Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.112 n.1-2 Pretoria Jan./Feb. 2016
http://dx.doi.org/10.17159/sajs.2016/20150165
RESEARCH ARTICLE
Australopithecus robustus societies - one-male or multimale?
Katarzyna A. Kaszycka
Department of Human Evolutionary Ecology, Institute of Anthropology, Adam Mickiewicz University, Poznan, Poland
ABSTRACT
Determining the sex of individual specimens is important in estimating the degree of sexual dimorphism. Sexual dimorphism, in turn, provides clues for reconstructing the social organisation and mating systems of extinct species. In an article published in Science, Lockwood et al. (Lockwood CA, Menter CG, Moggi-Cecchi J, Keyser AW. Extended male growth in a fossil hominin species. Science. 2007;318:1443-1446.) suggested an uneven sex ratio (in favour of males) for the known individuals of the South African Pleistocene hominid, Australopithecus robustus, and claimed evidence of an extended period of growth (delayed maturity) for the males of this species. They concluded that this finding, combined with estimates of sexual size dimorphism, suggests a polygynous reproductive strategy, and a social system similar to that of silverback gorillas (i.e. one-male harems). On re-examination of these claims, and based on further analysis, I agree with Lockwood et al. that morphologically A. robustus exhibits an increased (almost gorilla-like) level of facial dimorphism, but propose using an alternate (clustering) technique for grouping the specimens of highly dimorphic species into sexes, and argue that their pronouncements regarding a polygynous social structure of these early hominids are inconclusive. I contend instead that the habitat occupied by this species suggests rather that a one-male harem social structure would have been counterproductive.
Keywords: australopithecine; sexing fossils; size dimorphism; mating system; social structure; k-means clustering
Introduction
Sexual dimorphism (genetically determined differences between the sexes) is a common phenomenon among both extant and extinct primates.1 The dimorphism in secondary sexual characteristics (i.e. those not directly related to reproduction) can be found in a variety of morphological features. In primates, these characteristics are primarily differences in overall body size and dimensions of the canine teeth and are among the skeletal features which can also be observed in fossils. Sexual dimorphism correlates with patterns of social organisation and mating systems.2-5 As a general rule, size sexual dimorphism is lacking among monogamous primate species, while polygynous species (showing intensive male-male competition) are dimorphic,6 with males commonly being larger than females. There are, however, two ways in which a difference in body size between males and females at adulthood (i.e. sexual maturity) can be attained during development7,8: difference in rate of growth - males growing faster than females, and difference in duration of growth - males maturing, breeding and achieving full body weight later than females (so-called bimaturism). For example, female gorillas start breeding at the age of 10, while male gorillas only 5 years later (at about 11 years of age, males start becoming larger than females).9
Sexual dimorphism provides clues for reconstructing the social structure and mating systems of extinct species.4,10 The current data for early hominids - the australopithecines - are not unequivocal in this regard, suggesting a unique (among primates) combination of small canine size dimorphism and marked body mass dimorphism4 which makes inferring of social behaviour difficult. In addition, there is also disagreement about the degree of body size dimorphism. Depending on the australopithecine species, the literature provides data of assessed sexual dimorphism ranging in values from characteristics of gorillas4,11 to those of chimpanzees12,13 or even modern humans.14,15 (I use the term 'hominid' rather than 'hominin' to refer to the human clade after the split from the chimpanzee line in its classical, narrow meaning - as a common name (such as 'ape') and not in a taxonomic sense. I use the term 'australopithecines' to refer to a group of species of Plio-Pleistocene extinct relatives of humans, which comprise the so-called 'gracile' and 'robust' forms. Because I disagree with the generic separation of the two groups, I have used the species name Australopithecus robustus and not Paranthropus robustus.)
The lack of agreement about the degree of size skeletal dimorphism occurs not only for different species of early hominids, but also within the same species and even the same samples. For example, Reno14 claimed that sexual dimorphism for Australopithecus afarensis was not pronounced, i.e., gorilla-like (as generally believed), but only moderate, i.e., contemporary human-like, and implied a principally monogamous mating system for this species - conclusions that were both criticised by Plavcan et al.16 Lee17, on the other hand, argued that size sexual dimorphism of A. afarensis varies, depending on the skeletal elements taken into consideration, i.e. in femoral variables it is similar to gorillas, in humeral variables, similar to humans, and in canine variables, similar to chimpanzees. Yet, whatever the degree of dimorphism in the postcranial skeleton of the South African 'robust' australopithecines, it at least seems likely that they had markedly dimorphic faces.18-20
In 2007, Lockwood et al.18 published a paper claiming evidence of an extended period of growth (delayed maturity) for males in the South African Australopithecus robustus sample from approximately 1.5-2.0 million years ago, along with its implications. They argued that ranking a sample of early hominid cranial remains on the basis of the stages of tooth wear revealed a difference in size between young and old adult males. The authors18 stated that their finding, combined with estimates of gorilla-like level of sexual size dimorphism (based on dimensions of the face), suggests a reproductive strategy in which males control mating access to multiple females in a manner similar to that of silverback gorillas. Males, however, experience the costs of such behaviour in the form of high rates of predation, i.e. the peripheral, solitary life of young adult males places them at greater risk of becoming victims of a predator attack.
These claims were then highly publicised and commented upon,21 but warrant re-examination, as the line of reasoning taken (both for the methods used and interpretation of the results) was somewhat speculative and raises several questions. These concern not only sexing individual australopithecine specimens (the actual sex ratio in the sample, the relation between sex and size, and sex and range of variation), but also the supposed evidence for bimaturism and one-male-multifemale social grouping and lastly, the possibility of a polygynous gorilla-like harem pattern of social structure on savannah.
Re-examination of Lockwood's claims
'Sexing' australopithecine specimens
Sex ratio in the A. robustus sample
Lockwood et al.18, on the basis of size and morphology, concluded that the known craniofacial fragments of the South African robust australopithecines represent individuals in an uneven sex ratio of 4:1 in favour of males. According to the authors,18 the sample included as many as 15 males and only 4 females. (Previous estimates for the Swartkrans plus Kromdraai sample22,23 were shown to be biased toward females). The approach I used to determine the actual sex ratio in the sample of A. robustus involved sorting the individuals into two groups (presumably sexes) using cluster analysis (a k-means clustering algorithm).24
Materials: samples and measurements
The materials used in my analysis consisted mostly of the same fossil sample of Pleistocene hominids as studied by Lockwood et al.18 -Australopithecus robustus from South African sites of the Gauteng area, housed in the Ditsong National Museum of Natural History (formerly known as the Transvaal Museum) in Pretoria, and the University of the Witwatersrand, Johannesburg. I also used comparative samples consisting of extant African apes (see Figure 1) - western gorillas (Gorilla gorilla) and chimpanzees (Pan troglodytes) of known sex, both from the Powell-Cotton collection in the Quex Museum, Birchington, Kent, England. (These apes were shot in the wild during the 1920s and 1930s in the French Congo, now known as the Democratic Republic of Congo, and Cameroon).
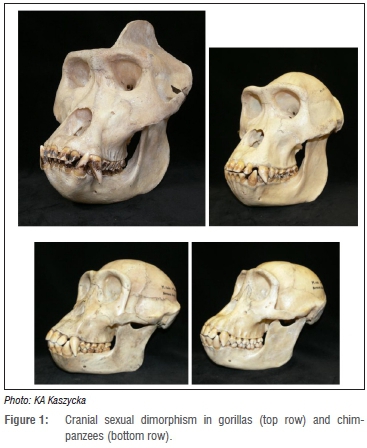
Lockwood et al.18 examined 12 measurements (selected dimensions of the face and palate) of 19 A. robustus specimens (17 from Swartkrans, 1 from Kromdraai and 1 from Drimolen). The facial measurements used were: orbito-alveolar height (OALH), orbito-jugal height (OJUH), foraminal height (FORH), alveolar height (ALVH; ns-pr), bimaxillary breadth (BMAB; zm-zm), interforaminal breadth (IFOB), nasal aperture breadth (NASB; apt-apt), and snout breadth (SNOB). The palatal measurements used were: anterior maxillo-alveolar breadth (ANMB), maxillo-alveolar breadth (MAXB; ekm-ekm); palatal breadth (PALB; enm-enm), and postcanine maxillo-alveolar length (PMXL) (see Figure 2 and Lockwood19 for measurement definitions).

Eleven of these dimensions (excluding snout breadth) were also measured by me. I, however, omitted foraminal height, as I considered this measurement to be not only correlated with the two other height measures of the face (orbito-alveolar height, and orbito-jugal height), but also of little potential use in describing sexual dimorphism. To equate to the same number of dimensions used by Lockwood et al.18 - i.e., twelve, I added two measurements which were highly dimorphic in apes20 and of sufficient number in my fossil sample. These two additional dimensions were: maxillary canine (or canine socket) bucco-lingual breadth and palate length, i.e. length of the maxillary palatal process (orale-staurion; ol-sr). The above-mentioned bucco-lingual canine dimension also appeared to be quite dimorphic for A. robustus (as assessed by the CV method26 - the index of sexual dimorphism ISD=1.10; n=26).20 To make all dimensions comparable, they were standardised for mean values and standard deviations.
My sample of Australopithecus robustus included 17 maxillofacial specimens: 14 from Swartkrans Member 1 (dated to ~ 1.6-1.8 Ma) (SK 11, SK 12, SK 13/14, SK 46, SK 48, SK 52, SK 79, SK 83, SK 845, SKW 8, SKW 11, SKW 12, SKW 29, and SKX 265), and one each from Kromdraai (TM 1517), Coopers (COB 101) and Drimolen (DNH 7). Although two specimens included in my analysis - SK 13/14 and SK 52 - were subadults (their third molars were only just erupting), I decided to use them because the dimensions of their canine sockets seemed to sufficiently distinguish them with regard to sex, and their face/palatal measurements were considered to be probably close enough to the minimal values for young adults.
Of the sample of 62 gorilla cranial specimens measured by me,20 48 complete (based on possession of at least 10 out of the 11 above-mentioned facial measurements plus the maxillary canine breadth) adult individuals were used for this analysis (26 females and 22 males). In the case of the chimpanzees, 38 out of 56 measured cranial specimens20 were used (22 females and 16 males). All the apes were adults, based on the eruption of the third molar. All dimensions were measured by the current author, with the exception of the DNH 7 specimen from Drimolen, for which I used Lockwood's18 and Moggi-Cecchi's27 data.
Methods
My approach to the issue of 'sexing' the australopithecine specimens, and determining the actual sex ratio in the fossil sample of A. robustus, was to partition the individuals into two groups (presumably sexes) using cluster analysis (STATISTICA 9.1). The k-means clustering technique, through the Hartigan and Wong algorithm, was used24 (where k is the number of clusters desired, while the action in the algorithm centres around finding the k-means). Cluster analysis is a multivariate analysis technique that seeks to organise information about variables so that relatively homogeneous groups (clusters) can be formed. From the point of view of the calculation, the k-means method is a 'reversal' of analysis of variance (ANOVA). In the k-means clustering, the program transfers objects (cases) between k randomly formed groups (clusters), to achieve a minimum variation within each group and the maximum variation between groups - so as to form groups (clusters) which will be as different as possible.
The accuracy of the k-means clustering was tested on samples of African apes of known sex, which made it possible to compare the results of the cluster analysis with actual data on the sex of the individuals, using the same measurements taken for the australopithecines (i.e. 11 face measurements plus one canine measurement).20 To assess whether this technique is (or is not) biased towards recovering groups of equal size,28 and sensitive to the order the algorithm is applied to the cases,28 several additional analyses were performed using different variants: (1) two, three, and four times as many representatives of one sex than representatives of the other sex in the sample, and (2) different orders of case entry: (a) all representatives of one sex, then all representatives of the other sex, (b) one representative of each sex one after another, and (c) five representatives of one sex, and five representatives of the other sex.
Results
By applying cluster analytic techniques for the apes, I obtained accurate results for highly dimorphic gorillas, but the method was not as statistically robust for the much less dimorphic chimpanzees. Thus the gorillas were partitioned into two sexes with a high level of accuracy: as many as 94% of the individuals classed among members of the larger and the smaller groups were actually males and females, respectively. Out of 48 individuals only three (6%) were assigned the incorrect sex group: one large gorilla female was grouped with males, and two small gorilla males were grouped with females. Average measurements for actual sexes and the calculated 'sexes' (i.e. those obtained using the clustering algorithm) were insignificantly different from each other. The results for weakly dimorphic chimpanzees, on the other hand, were not accurate, with nearly 30% of individuals (11 out of 38) assigned to the incorrect sex group. There is a significant difference in the frequency of correct sex assignment between gorillas and chimpanzees - according to Fisher's exact test; p=0.007 (Table 1).
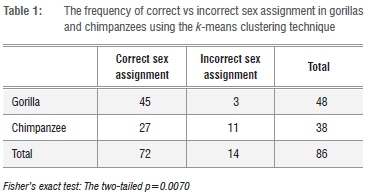
Having an unequal sex ratio in the gorilla sample: twice as many males than females (22 M vs2 11 F), and twice as many females than males (26 F vs. 13 M) had no bearing on the results. Likewise, having three (21 M vs. 7 F), and even four-times (20 M vs. 5 F) as many males than females in the sample also did not significantly change the final classification - each time from zero up to no more than three individuals were assigned the incorrect sex group (0-10%). I therefore reject the hypothesis that the k-means clustering algorithm used here is biased towards recovering clusters of roughly equal size. Changing the order of a dataset entry (either the cases or the variables), did not influence the classification at all. Therefore, I contend that k-means clustering is a reliable technique for sorting individual specimens of highly dimorphic species (such as the gorilla) into sexes.
The degree of size sexual dimorphism for A. robustus seems to correspond with that for gorillas (the male facial features being on average 17% larger than in females),18,20 so the k-means clustering technique, by assumption, should also be useful in grouping this fossil species into two subsets (sexes). The program divided the A. robustus sample into two groups (which differed significantly in 6 out of 12 features20) of roughly equal numbers of individuals of both sexes, and not a ratio of 4:1 in favour of the males. In the first group, consisting of the smaller individuals (presumably females), the following specimens were found: SK 13/14, SKW 11, SK 48, DNH 7, COB 101, SK 79, SKW 12 and SKW 8 (n=8; Figure 3). In the second group, composed of the larger individuals (presumably males), the following specimens were found: SK 52, SKX 265, TM 1517, SKW 29, SK 845, SK 11, SK 83, SK 46 and SK 12 (n=9; Figure 4). One may conclude therefore that either the size sexual dimorphism in A. robustus was not as pronounced, and the clustering method used here inadequate, or that the sex ratio reported in the Lockwood et al.18 study is not necessarily correct.
Other factors used as a 'guide' to variation and sex
Relation between sex and size
In light of the foregoing, the sex assignment for the specimens of Australopithecus robustus in the Lockwood et al.18 study appears unconvincing. Furthermore, sex assignment can also be questioned from standpoints other than research above. In the Lockwood et al18 study, only individuals of overall size categories 1-2 were considered to be females and all others (size categories 3-9) to be males (see Figure 5). In Lockwood's 'Supporting Online Material'18, this step was justified in morphological and metric terms, for example, by referring to the smallest (and the youngest) A. robustus individual identified as male (SKW 11) and its measurements. These measurements were supposedly found to be closer to that of five other specimens ('males') than they were to the small Drimolen female - DNH 7 - even though the authors18 recognised that DNH 7 is substantially smaller than other well-preserved skulls of A. robustus. Listed in Table 2 are three facial measurements cited by Lockwood et al.18 to support their statements [see Lockwood's 'Supporting Online Material' p. 5, and Table S118]. In these examples [at least for AnMB and IFOB - see Table 2], it appears that the claim for the measurements of Swartkrans specimen SKW 11 being closer to those of 'other males' than those of the Drimolen female, is an over-interpretation.


Relation between sex and range of variation
Lockwood et al.18 also asserted that 'it is unlikely that any of the other relatively complete skulls are female' (p. 1444), including the smallest of the previously known ones: Kromdraai TM 1517 and Swartkrans SK 48, because the size differences between either of these and Drimolen DNH 7 is too large (as is the range of variation among the supposed females).
In contrast, firstly, cluster analysis places the individual SK 48 in the same group as DNH 7, therefore these specimens can well be regarded as large and small adult females, respectively. It is worth mentioning that SK 48 was grouped with females in spite of a low sagittal crest. (While crests are commonly found in gorillas males, gorilla females with crests are known to occur, as was the case for the largest gorilla female in my sample).
Secondly, the range of variation expressed by the ratio of size: 'Mean of a larger individual to the mean of a smaller individual' is greater between the largest and the smallest western gorilla females in a sample n=26, shot two years apart in Cameroon (ratio=1.15), than between the australopithecine individuals: TM 1517 / DNH 7 (ratio=1.131) or SK 48 / DNH 7 (ratio=1.128), which may be tens, or even hundreds of thousands years apart. The chance of sampling two gorilla females, whose ratio of size is equal to or greater than 1.13, is p=3.4% (probability based on the exact resampling of all 325 possible combinations from a sample of n=26). This probability exceeds 2 standard errors (SE=1.01) and therefore cannot be discounted. It should also be mentioned that in Lockwood et al.'s Table S218, the given chance of sampling two gorilla females, whose ratio of size is equal to or larger than 1.13, is even greater (p=5.4% probability based on 5000 random samples with replacement in a sample n =37).
Bimaturism and social structure
Relation between age of adults and size - evidence for bimaturism?
There are several problems with the assessment of correlation between the age of adults and overall size (small size = young adults, large size = old adults) in the A. robustus sample, and the claim of evidence of extended male growth. Firstly, when calculating the correlation between age and size of the male specimens, the assumption was made that size categories 3 and 4 are male. In light of the results of the k-means clustering analysis I performed, this assumption is not necessarily correct. Furthermore, it is also noteworthy that size-3, age-1 Swartkrans SKW 11 specimen was shown here to be well within the female size range (see Table 2).
Secondly, there may be a problem with assessing the level of statistical significance of the above-mentioned correlation. Lockwood et al. claim that: 'When a randomisation test of correlation coefficient is used, age and size are significantly correlated among the male maxillofacial specimens (r=0.52; p=0.027, one-tailed test)' (p. 1445).18 Whether or not the one-tailed test should have been used is a matter for debate. The p-value of the two-tailed test (p=0.054) shows a non-significant correlation between age and size, as does the Spearman's rank correlation coefficient (rS)=0.51.
Finally, it should also be noted that an age-size relation within the sexes (among females: no correlation; among males: positive correlation) was not shown for the mandibular specimens (even though mandibular measurements are generally thought to show quite a high degree of sexual dimorphism in various primate and fossil hominid species).1,5,19,23 Lockwood et al.18 did not assign sexes to A. robustus mandibles, stating that 'there is greater overlap in male and female mandible size in sexually dimorphic hominoids' (p. 1445) and that 'the available specimens preserve less information than the maxillas' [Lockwood et al.18 'Supporting Online Material', p. 3]. However, in contrast to the maxillary specimens, there is a significant correlation between age and size for the whole sample of mandibular specimens (rS=0.66; p<0.05, based on data from Lockwood's Fig. 118), which should not be the case for both sexes together, unless all of them were males.
Evidence for one-male polygynous groups?
Sex differences in predation risk
Lockwood et al.18 argued that in sexually dimorphic primates, non-dominant adult males spend more time alone, on the periphery of a social group, or in small all-male bands.29 A more peripheral, solitary life places males at greater risk of becoming victims of a predator attack.30-32 An example adduced to illustrate this claim is the behaviour of Papio cynocephalus baboons, in which the mortality rate for dispersing males compared to males living in groups increases by at least 2-3 times.32 This difference in mortality was said to match the 4-fold difference in the sex ratio, as claimed for the Swartkrans australopithecines.18
Dunbar33 emphasised that although primate males frequently suffer higher mortality rates than females, this occurs at any given age, and irrespective of whether they belong to multimale or one-male groups. Besides being exposed to a higher risk of predation, males also have a greater likelihood of sustaining fatal wounds as a result of fighting over mates, or entering new groups. It was shown by Brain34,35 that the bone accumulations in the Swartkrans cave can indeed, largely be attributed to predator behaviour (e.g. the Swartkrans leopard hypothesis34). Besides leopards, the primary predators suggested for the australopithecines were sabre-toothed felids and hunting hyenas.35 Extant savannah baboons face threats from very predators, namely leopards, lions and hyenas,31 particularly when visibility is limited. Sex differences in predation risk by large felids were investigated in these baboon populations, and while leopards were indeed shown to prey on males more frequently than on females, the actual proportion of attacked and killed males to females was about 60% vs 40%31 - i.e. only 1.5 times (and not 4 times) more often.
Association between delayed maturity and group composition
Taking the gorilla as a model, Lockwood et al.18 also implied a relationship between extended male growth of A. robustus and group composition. While an association between modes of dimorphic growth (different duration vs different rate) and different kinds of group compositions in anthropoid primates has previously been suggested,8 it is the multimale, rather than the one-male species that tends to attain dimorphism through bimaturism. Although similar levels of adult body size dimorphism in gorillas and A. robustus might still be produced through the same ontogenetic processes, the conclusions reached regarding the same social structure of those species18 are not justified. Examples of primates showing a similarly high level of bimaturism (4 years), though not quite the same group composition, are gorilla (G. gorilla) and rhesus monkey (Macaca mulatta)36 - the latter composed of large multimale-multifemale social groups.
It would appear, therefore, that the Lockwood et al.18 argument that difference in sex bias of male deaths resulting from predation and extended male growth be taken as evidence of one-male Australopithecus robustus social groups, seems dubious.
Clues to A. robustus societies from ecological factors
As the arguments by Lockwood et al.18 regarding the gorilla-like polygynous social structure of Australopithecus robustus appear to be inconclusive, one can speculate about an alternative, given the extreme sexual dimorphism of this species.18,20 In doing so, it is useful to consider ecological pressures as an important factor in determining the pattern of social organisation of a species. As is well-known, even small ecological differences can bring about large consequences for social behaviour. It seems beyond doubt that the observed pronounced sexual dimorphism in the craniofacial features of A. robustus and the consequent dimorphism in body mass5 imply either polygyny (one-male-multifemale harems) or multimale-multifemale groups with a dominance hierarchy. However, is it possible there existed uni-male 'female-defence polygyny' hominid societies on savannah?
In attempting to answer this, one additional point needs to be made. As argued by Dixson37, the social organisation of a primate group and its mating system are not necessarily synonymous. Dixson37 gives the example of mountain gorilla to illustrate that major differences may exist between the two. Mountain gorilla groups may consist of up to 3-4 adult silverback males, forming therefore a multimale social group, yet the majority of copulations are performed by a single, dominant male, so that the mating system is one-male polygynous. Furthermore, in some cases, different social groups and more than one mating system does occur within the same primate species (e.g. one-male and multimale-multifemale social units in the hanuman [gray] langur, or monogamy and polyandry in some calitrichids).37 This occurrence may reflect lability in the mating systems of different populations of a given species under different circumstances or environmental conditions (e.g. different food availability or density situation).38
The South African Australopithecus robustus occupied a different habitat to the gorillas with regard to diet, spatial distribution of resources, food availability and predation risk. Gorillas, despite the marked differences in habitats between the lowland and the mountain populations, live in forests where visibility is limited because of dense vegetation, while the 'robust' australopithecines lived out on the open savannah. In terms of food quality, A. robustus was most probably an omnivore, inclined towards herbivory, feeding on diverse plant foods requiring intensive mastication. They must have eaten seasonal fruits, but might also have significantly fed on hard food objects such as seeds, roots and nuts,39,40 at least during times of food scarcity. As the southern African australopithecines appear to have enamel isotope values, indicating a considerable amount (about one-third)41 of C4-derived food in their diet,42 it has been suggested that savannah-based foods such as tropical grasses and sedges, and/or the animals and insects that themselves eat those plants, made up an important but variable part of its diet.41-44 Others45 have concluded that the C4 plant underground storage organs for hominid diets was a possible source of nutrition.
There is a marked difference between the diets (staple foods) of the savannah-dwelling 'robust' australopithecines and the mostly tropical forest-dwelling African apes. Gorillas, despite their regional differences, primarily eat lower quality but abundant foods such as leaves, bark, bamboo shoots and herbs that they forage for mostly on the ground (lowland gorillas also forage on fruit).46-49 Chimpanzees, on the other hand, are obligated fruit eaters48 (for which they forage high in trees),49 consuming predominantly C3-derived foods.50 They are also meat-consumers.51 The diet of the 'robust' australopithecines resembled rather more that of savannah baboons,52 which consume significant quantities of C4 resources such as grass seeds and roots.53 If A. robustus competed with these terrestrial monkeys, they would have gained an advantage over the baboons in those parts of the trophic niche that contained fallback foods, which required heavy chewing and grinding.
The South African 'robust' australopithecines appear to have consumed food that occurred in scattered patches and their daily path lengths must therefore have been relatively large, unlike gorillas, who travel short distances (usually 1-2 km/day).9,47,49 It also seems unlikely that A. robustus females fed dispersed from each other (as do females of the great apes) as this would have been too risky on the open savannah. For the australopithecines, being medium-sized12,13,54,55 savannah inhabitants, predator pressure is not sufficiently suggestive of one-male harems because of the high risks involved. Rather, it would have been more advantageous if they were organised as cohesive multimale-multifemale groups in which extra males were of value in protecting the females and offspring. Even for much larger sized gorillas (i.e. mountain gorillas), about one-third of their social groups are multimale groups.9,56 It has been suggested that the existence of multimale mountain gorilla groups increases the chances of survival and future reproductive success of all age categories of specimens, namely infants, subadults and adults.57 Gorilla multimale teams can resist male outsiders more powerfully than each can achieve individually.58
In most species of primates living in groups, it is the males, not females, that tend to leave the natal groups and transfer between groups.29,30,59 However, in gorillas, chimpanzees and hamadryas baboons,9,60 it is the females that transfer between groups. While authors usually invoke the fundamental difference between the social structures of monkeys and apes, there are notable exceptions to this rule. For example, while savannah species of baboons: Papio papio, P. anubis, P. cynocephalus and P. ursinus form multimale-multifemale groups (based on related females), arid country Papio hamadryas forms one-male harems.61 A few one-male units, however, frequently keep the company of one another, forming higher level social units, called clans, and several clans form bands, thus displaying a multileveled organisation of society.62,63 As such, the social structure of hamadryas baboons appears to exhibit features of both types of primate social organisation: one-male and multimale. It has therefore been suggested that the multileveled organisation might be a more suitable model (than either of these two systems) for investigating the emergence of some of the aspects of early hominid societies.62 Yet, on the other hand, Smuts' observations64,65 of olive baboons (P. anubis) are also very suggestive in the context of the evolution of male-female relationships in the human lineage. She reported that in groups of these baboons (which have multimale-multifemale societies), both females and males often develop strong preferences towards certain sexual partners in the context of long-term social bonds (which may last for years).64,65
Concluding remarks
Ecological factors, on the one hand, and evolutionary/phylogenetic history, on the other, determine the pattern of social organisation of species66 and impose limits on the range of types of this organisation that will be adaptive.38 If the set of ecological variables (habitat, diet, spatial and temporal distribution of food resources, its degree of stability and predictability, and predator pressure) of hominids changed from those of their African ancestors (that which was beneficial in the forest was not so in the savannah), it can be expected that their social organisation changed too to meet the environmental changes.
I have re-examined the evidence presented by Lockwood et al.18 for a polygynous, one-male harem social structure in the South African Pleistocene hominid, Australopithecus robustus, and have shown that the conclusions reached in the Lockwood et al. paper18 can be questioned. Although A. robustus exhibited an increased (almost gorilla-like) degree of facial size dimorphism, I have argued that the assertions regarding evidence of a highly uneven sex ratio and the hypothesised polygynous societies of these early hominids are dubious. The approach I have tested here to determine the sex ratio in highly dimorphic species involved sorting the specimens into sexes using a k-means clustering technique - a method that I have demonstrated to be highly accurate for gorillas.
While Lockwood et al.18 may still be correct that the mating system of Australopithecus robustus was one-male, I suggest that the social system of this species was more likely a multimale-multifemale one. The typical one-male harem organisation of forest-dwelling gorillas seems to be a counterproductive model for interpreting the ecology and social life of the savannah-dwelling 'robust' australopithecines. It is difficult to determine whether the 'robust' groups were based on either the kinship of females or the kinship of males. Australopithecines were unlike any of the present-day hominoids, and thus it can hardly be expected that we would be able to perfectly fit them into behavioural patterns we know today.
Acknowledgements
I would like to thank Milford H. Wolpoff and Jan Strzatko for constructive discussions, as well as the anonymous reviewers for their useful comments on earlier drafts of the manuscript. I also thank J.F. Thackeray, formerly of the Transvaal Museum and M. Harman of the Powell-Cotton Museum, for assistance with the specimens, and D.J. Chorn for proofreading the manuscript.
References
1. Plavcan JM. Sexual dimorphism in primate evolution. Yrbk Phys Anthropol. 2001;44:25-53. http://dx.doi.org/10.1002/ajpa.10011 [ Links ]
2. Leutenegger W, Kelly JT. Relationship of sexual dimorphism in canine size and body size to social, behavioral and ecological correlates in anthropoid primates. Primates. 1977;18:117-136. http://dx.doi.org/10.1007/BF02382954 [ Links ]
3. Mitani J, Gros-Louisi J, Richards AF. Sexual dimorphism, the operational sex ratio, and the intensity of male competition in polygynous primates. Am Nat. 1995;147:966-980. http://dx.doi.org/10.1086/285888 [ Links ]
4. Plavcan JM, Van Schaik CP Interpreting hominid behavior on the basis of sexual dimorphism. J Hum Evol. 1997;32:345-374. http://dx.doi.org/10.1006/jhev.1996.0096 [ Links ]
5. Plavcan JM. Scaling relationships between craniofacial sexual dimorphism and body mass dimorphism in primates: Implications for the fossil record. Am J Phys Anthropol. 2003;120:38-60. http://dx.doi.org/10.1002/ajpa.10154 [ Links ]
6. Harvey PH, Kavanagh M, Clutton-Brock TH. Sexual dimorphism in primate teeth. J Zool Lond. 1978;186:474-485. http://dx.doi.org/10.1111/j.1469-7998.1978.tb03934.x [ Links ]
7. Martin RD, Willner LA, Dettling A. The evolution of sexual size dimorphism in primates. In: Short RV, Balaban E, editors. The differences between the sexes. Cambridge: Cambridge University Press; 1994. p. 159-200. [ Links ]
8. Leigh SR. Socioecology and the origin of sexual dimorphism in anthropoid primates. Am J Phys Anthropol. 1995;97:339-356. http://dx.doi.org/10.1002/ajpa.1330970402 [ Links ]
9. Stewart KJ, Harcourt AH. Gorillas: Variation in female relationships. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 155-164. [ Links ]
10. Plavcan JM. Inferring social behavior from sexual dimorphism in the fossil record. J Hum Evol. 2000;39:327-344. http://dx.doi.org/10.1006/jhev.2000.0423 [ Links ]
11. Lockwood CA, Richmond BG, Jungers WL, Kimbel WH. Randomization procedures and sexual dimorphism in Australopithecus afarensis. J Hum Evol. 1996;31:537-548. http://dx.doi.org/10.1006/jhev.1996.0078 [ Links ]
12. McHenry HM. Petite bodies of the 'robust' australopithecines. Am J Phys Anthropol. 1991;86:445-454. http://dx.doi.org/10.1002/ajpa.1330860402 [ Links ]
13. McHenry HM. Body size and proportions in early hominids. Am J Phys Anthropol. 1992;87:407-431. http://dx.doi.org/10.1002/ajpa.1330870404 [ Links ]
14. Reno PL, Meindl RS, McCollum MA, Lovejoy CO. Sexual dimorphism in Australopithecus afarensis was similar to that of modern humans. Proc Natl Acad Sci USA. 2003;100:9404-9409. http://dx.doi.org/10.1073/pnas.1133180100 [ Links ]
15. Reno PL, Meindl RS, McCollum MA, Lovejoy CO. The case is unchanged and remains robust: Australopithecus afarensis exhibits only moderate skeletal dimorphism. A reply to Plavcan et al. (2005). J Hum Evol. 2005;49:279-288. http://dx.doi.org/10.1016/j.jhevol.2005.04.008 [ Links ]
16. Plavcan JM, Lockwood CA, Kimbel WH, Lague MR, Harmon EH. Sexual dimorphism in Australopithecus afarensis revisited: How strong is the case for a human-like pattern of dimorphism? J Hum Evol. 2005;48:313-320. http://dx.doi.org/10.1016/j.jhevol.2004.09.006 [ Links ]
17. Lee S-H. Patterns of size sexual dimorphism in Australopithecus afarensis: Another look. Homo. 2005;56:219-32. http://dx.doi.org/10.1016/j.jchb.2005.07.001 [ Links ]
18. Lockwood CA, Menter CG, Moggi-Cecchi J, Keyser AW. Extended male growth in a fossil hominin species. Science. 2007;318:1443-1446. http://dx.doi.org/10.1126/science.1149211 [ Links ]
19. Lockwood CA. Sexual dimorphism in the face of Australopithecus africanus. Am J Phys Anthropol. 1999;108:97-127. http://dx.doi.org/10.1002/(SICI)1096-8644(199901)108:1 <97::AID-AJPA6>3.0.CO;2-O [ Links ]
20. Kaszycka KA. Dymorfizm plclowy potudniowoafrykanskich australopiteków [Sexual dimorphism in the South African australopithecines]. [ Links ] Poznan: Wyd. Naukowe UAM; 2009. Polish.
21. Gibbons A. Hominid harems: Big males competed for small australopithecine females. Science. 2007;318:1363. http://dx.doi.org/10.1126/science.318.5855.1363a [ Links ]
22. Wolpoff MH. Sexual dimorphism in the australopithecines. In: Tuttle RH, editor. Paleoanthropology: morphology and paleoecology. The Hague: De Gruyter Mouton; 1975. p. 245-284. http://dx.doi.org/10.1515/9783110810691.245 [ Links ]
23. Wolpoff MH. Some aspects of the evolution of early hominid sexual dimorphism. Curr Anthropol. 1976;17:579-606. http://dx.doi.org/10.1086/201798 [ Links ]
24. Hartigan JA, Wong MA. Algorithm AS 136 A K-means clustering algorithm. Appl Stat J Roy St C. 1979;28:100-108. http://dx.doi.org/10.2307/2346830 [ Links ]
25. Kaszycka KA. Status of Kromdraai: Cranial, mandibular and dental morphology, systematic relationships, and significance of the Kromdraai hominids. Paris: CNRS; 2002. p.130 [ Links ]
26. Plavcan JM. Comparison of four simple methods for estimating sexual dimorphism in fossils. Am J Phys Anthropol. 1994;94:465-476. http://dx.doi.org/10.1002/ajpa.1330940403 [ Links ]
27. Keyser AW. The Drimolen skull: The most complete australopithecine cranium and mandible to date. S Afr J Sci. 2000;96:189-193. [ Links ]
28. Morissette L, Chartier S. The k-means clustering technique: General considerations and implementation in Mathematica. Tutorials Quant Meth Psych. 2013;9:15-24. [ Links ]
29. Pusey AE, Packer C. Dispersal and philopatry. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 250-266. [ Links ]
30. Harcourt AH. Strategies of emigration and transfer by primates, with particular reference to gorillas. Z Tierpsychol. 1978;48:401-420. http://dx.doi.org/10.1111/j.1439-0310.1978.tb00267.x [ Links ]
31. Cowlishaw G. Vulnerability to predation in baboon populations. Behaviour. 1994;131:293-304. http://dx.doi.org/10.1163/156853994X00488 [ Links ]
32. Alberts SC. Altmann J. Balancing costs and opportunities: Dispersal in male baboons. Am Nat. 1995;145:279-306. http://dx.doi.org/10.1086/285740 [ Links ]
33. Dunbar RIM. Demography and reproduction. In: Smuts BB et al., editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 240-249. [ Links ]
34. Brain CK. New finds at the Swartkrans australopithecine site. Nature. 1970;225:1112-1119. http://dx.doi.org/10.1038/2251112a0 [ Links ]
35. Brain CK. The hunters or the hunted. An introduction to African cave taphonomy. Chicago, IL: University of Chicago Press; 1981. [ Links ]
36. Leigh SR. Patterns of variation in the ontogeny of primate body size dimorphism. J Hum Evol. 1992;23:27-50. http://dx.doi.org/10.1016/0047-2484(92)90042-8 [ Links ]
37. Dixson AF. Primate sexuality. Comparative studies of the prosimians, monkeys, apes, and human beings. Oxford: Oxford University Press; 1998. [ Links ]
38. Emlen ST, Oring LW. Ecology, sexual selection, and the evolution of mating systems. Science 1977;197(4300):215-223. http://dx.doi.org/10.1126/science.327542 [ Links ]
39. Grine FE. Dental evidence for dietary differences in Australopithecus and Paranthropus: A quantitative analysis of permanent molar microwear. J Hum Evol. 1986;15:783-822. http://dx.doi.org/10.1016/S0047-2484(86)80010-0 [ Links ]
40. Kay RF, Grine FE. Tooth morphology, wear and diet in Australopithecus and Paranthropus from southern Africa. In: Grine FE, editor. Evolutionary history of the 'robust' australopithecines. New York: Aldine de Gruyter; 1988. p. 427-447. [ Links ]
41. Lee-Thorp JA, Sponheimer M. Contributions of biogeochemistry to understanding hominin dietary ecology. Year Phys Anthropol. 2006;49:131-148. http://dx.doi.org/10.1002/ajpa.20519 [ Links ]
42. Lee-Thorp JA, Van der Merwe NJ, Brain CK. Diet of Australopithecus robustus at Swartkrans from stable carbon isotopic analysis. J Hum Evol. 1994;27:361-372. http://dx.doi.org/10.1006/jhev.1994.1050 [ Links ]
43. Sponheimer M, Alemseged Z, Cerling TE, Grine FE, Kimbel WH, Leakey MG, et al. Isotopic evidence of early hominin diets. P Natl Acad Sci USA, 2013;110(26):10513-10518. http://dx.doi.org/10.1073/pnas.1222579110 [ Links ]
44. Sponheimer M, Passey BH, De Ruiter DJ, Guatelli-Steinberg D, Cerling TE, Lee-Thorp JA. Isotopic evidence for dietary variability in the early hominin Paranthropus robustus. Science. 2008;314:980-982. http://dx.doi.org/10.1126/science.1133827 [ Links ]
45. Yeakel JD, Bennett NC, Koch PL, Dominy NJ. The isotopic ecology of African mole rats informs hypotheses on the evolution of human diet. Proc R Soc B. 2007;274(1619):1723-1730. http://dx.doi.org/10.1098/rspb.2007.0330 [ Links ]
46. Rogers ME, Abernethy K, Bermejo M, Cipolletta C, Doran D, McFarland K, et al. Western gorilla diet: A synthesis from six sites. Am J Primatol. 2004;64:173-192. http://dx.doi.org/10.1002/ajp.20071 [ Links ]
47. Doran-Sheehy DM, Greer D, Mongo P, Schwindt D. Impact of ecological and social factors on ranging in western gorillas. Am J Primatol. 2004;64:207-222. http://dx.doi.org/10.1002/ajp.20075 [ Links ]
48. Yamagiwa J, Basabose AK. Diet and seasonal changes in sympatric gorillas and chimpanzees in Kahuzi-Biega National Park. Primates. 2006;47:74-90. http://dx.doi.org/10.1007/s10329-005-0147-7 [ Links ]
49. Stanford CB. The behavioral ecology of sympatric African apes: Implications for understanding fossil hominoid ecology. Primates. 2006;47:91-101. http://dx.doi.org/10.1007/s10329-005-0148-6 [ Links ]
50. Sponheimer M, Loudon JE, Codron D, Howells ME, Pruetz JD, Codron J, et al. Do 'savanna' chimpanzees consume C4 resources? J Hum Evol. 2006;51:128-133. http://dx.doi.org/10.1016/j.jhevol.2006.02.002 [ Links ]
51. Stanford CB, Wallis J, Matama H, Goodall J. Patterns of predation by chimpanzees on red colobus monkeys in Gombe National Park, Tanzania, 1982-1991. Am J Phys Anthropol. 1994;94:213-228. http://dx.doi.org/10.1002/ajpa.1330940206 [ Links ]
52. Strum SC. Processes and products of change: Baboon predatory behavior at Gilgil, Kenya. In: Harding R, Teleki G, editors. Omnivorous primates: Gathering and hunting in human evolution. New York: Columbia University Press; 1981. p. 255-302. [ Links ]
53. Codron D, Lee-Thorp JA, Sponheimer M, De Ruiter D, Codron J. Inter- and intrahabitat dietary variability of chacma baboons (Papio ursinus) in South African savannas based on fecal d13C, d15N, and %N. Am J Phys Anthropol. 2006;129:204-214. http://dx.doi.org/10.1002/ajpa.20253 [ Links ]
54. Steudel K. New estimates of early hominid body size. Am J Phys Anthropol. 1980;52:63-70. http://dx.doi.org/10.1002/ajpa.1330520109 [ Links ]
55. Spocter MA, Manger PR. The use of cranial variables for the estimation of body mass in fossil hominins. Am J Phys Anthropol. 2007;134:92-105. http://dx.doi.org/10.1002/ajpa.20641 [ Links ]
56. Harcourt AH, Stewart KJ. Gorilla society: What we know and don't know. Evol Anthropol. 2007;16:147-158. http://dx.doi.org/10.1002/evan.20142 [ Links ]
57. Robbins MM. A demographic analysis of male life history and social structure of mountain gorillas. Behaviour. 1995;132:21-47. http://dx.doi.org/10.1163/156853995X00261 [ Links ]
58. Strier KB. Beyond the apes: Reasons to consider the entire primate order. In: De Waal FBM, editor. Tree of origin. What primate behaviour can tell us about human social evolution. Cambridge, MA: Harvard University Press; 2002. p. 70-93. [ Links ]
59. Packer C. Inter-troop transfer and inbreeding avoidance in Papio anubis. Anim Behav. 1979;27:1-36. http://dx.doi.org/10.1016/0003-3472(79)90126-X [ Links ]
60. Wrangham RW. An ecological model of female-bonded primate groups. Behaviour. 1980;75:262-300. http://dx.doi.org/10.1163/156853980X00447 [ Links ]
61. Stammbach E. Desert, forest and montane baboons: Multilevel-societies. In: Smuts BB, et al., editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 112-120. [ Links ]
62. Sigg H, Stolba A, Abegglen J-J, Dasser V. Life history of hamadryas baboons: Physical development, infant mortality, reproductive parameters and family relationships. Primates. 1982;23:473-487. http://dx.doi.org/10.1007/BF02373959 [ Links ]
63. Abegglen J-J. On socialization in hamadryas baboons. A field study. Cranbury: Associated University Press; 1984. [ Links ]
64. Smuts BB. Sex and friendship in baboons. New York: Aldine; 1985. [ Links ]
65. Smuts BB. Sexual competition and mate choice. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 385-399. [ Links ]
66. Wrangham RW. Evolution of social structure. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago, IL: University of Chicago Press; 1987. p. 282-296. [ Links ]
 Correspondence:
Correspondence:
Katarzyna A. Kaszycka
Institute of Anthropology, Adam Mickiewicz University
Umultowska 89, Collegium Biologicum, Poznan 61-614, Poland
kaszycka@amu.edu.pl
Received: 04 May 2015
Revised: 25 July 2015
Accepted: 03 Aug. 2015














