually applying sufficient pressure while gently tapping the syringe on a bench until the contents were packed tightly. Direct density was determined by adding fibre to the 5 mL mark in a 10 mL graduated cylinder. Care was taken to avoid shaking the cylinder so as to avoid settling of the fibre.5 The dietary fibre was then emptied and weighed. Bulk and direct densities were expressed in g/mL.
Oil binding capacity of BGN fibres
The method described by Dalgetty and Baik5 was applied to determine the oil binding capacity (ObC) of the BGN fibres with modifications. Fibre (1 g) was mixed with 5 g of canola oil in a 50 mL centrifuge tube. The mixture was vortexed for 30 sec at 5 min intervals for 30 min. The mixture was then centrifuged (1600 x g, 25 min, 23 °C). After centrifugation, the supernatant (free oil) was decanted and weighed. OBC was expressed as grams of oil retained/grams of fibre.
Colour measurements of BGN fibres
Colour attributes of BGN dietary fibres were determined using a spectrophotometer (Model CM-5, Konica Minolta Sensing, Osaka, Japan) set at standard observer 10° and D65. The spectrophotometer was calibrated with black and white plates followed by zero calibration. BGN fibres (3 g of IDF and 0.6-0.8 g SDF) were placed in a glass sample holder (diameter 30 mm). Lightness (L*), redness/greenness (a*) and yellowness/blueness (b*), hue and chromacity were assessed through L*C*h* and CIE-L*a*b* colour space systems. Each variety was analysed in triplicate with each individual sample giving three readings. Colour differences amongst the fibre samples were calculated using the colour difference equation:

where L* is lightness, a* is redness/greenness and b* is yellowness/blueness
Assessment of polyphenolic compounds in BGN fibres
The method of Diedericks15 was adopted in the assessment of polyphenolic compounds. Condensed tannins were determined in IDFs while hydrolysable polyphenols (HPPs) were determined in both SDFs and IDFs. For determination of HPPs in BGN IDFs, samples (250 mg) were mixed with 10 mL of methanol and 1 mL of H2SO4 in 14 mL centrifuge tubes. The samples were incubated at 80 °C for 20 h. The samples were then centrifuged (4000 x g, 5 min, 21 °C) and the residues were analysed using the Folin-Ciocalteu assay by mixing 25 μL of sample with 125 μL of 0.2 M Folin-Ciocalteu and 100 μL of 7.5% Na2CO3 solution. The mixtures were left to stand for 2 h then the absorbance was measured using a spectrophotometer at 750 nm using a gallic acid standard calibration curve. The results were expressed as mg/g gallic acid equivalents (GAE). For determination of HPPs, BGN SDF samples (250 mg) were dissolved in 10 mL distilled water, centrifuged (4000 x g, 5 min, 21 °C) and the supernatant was subjected to the Folin-Ciocalteu assay. Tannins were determined by treating IDF samples (250 mg) with a 1:1 mixture of 5 mL/L HCl-Butanol. The mixture was incubated at 100 °C for 1 h then centrifuged (4000 x g, 5 min, 21 °C). Tannins were calculated from the anthocyanidin solutions absorbance at a wavelength of 555 nm using a standard curve of 0.0072 ppm and an absorbance of +0.0072.
Assessment of neutral sugars and uronic acids in BGN fibres
BGN fibres were subjected to acid hydrolysis prior to analysis of neutral sugars and uronic acids. SDFs were hydrolysed with 1 M H2SO4 at 100 °C for 90 min and IDFs were first hydrolysed with 12 M H2SO4 at 30 °C for 90 min and then with 1 M H2SO4 at 100 °C for 90 min to yield monomers. After hydrolysis, samples were centrifuged (3000 x g, 15 min, 21 °C). IDF residues were washed twice with 2 mL distilled water and SDFs were filtered to remove any suspensions. Uronic acids and neutral sugars were then analysed in the IDF and SDF supernatants by spectrophotometry (340 nm) using K-Arga, K-Fucose, K-Mangl, K-Rhan, K-Uronic and K-Xylose assay kits (Megazyme International, Wicklow, Ireland).
Data analysis
For statistical analysis, IBM Statistical Package for the Social Science (IBM SPSS, version 22) was used. The results were subjected to Multivariate Analysis of Variance (MANOVA) to determine mean differences between treatments. Duncan's multiple range test was conducted to separate mean differences where differences existed.
Results and Discussion
Yield of BGN fibres
Soluble and insoluble dietary fibres were successfully isolated from four varieties of BGN using the modified wet milling method (Figure 1) and the yield of each dietary fibre is given in Table 1. Scanning electron micrographs of BGN fibres are shown in Figure 2. The yield of SdFs was in the range 15.4% (red) to 17.1% (brown-eye) and that of IDFs was in the range 12.0% (brown-eye) to 15.6% (red). There was no significant difference (p>0.05) in the yield of SDFs as well as among the IDFs.
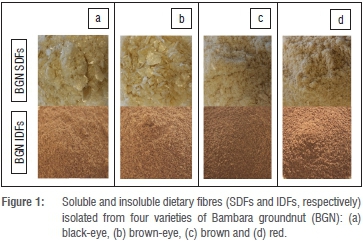
Using the enzymatic-gravimetric method to extract DFs from legumes, a lower yield of both IDFs and SDFs has been reported. The lower yield of DFs obtained using the enzymatic-gravimetric-method may be attributed to the fact that chemicals used in this method result in the loss of some IDFs and most SDFs.18 The yield of BGN DFs in this study was considered high as several researchers have reported BGN DF content in the range 5.2% to 6.4%.19-21 The variations in yield among researchers may be attributed to differences in BGN varieties, climatic conditions, type of soil grown on, processing and determination methods used. The SDF content of most edible legumes such as pea, broad pea and soybean cotyledons range between 3.3% and 13.8%.10,22 The yield of SDFs in this study was higher than the reported range. This increase in yield could be an indication that BGN has a higher SDF content than previously studied legumes. In addition, the use of different extraction methods could be responsible for the differences in yield; the wet milling method could have favoured the extraction of soluble fractions of DF more than other methods.
Hydration properties of BGN dietary fibres
Swelling capacity of BGN fibres
The swelling capacity of IDFs ranged from 6.50 mL/g (brown-eye) to 5.50 mL/g (brown) as shown in Table 1. There were no significant (p>0.05) differences between brown and red IDFs and also among black-eye, brown-eye and red IDFs in terms of swelling capacity. Brown-eye and black-eye IDFs had higher (p<0.05) swelling capacities than brown and red IDFs. Swelling capacities in the range 4.28-5.51 mL/g were reported for chickpea and pea IDFs.5,23 These values are comparable to those obtained in this study. A swelling capacity of 5.51 mL/g was reported for mung bean hulls sieved through a 50 μm mesh which is similar to the sieve size used in this study.24 The similarities in the results of the two studies suggest that particle size plays a major role in determining the swelling capacity of fibres.24 Increasing swelling capacity with decreasing particle size has been reported.24 Thus it can be deduced that particle size has an inverse relationship with swelling capacity.
The swelling capacities of BGN fibres are comparable to that of cellulose (6.2 mL/g), a dietary fibre constituent that is widely used in food products as a bulking agent, stabiliser, thickener and anti-caking agent owing to its hydration properties.25 Reduced cooking losses, decreased firmness, decreased adhesiveness and reduced stickiness in pea and inulin fibre enriched pastas have been reported.26 The researchers reported that the increase in swelling capacities of the fibres imparted these desirable characteristics in the pastas.
This study suggested that BGN fibres would make suitable substitutes for cellulose, inulin and pea fibres in food systems such as pasta because of to their swelling capacities. Physiologically, the swelling capacity of fibres is important in the control of blood glucose levels and also contributes to proper gut function.7,9
Water holding capacity of BGN fibres
The water holding capacity (WHC) of BGN fibres ranged from 2.41 g water/g sample (red) to 2.84 g water/g sample (black-eye) as shown in Table 1. The WHCs of black-eye and brown-eye IDF were significantly (p<0.05) higher than the WHC of brown IDF and brown IDF WHC in turn was higher (p<0.05) than red IDF WHC.
The WHC of passion fruit seed IDF was reported as 2.37 g water/g sample.27 Passion fruit seed fibre has been described as a functional ingredient that improves the health and functioning of the gut attributing these characteristics to its WHC.28,29 As the WHC of BGN IDF is comparable to that of passion fruit seed IDF, it can be deduced that BGN IDF can play a similar physiological role.
Several researchers reported the WHC of various legumes in the range 3.13 g water/g sample to 13.4 g water/g sample.5,13,23,24 The higher values obtained by these researchers could be because of different particle sizes, as well as differences in the chemical nature, composition and processing history of the fibres.30 The differences can also be attributed to the difference in legume species. The WHC of BGN fibres could find use in the meat, dairy and bakery industries.31
Densities of BGN fibres
The bulk and direct densities of BGN SDFs and IDFs were evaluated and the findings are given in Table 1. SDFs had bulk densities in the range 0.46 g/mL (black-eye and brown-eye) to 0.57 g/mL (brown) and direct densities in the range 0.05 g/mL (black-eye) to 0.11 g/mL (brown). Red SDF was significantly (p < 0.05) higher than both brown-eye and black-eye SDFs but lower (p <0.05) than brown SDF in terms of bulk density while all SDFs differed significantly (p <0.05) in direct density. IDFs had bulk densities in the range 0.57 g/mL (red and black-eye) to 0.67 g/mL (brown-eye) and direct densities in the range 0.45 g/mL (brown) to 0.53 g/mL (black-eye). The IDFs of black-eye and brown-eye as well as black-eye and red varieties did not differ significantly (p > 0.05) in their direct densities. Brown IDF had a significantly (p < 0.05) lower direct density than the other three fibres.
Direct density is measured without compressing the fibres while bulk density is measured after compressing the fibres. Consequently, bulk density measurements yielded higher values than direct densities. Dalgetty and Baik5 concluded that SDFs have higher densities than IDFs. The results obtained in the current study disagree with Dalgetty and Baik's5 conclusion as IDFs had higher bulk and direct densities than SDFs. The bulk densities of BGN IDFs were comparable to those of IDFs from passion fruit seeds (0.68 g/mL), soybean (0.43 g/mL), peas (0.54-0.56 g/mL), pigeon pea (0.47 g/mL) and chickpea (0.65 g/mL).27,32 These fibres are commercially available hence this is one of the indications that BGN dietary fibres have the potential to successfully compete commercially with other fibres. Differences in bulk densities with other leguminous fibres could be because of different structural compositions owing to different leguminous species.13
Density is of importance in packaging, with higher densities resulting in a reduced ability to compress. Therefore, the densities of BGN fibres could be an advantage as they will pack closely together, hence requiring less packaging material, resulting in cost saving.15
Oil binding capacity of BGN fibres
The oil binding capacities (OBCs) of SDFs ranged from 2.78 g oil/g sample (brown) to 4.03 g oil/g sample (brown-eye) (Table 1). Black-eye and red SDFs did not differ significantly in OBC, brown-eye SDF was significantly higher than the other three SDFs and brown SDF was significantly lower than all three SDFs. Among the IDFs, brown IDF had the highest OBC of 1.52 g oil/g sample, while brown-eye and black-eye IDFs both had the lowest OBC of 1.40 g oil/g sample. All four IDFs did not differ (p > 0.05) in terms of OBC. SDFs showed higher OBCs than IDFs.
Higher IDF OBC values were reported for pea (6.93 g oil/g sample), chickpea (4.25 g oil/g sample) and lentil fibres (4.01 g oil/g sample)5. These differences can be attributed to structural differences and compositional variation of the fibres. OBC of 1.49 g oil/g sample to 1.83 g oil/g sample were reported for mung bean hulls which compares fairly with the OBC of BGN IDFs.24
A total of 11 commercial fibres were studied and low OBC values were reported with the highest being 0.02 g oil/g sample from bamboo.25 The low OBC values indicated that BGN fibres can compete commercially with other fibres in stabilising high fat foods and emulsions.23 Lower OBC values for SDFs derived from pea (1.15 g oil/g sample), chickpea (1.14 g oil/g sample) and lentil fibres (0.89 g oil/g sample) have been reported.5
This study indicated that the use of BGN fibres would be economical as less BGN fibre would be used to render the desirable properties compared to other leguminous fibres. The ability of fibres to bind oil can be harnessed by the food industry to reduce fat losses upon cooking and in stabilising emulsions.23,33 Physiologically, the OBCs of BGN fibres would allow them to play a role in bile acid absorption and consequently cholesterol reduction.30 OBC would also be significant in reducing fat absorption by the body.
Colour characteristics of BGN fibres
The colour attributes of BGN measured were lightness (L*), redness (+a*) / greenness (-a*), blueness (-b*) / yellowness (+b*), hue and chroma (Table 2). SDFs were lighter than IDFs and all the BGN fibres had +a* and +b* values indicating that they were more associated with redness and yellowness. The redness and yellowness of these fibres suggests their antioxidant properties.34,35 BGN fibres are high in polyphenolic compounds (Table 2). Chroma describes the vividness or dullness of a colour and hue is how the colour of an object is perceived.34 The hue angle of BGN DFs indicated a yellowish-red colour associated with these fibres.
Lightness of dietary fibres is of importance in food products as it determines the extent to which the original colour of the food is affected.25,30 The varying colours of the BGN dietary fibres are advantageous as the manufacturer will have a choice of a fibre variety that best suits the colour of their product. IDFs had darker colours hence could find use in products such as meat emulsions and brown bread, while the lighter coloured SDFs could be used in food products such as white bread and beverage emulsions.
A colour difference (AE) of 1 is the threshold at which a trained observer would notice the difference between two colours, a AE between 4 and 8 is deemed acceptable and above 8 is deemed unacceptable and likely to be rejected by consumers.36Table 3 gives the colour difference between BGN fibres. All the SDFs showed acceptable differences with AE ranging between 0.81 and 3.08.
Hence, they could be used interchangeably in products without a noticeable difference. IDFs, with the exception of black-eye - brown-eye and brown - red comparisons had AE above 8 meaning their colour differences were very apparent and if used interchangeably, a perceivable difference would be expected.
Phenolic content of BGN fibres
The antioxidant capacity of BGN fibres, as represented by hydrolysable phenols (HPPs) and tannins, is shown in Table 1. The HPP content of SDFs ranged from 6.89 mg/g GAE (brown) to 20.86 mg/g GAE (brown-eye). All four SDFs differed significantly in their HPP content. The HPP content of IDFs ranged from 10.96 mg/g GAE (black-eye) to 14.43 mg/g GAE (brown). Black-eye and brown-eye IDFs as well as brown and red IDFs did not differ significantly in their HPP content. The tannin content of IDFs ranged from 1.12 mg/g (black-eye) to 2.1 mg/g (brown). Black-eye and brown-eye IDFs were both significantly higher (p < 0.05) in tannin content than red and brown IDFs. Brown IDF was significantly lower than the other three fibres in terms of tannin content. The difference in phenolic content of the BGN fibres was in agreement with Nti35 who reported that tannin content differs from one BGN variety to another.
The high polyphenolic composition of BGN reveals their potential antioxidant properties. It has been suggested that fibres can be exploited as novel antioxidants and would be of importance in protecting against superoxide radicals, hydroxyl free radicals and lipid peroxidation.23 They would thus find use as ingredients in fatty foodstuffs to improve oxidative stability, hence improving their shelf life. Antioxidants are important for human health as they prevent some degenerative diseases like cancers and decrease the oxidation of low density lipoproteins, thereby avoiding arteriosclerosis and related coronary heart diseases.37-39 Antioxidants carry out these functions by reacting with free radicals forming stable or non-reactive radicals.39 BGN fibres can be a useful source of natural antioxidants as alternatives to artificial antioxidants; artificial antioxidants have been shown to be carcinogenic and teratogenic.39 The low tannin content observed in BGN fibres could be advantageous as tannins have been associated with anti-nutritional properties because of their ability to form complexes with some nutrients, including divalent minerals and proteins, rendering them bio-unavailable.15,40
Neutral sugars and uronic acids in BGN fibres
Seven neutral sugars were analysed for in BGN fibres and the results are given in Table 4. Arabinose and galactose coeluted in this study and therefore are presented as arabinose/galactose in Table 4. The percentage of arabinose/galactose in SDFs was in the range 9.4% (brown) to 19.6% (black-eye). The percentage of fructose in SDFs ranged from 1.3% (black-eye) to 1.7% (brown). Fucose and glucose were obtained in low amounts (below 1%) in SDFs while relatively higher percentages of xylose were obtained in the range 13.0% (brown) to 16.6% (black-eye) (Table 4). Brown-eye and black-eye SDFs did not differ significantly (p > 0.05) in their sugar composition with the exception of arabinose/ galactose. Rhamnose was absent in all SDFs. This finding is in agreement with Dalgetty and Baik5 who reported the absence of rhamnose in SDFs of pea, lentil and chickpea. The researchers also reported the absence of arabinose and mannose in SDFs. In the current study however, these two sugars were present. These authors further reported higher values of xylose (32%) in chickpea SDF. These differences can be attributed to the different analytical methodologies adopted, with sugar assay having been used in the current study and HPLC having been used in the study by Dalgetty and Baik.5
The presence of these sugars in BGN SDFs suggests the possible presence of galactomannans, arabinoxylans and arabinogalactans. Galactomannans are related to locust bean and guar gums and their solubility in water increases with increasing galactose content. BGN SDFs had higher quantities of galactose compared to mannose hence the solubility of galactomannans would be elevated.41 Arabinoxylans possess antioxidant capabilities and influence water balance and rheology42 and arabinogalactans possess similar characteristics as gum Arabic.43-45 The suggestive presence of these hydrocolloids in BGN fibres could mean that BGN fibres possess similar beneficial characteristics and thus can be classified with them.
The uronic acid content of SDFs did not differ significantly (p>0.05) and ranged from 10.6% (brown) to 11.5% (red). Dalgetty and Baik5 reported lower uronic acids in pea, lentil and chickpea SDFs in the range 0.2% to 1.3%. Uronic acids form salts with some wastes in the human body thereby facilitating their excretion.46 Hence, their presence in BGN DFs would contribute to the body's detoxification.
In IDFs, arabinose/galactose was in the range 2.3% (brown-eye) to 2.8% (red). The percentage of fructose in IDFs ranged from 1.5% (brown) to 1.9% (red). Fucose and glucose were obtained in low amounts (below 1%) while rhamnose ranged from 1.0% (brown-eye) to 2.6% (black-eye). Relatively high percentages of xylose were obtained in IDFs (Table 4). The presence of these in BGN IDFs is an indication of the presence of some polysaccharides such as cellulose (glucose) and hemicellulose (xylose, glucose, arabinose, galactose and mannose).15,47
The presence of these sugars in BGN IDFs suggests the presence of some polysaccharides such as pectic substances (rhamnose and galactose)24 which in turn suggests the presence of rhamnogalacturonans.15 Rhamnogalacturonans have been reported to bind heavy metals in the human body as well as lower blood cholesterol.43,44 The presence of arabinose and xylose in IDFs suggest the presence of arabinoxylans. Low quantities of arabinose and galactose in IDFs suggest low quantities of arabinogalactans.
The uronic acid content of IDFs ranged from 6.7% (black-eye) to 10.6% (red). There was no significant difference among the red, brown and black-eye IDFs as well as between brown and brown-eye IDFs in uronic acid content. Dalgetty and Baik5 reported lower uronic acid of pea, lentil and chickpea IDFs in the range 2.0% to 2.8% indicating the superiority in uronic acid content of BGN IDFs over other leguminous IDFs.
Conclusions
The wet milling method was successfully applied in the extraction of BGN SDFs and IDFs yielding an appreciable amount of both fractions. Black-eye and brown-eye fibres have superior physicochemical properties compared to the brown and red fibres as evidenced by their higher swelling capacities, water-holding capacities, oil binding capacities, phenolic as well as total sugar content. Brown-eye and black-eye IDFs were lighter in colour, yellower, redder, more saturated and had higher hues compared to the red and brown IDFs. The physicochemical properties of BGN fibres make them valuable to the food industry as thickening agents, stabilisers, health ingredients as well as cryoprotectants in frozen dairy products. BGN fibres can be considered suitable alternatives for commercial fibres such as pea, chickpea and lentil fibres as they have been shown to possess similar qualities to these fibres.
Authors' contributions
The authors contributed equally to the work presented in this paper. YM. was responsible for the experimental work and wrote the manuscript. V.A.J. supervised the project, carried out the statistical analysis and proofread the manuscript.
Acknowledgements
The authors acknowledge the Cape Peninsula University of Technology (CPUT) University Research Funding and the National Research Foundation for financial assistance towards the research running costs. Bambara groundnut dietary fibre is a patent of CPUT; Dietary fibre supplement: South Africa complete patent (2014/04371).48
References
1. Jideani VA, Diedericks CF. Nutritional, therapeutic, and prophylactic properties of Vigna subterranea and Moringa oleífera. In: Oguntibeju O. editor. Antioxidant-antidiabetic agents and human health. Croatia: InTech; 2014. p. 187. http://dx.doi.org/10.5772/57029 [ Links ]
2. Jideani VA, Mpotokwana SM. Modelling of water absorption of Botswana Bambara varieties using Peleg's equation. J Food Eng. 2009;92(2):182-188. http://dx.doi.org/10.1016/j.jfoodeng.2008.10.040 [ Links ]
3. Fasoyiro SB, Ajibade SR, Omole AJ, Adeniyan ON, Farinde EO. Proximate, mineral and anti-nutritional factors of some underutilized grain legumes in South-Western Nigeria. Nutr Food Sci. 2006;38:18-23. http://dx.doi.org/10.1108/00346650610642151 [ Links ]
4. Fasoyiro S, Yudi W, Taiwo K. Processing and utilization of legumes in the tropics. In: Eissa AA, editor. Trends in vital food and control engineering. Croatia: InTech; 2012. [ Links ]
5. Dalgetty DD, Baik B. Isolation and characterisation of cotyledon fibers from peas, lentils, and chickpeas. Cereal Chem. 2003;80(3):310-315. [ Links ]
6. Ojimelukwe PC. Sourcing and processing of legumes. In: Onwualu PA, Obasi SC, Ukpabi, UJ, editors. Nigerian agro raw materials development. Abuja: Raw Materials Research and Development Council, 2009. [ Links ]
7. Daou C, Zhang H. Physico-chemical properties and antioxidant activities of Dietary Fiber derived from defatted rice bran. Adv J Food Sci Technol. 2011;3(5):339-347. [ Links ]
8. Hawkes C. Uneven dietary development: Linking the policies of globalization with the nutrition transition, obesity, and diet-related chronic diseases. Global Health. 2006;2:4. http://dx.doi.org/10.1186/1744-8603-2-4 [ Links ]
9. Wood JA, Grusak MA. Nutritional value of chickpea. In: Yadav SS, Redden R, Chen W, Sharma B, editors. Chickpea breeding and management. Trowbridge: Cromwell Press; 2007. p.119. http://dx.doi.org/10.1079/9781845932138.005 [ Links ]
10. Khan AR, Alam S, Ali S, Bibi S, Khalil IA. Dietary fiber profile of food legumes. Sarhad J Agric. 2007;23(3):763-766. [ Links ]
11. USA Dry Pea and Lentil Council. Processing methods for dry peas, lentils and chickpeas. In: Pulse processing technical manual. Moscow: USA Dry Pea and Lentil Council; 2010. [ Links ]
12. Bogracheva T, Cserhalmi Z, Czukor B, Fornal J, Schuster-Gajzago I, Kovacs ET, et al. Processing. In: Henley CL, editor. Carbohydrates in grain legume seeds: Improving nutritional quality and agronomic characteristics. New York: CABI Publishing; 2001. p. 89-92. [ Links ]
13. Tiwari U, Cummins E. Functional and physicochemical properties of legume fibers. In: Tiwari BK, Gowen, A, McKenn B, editors. Pulse foods: Processing, quality and nutraceutical applications. London: Academic Press; 2011. p. 139, 144. http://dx.doi.org/10.1016/B978-0-12-382018-1.00005-8 [ Links ]
14. Urriola PE, Cervantes-Pahm SK, Stein HH. Fiber in swine nutrition. In: Chiba LI, editors. Sustainable swine nutrition. West Sussex: Wiley-Blackwell; 2013. p. 259. http://dx.doi.org/10.1002/9781118491454.ch11 [ Links ]
15. Diedericks CF. Functional properties of Bambara groundnut (Vigna Subterranea (L.) Verdc.) non-starch polysaccharides in model and food systems [MTech dissertation]. [ Links ] Cape Town: Cape Peninsula University of Technology; 2014.
16. Wang N, Toews, R. Certain physicochemical and functional properties of fibre fractions from pulses. Food Res Int. 2011;44(8):2515-2523. http://dx.doi.org/10.1016/j.foodres.2011.03.012 [ Links ]
17. Parrott ME, Thrall BE. Functional properties of various fibers: Physical properties. J Food Sci. 1978;43:759-763. http://dx.doi.org/10.1111/j.1365-2621.1978.tb02412.x [ Links ]
18. Gordon DT, Okuma K. Determination of total dietary fiber in selected foods containing resistant maltodextrin by enzymatic-gravimetric method and liquid chromatography: Collaborative study. J AOAC Int. 2002;85:435-444. [ Links ]
19. Baryeh EA. Physical properties of Bambara groundnuts. J Food Eng. 2001;47:321-326. http://dx.doi.org/10.1016/S0260-8774(00)00136-9 [ Links ]
20. Mkandawire CH. Review of Bambara groundnut (Vigna subterranea (L.) Verdc.) production in Sub-Sahara Africa. Agric J. 2007;2(4):464-470. [ Links ]
21. Murevanhema YY, Jideani VA. Potential of Bambara groundnut (Vigna subterranea (L.) Verdc) milk as a probiotic beverage: A review. Crit Rev Food Sci. 2013;53(9):954-967. http://dx.doi.org/10.1080/10408398.2011.574803 [ Links ]
22. Guillon F, Champ M. Structural and physical properties of dietary fibres, and consequences of processing on human physiology. Food Res Int. 2002;33:233-245. http://dx.doi.org/10.1016/S0963-9969(00)00038-7 [ Links ]
23. Elleuch M, Bedigian D, Roiseux O, Besbes S, Blecker C. Dietary fibre and fibre-rich by-products of food processing; Characterisation technological functionality and commercial applications: A review. Food Chem. 2011;124:411-421. http://dx.doi.org/10.1016/j.foodchem.2010.06.077 [ Links ]
24. Huang SC, Lia TS, Cheng TC, Chan HY, Hwang SM, Hwang DF. In vitro interactions on glucose by different fibre materials prepared from mung bean hulls, rice bran and lemon pomace. J Food Drug Anal. 2009;17:307-314. [ Links ]
25. Rosell CM, Santos E, Collar C. Physico-chemical properties of commercial fibres from different sources: A comparative approach. Food Res Int. 2009;42:176-184. http://dx.doi.org/10.1016/j.foodres.2008.10.003 [ Links ]
26. Tudorica CM, Kuri V, Brennan CS. Nutritional and physicochemical characteristics of dietary fiber enriched pasta. J Agric Food Chem. 2002:50(2):347-356. http://dx.doi.org/10.1021/jf0106953 [ Links ]
27. Chau CF, Huang YL. Characterization of passion fruit seed fibres - a potential fibre source. Food Chem. 2004;85:189-194. http://dx.doi.org/10.1016/j.foodchem.2003.05.009 [ Links ]
28. Chau CF, Huang YL, Chang FY Effects of fibre derived from passion fruit seed on the activities of ileum mucosal enzymes and colonic bacterial enzymes in hamsters. J Sci Food Agric. 2005;85:2119-2124. http://dx.doi.org/10.1002/jsfa.2230 [ Links ]
29. Esposito F, Arlotti G, Bonifati AM, Napolitano A, Vitale D, Fogliano V. Antioxidant activity and dietary fibre in durum wheat bran by-products. Food Res Int 2005;38:1167-1173). http://dx.doi.org/10.1016/j.foodres.2005.05.002 [ Links ]
30. Tosh SM, Yada S. Dietary fibres in pulse seeds and fractions: Characterisation, functional attributes and applications. Food Res Int. 2010;43(2):450-460. http://dx.doi.org/10.1016/j.foodres.2009.09.005 [ Links ]
31. Kohajdova Z, Karovicova J, Magala M. Effect of lentil and bean flours on rheological and baking properties of wheat dough. Chem Pap. 2013;67(4):398-407. http://dx.doi.org/10.2478/s11696-012-0295-3 [ Links ]
32. Maskus H. Pulse processing, functionality and application. Manitoba: University of Winnipeg; 2010. [ Links ]
33. Slavin J. Fiber and prebiotics: Mechanisms and health benefits. Nutrients. 2013;5(4):1417-1435. http://dx.doi.org/10.3390/nu5041417 [ Links ]
34. Murevanhema YY. Evaluation of Bambara groundnut (Vigna subterranea (L.) Verdc) milk fermented with lactic acid bacteria as a probiotic beverage [MTech dissertation]. Cape Town: Cape Peninsula University of Technology; 2012. [ Links ]
35. Nti CA. Effects of Bambara groundnut (Vigna subterranea) variety and processing on the quality and consumer appeal for its products. Int J Food Sci Tech. 2009;44(11):2234-2242. http://dx.doi.org/10.1111/j.1365-2621.2009.02064.x [ Links ]
36. Sharma A. Understanding color management. USA: Thomson Delmar Learning, 2004. [ Links ]
37. Tomas M, Latorre G, Senti M, Marrugat J. The antioxidant function of high density lipoproteins: A new paradigm in atherosclerosis. Rev Esp Cardiol. 2004;57(6):557-569. http://dx.doi.org/10.1016/s1885-5857(06)60630-0 [ Links ]
38. Tsimikas S. Lipoproteins and oxidation. In: Bourassa MG, Tardif JC, editors. Antioxidants and cardiovascular disease. 2nd ed. Montreal: Springer; 2006. p 17. http://dx.doi.org/10.1007/0-387-29553-4_2 [ Links ]
39. Betancur-Ancona D, Perza-Mercado G, Moguel-Ordonez Y, Fuertes-Blanco S. Physicochemical characterisation of lima beans (Phaselous lunatus) and jack bean (Canavalia ensiformis) fibrous residues. Food Chem. 2004;84:287-295. http://dx.doi.org/10.1016/S0308-8146(03)00213-9 [ Links ]
40. Saura-Calixto FD, Bravo L. Dietary fiber-associated compounds: Chemistry, analysis, and nutritional effects of polyphenols. In: Cho SS, Dreher ML. editors. Handbook of dietary fiber. New York: Marcel Dekker Inc; 2001. http://dx.doi.org/10.1201/9780203904220.ch22 [ Links ]
41. Silveira JLM, Bresolin TMB. Pharmaceutical use of galactomannans. Quim Nova. 2011;34(2):292-299. http://dx.doi.org/10.1590/S0100-40422011000200023 [ Links ]
42. Saeed F, Pasha I, Anjum FM, Sultan MT. Arabinoxylans and arabinogalactans: A comprehensive treatise. Crit Rev Food Sci Nutr. 2011;51(5):467-476. http://dx.doi.org/10.1080/10408391003681418 [ Links ]
43. Sivam AS, Sun-Waterhouse D, Quek SY, Perera CO. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J Food Sci. 2010;75(8):163-174. http://dx.doi.org/10.1111/j.1750-3841.2010.01815.x [ Links ]
44. Khotimchenko Y, Khozhaenko E, Kovalev V, Khotimchenko M. Cerium binding activities of pectins isolated from the seagrasses Zostera marina and Phyllospadix iwatensis. Mar Drugs. 2012;10(4):834-848. http://dx.doi.org/10.3390/md10040834 [ Links ]
45. Golenser J, Frankenburg S, Ehrenfreund T, Domb AJ. Efficacious treatment of experimental leishmaniasis with amphotericin B-arabinogalactan water-soluble derivatives. Antimicrob Agents Chemother. 1999;43(9):2209-2214. [ Links ]
46. Vazquez JA, Rodriguez-Amado I, Montemayor MI, Fraguas J, Gonzalez MDP, Murado MA. Chondroitin sulfate, hyaluronic acid and chitin/chitosan production using marine waste sources: Characteristics, applications and eco-friendly processes: A review. Mar Drugs. 2013;11(3):747-774. http://dx.doi.org/10.3390/md11030747 [ Links ]
47. Dhingra D, Michael M, Rajput H, Patil RT. Dietary fibre in foods: A review. J Food Sci Technol. 2012;49:255-266. http://dx.doi.org/10.1007/s13197-011-0365-5 [ Links ]
48. Jideani VA, Diedericks CF. Dietary fibre supplement from Bambara groundnuts (Vigna subteranea). South Africa - Complete patent (2014/04371); 2014 June 19. Pretoria: Companies and Intellectual Property Division; 2014 [ Links ]
 Correspondence:
Correspondence:
Yvonne Maphosa
Department of Food Technology, Cape Peninsula University of Technology
PO Box 1906, Bellville 7535, South Africa
yvonmaphosa@gmail.com
Received: 30 Mar. 2015
Revised: 17 May 2015
Accepted: 06 July 2015














