Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.111 n.11-12 Pretoria Nov./Dec. 2015
http://dx.doi.org/10.17159/sajs.2015/20140408
RESEARCH ARTICLE
In vitro sensitivity testing of Cladobotryum mycophilum to carbendazim and prochloraz manganese
Alinesi ChakwiyaI, II; Elna J. van der LindeIII; Lise KorstenI
IDepartment of Microbiology and Plant Pathology, University of Pretoria, Pretoria, South Africa
IIPlant Sciences Research Centre, National Institute for Scientific and Industrial Research, Kitwe, Zambia
IIIBiosystematics Division, Agricultural Research Council, Pretoria, South Africa
ABSTRACT
Limited information of fungicide efficacy on cultivated mushrooms and resistance development potential is available. Minor crop industries in general have a smaller arsenal of protectants to rely on and the likelihood of resistance build-up is of greater concern. This study focused on Cladobotryum mycophilum's sensitivity to carbendazim and prochloraz manganese following recent reports on decreased efficacy of both fungicides. The median effective dose (ED50) values for carbendazim ranged between 0.02 mg/L and 4.31 mg/L with 60% of the South African isolates being moderately resistant. The highest resistance factor for carbendazim was 215. Prochloraz manganese ED50 values varied from 0.00001 mg/L to 0.55 mg/L. A significant difference in mean ED50 values for both fungicides tested was observed. Using cluster analysis, no discrimination of isolates previously exposed and unexposed to prochloraz manganese was observed. A wide range of differences in ED50 values indicated moderate resistance to carbendazim and high sensitivity to prochloraz manganese among isolates under investigation. Discriminant analysis indicated significant differences between clusters contributed by one or a few variables. This study provided evidence that prochloraz manganese remains highly fungitoxic to C. mycophilum. However, prochloraz manganese is to be used in a disease management strategy in combination with strict farm hygiene management strategies to retain product efficacy and ensure crop protection.
Keywords: Agaricus bisporus; cobweb disease; fungicide effectiveness; fungicide resistance; fungicolous fungi
Introduction
Cobweb disease, caused by Cladobotryum dendroides (teleomorph Hypomyces rosellus) and Cladobotryum mycophilum (teleomorph Hypomyces odoratus), is one of the three major fungal diseases of the mushroom Agaricus bisporus (Lange) Sing.1Cladobotryum mycophilum is associated with cobweb disease in Australia, the mainland of Europe, the United States of America (USA) and South Africa. C. mycophilum is also the dominant species in the British Isles, where it is reportedly resistant to benzimidazoles.2,3Hypomyces aurantius, a teleomorph of Cladobotryum varium Nees, is another pathogen of mushrooms4, whose importance as well, as that of C. multiseptatum de Hoog, is not fully understood2.
Until the mid1990s, cobweb disease had not been a major problem in mushroom production in most countries. However, the disease has since become an important obstacle to viable mushroom production in countries such as Australia2, the UK and Serbia6. It is reported that the disease caused crop losses of up to 40% in 1994/1995 in the Irish and British mushroom industries as a result of serious spotting and early crop termination at the peak of the epidemic.5 According to Potocnik et al.6 and Back et al.7,8, cobweb disease was first reported on A. bisporus in Serbia and Korea in 2007 and 2009, respectively. Sporadic cases of cobweb disease have been reported on several mushroom farms in South Africa during the summer months.
Management of mushroom fungal diseases relies mainly on strict farm hygiene in combination with the use of fungicides.2 Although fungicides are used in the control of cobweb disease, there are certain inherent challenges including the fact that both the host and pathogen are fungi.9,10 In addition, very few studies on fungicide efficacy on cultivated mushrooms have been conducted and because of its minor crop status, only a few fungicides are currently registered for commercial use.11,12 For the South African mushroom industry, only prochloraz manganese, prochloraz zinc2 and thiabendazole13 are registered for use on mushrooms. Grogan14 proposed that the mushroom industry's heavy reliance on only two fungicides is a concern for sustainable production. Factors leading to resistance include (1) inherent risk factors relating to fungus biology and fungicide chemistry and (2) management risk factors relating to usage of fungicides in crop management in the wider sense. The inherent factors serve to assess the basic resistance risk for a fungicide/fungus combination in a given area and cannot be controlled. Management of risk factors can be controlled as they are in the hands of farmers, agriculture officials and distributors of fungicides.15
Benomyl, the first site-specific fungicide to be used in the mushroom industry, has been available from the late 1960s and provides good control of all fungal diseases.16 Resistance development in Lecanicillium fungicola Zare & Gams (syn. Verticillium fungicola (Preuss) Hassebrauk and H. rosellus was reported in 1974 and 1993, respectively.17,18 A few years later, resistance of Cladobotryum mycophilum to benzimidazole fungicides was reported in the UK.19
Prochloraz manganese, one of the demethylation inhibitors (DMIs), was introduced to the mushroom industry in the late 1980s to control wet bubble, dry bubble and cobweb diseases.1,20 This fungicide effectively controlled mushroom diseases but over time its effectiveness against Lecanicillium fungicola decreased. Since then, increased loss of sensitivity of the pathogen to the fungicide has been reported worldwide.21 Prochloraz manganese was the most effective fungicide in the prevention of cobweb disease,1 but in recent years, it has no longer been able to control the spotting symptoms caused by the disease.14
Monitoring of fungicide sensitivity within pathogen populations over time is important to ensure more effective management of the disease as well as retention of efficacy of the chemical.1,21 Distribution of sensitivity to DMI fungicides among individual strains in an unexposed pathogen population varies from highly sensitive to considerably less sensitive phenotypes. Therefore, exposure to a given DMI fungicide will provide selection pressure favouring the more resistant isolates in the population.20,22,23 Progressive reduction in DMI efficacy results in a practical resistance once the frequency of phenotypes prevents commercially acceptable disease control levels to be achieved under standard usage regimes.23 The fact that resistance has developed in L. fungicola21,24and many other pathogens of field crops,2527 combined with the fact that most Cladobotryum spp. produce abundant conidia and have repeated infection cycles, may suggest that Cladobotryum spp. might also be at risk.28
We investigated the sensitivity of 32 Cladobotryum isolates to prochloraz manganese in order to determine a possible population shift in South African isolates in comparison with isolates obtained from the USA which have not been exposed to this fungicide. The isolates were obtained over a period of time and preserved in a central collection. Carbendazim, although not registered for use on mushrooms in South Africa, but occasionally used, was included in this study to determine its potential fungi toxicity against these isolates.
Materials and methods
Origin and collection of fungal isolates
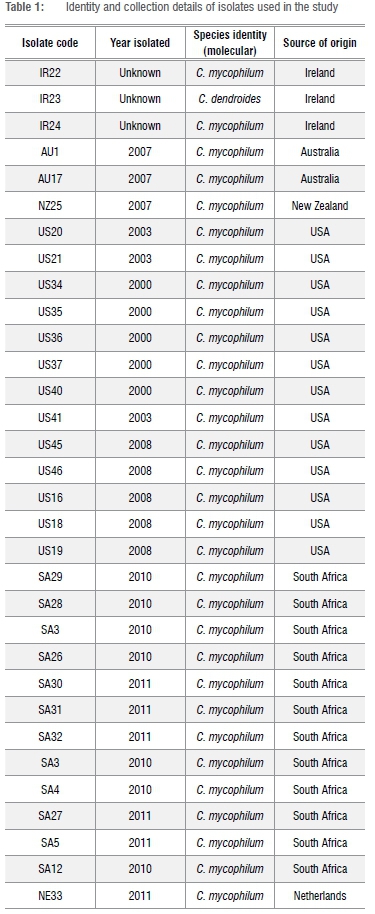
In total, 32 isolates of Cladobotryum spp. were obtained from mushroom farms in South Africa as well as from research institutes around the world (Table 1). Isolates collected from local mushroom farms were either sampled from diseased mushrooms (five) or collected as infected casing (seven), while those from research institutions around the world were received as cultures on agar slants. For each isolate, a single spore culture was prepared for use in this study and preserved in sterile distilled water and in 10% glycerol at -75 °C for short- and medium-term storage for future reference.
Confirmation of isolate identity
To confirm identity of isolates, conidia morphology was examined under a phase contrast microscope (Zeiss, Germany). Colony characteristics were observed on malt extract agar (MEA) (Merck, Johannesburg, South Africa) and potato dextrose agar (PDA) (Merck) upon receipt at the laboratory. Methods for pathogenicity tests were conducted by inoculating a pathogen spore suspension on the pilei of freshly harvested mushrooms according to the Serbian report by Potocnik et al.29 Molecular analysis was conducted to confirm the identity of all isolates through conventional polymerase chain reactions (PCR) using the partial internal transcribed spacer (ITS) region (ITS1 and ITS4) according to Tamm and Pôldmaa30. The DNA sequence was then blasted using the NCBI GenBank database and results were recorded.
Fungicides
Prochloraz manganese (Octave 500 g/kg WP Bayer Crop Science, Johannesburg, South Africa) and carbendazim (Bendazid 500 g/L EC, Plaaskem, Johannesburg, South Africa) were provided by mushroom farmers. To prepare stock solutions, prochloraz manganese was dissolved in sterile distilled water and then added to molten MEA cooled to approximately 50 °C at the desired test concentration. For carbendazim, the stock solution was added to MEA before sterilisation. For both fungicides, the MEA was poured into 90-mm sterile plastic Petri dishes and allowed to solidify before use.
Fungicide sensitivity test
Mycelial inhibition was assessed by transferring a 5-mm diameter disc taken from the edges of an actively growing single colony culture and placing it inverted in the centre of a plate. To assess the initial in vitro response of mycelial growth to different fungicide concentrations, all isolates were exposed to a concentration range of 1-100 mg/L for both products tested. From these observations, the isolates were grouped into two categories with reference to carbendazim concentration: low concentration (0.1-0.75 mg/L) and high concentration (5-20 mg/L). A series of MEA plates was subsequently prepared with concentrations of 0.0, 0.1, 0.2, 0.35, 0.5 and 0.75 mg/L carbendazim representing the low concentration category and concentrations of 5.0, 10.0, 15.0, 17.0 and 20.0 mg/L carbendazim representing the high concentration category. Concentrations used for the in vitro sensitivity test to prochloraz manganese were 0.0, 1.0, 2.0, 3.5, 4.5 and 5.0 mg/L. For both fungicide tests, each isolate was tested in triplicate per fungicide concentration and incubated at 25 °C for 96 h. All the media and the fungicide stocks used were prepared as a single batch and used within 24 h per trial. Perpendicular colony diameters were measured every day starting at 48 h of incubation. The experiment was repeated twice.
Statistical analysis
The mean effective concentration value (ED50) for each isolate at 96 h was calculated whenever possible by the regressing percentage inhibition against fungicide concentration. The ED50 values of some isolates could not be determined because their growth was too slow. The mean colony diameter for each treatment was expressed as percentage growth inhibition using a SAS general linear model procedure and the means were compared using a f-test. Multivariate analysis (Statistica version 10.0) was used to cluster and discriminate between and within groups among isolates tested.
Results
Confirmation of isolafe identify and pafhogenicify
Conidia of all isolates were 1-3 septate and mostly obvoid in shape except for isolate SA4 which had a few curved conidia. Conidia sizes ranged from 15 to 34 μηι long. On PDA, all isolates developed a yellowish colony colour after four days' incubation. On MEA, three distinct colony morphologies were observed in C. mycophilum. Colonies with a 'cotton-fluffy' texture were formed by US34, US35, US36, US37 and US41, unlike the more compact or 'felt-like' texture of US40 and IR23 (only H. rosellus), an ATCC isolate of Irish origin. The rest of the isolates were 'cotton-like' - neither 'fluffy' nor 'compacted'. Most isolates' colonies were initially yellowish to pinkish, turning purplish after more than 96 h of growth. Blasting of ITS sequence against GenBank gave a 98-99% similarity to C. mycophilum for all isolates and IR23 was confirmed as H. rosellus (Table 1). These results confirmed the isolates' identity as Cladobofryum species.
Pathogenicity of all isolates was confirmed by the development of disease symptoms (cobweb) on the mushroom pilei. The majority (85%) of isolates resulted in cobweb symptom development within 48 h while others, such as H. rosellus (IR23) and some C. mycophilum (SA3, SA4, SA27 and IR22), were less virulent and took between 72 and 96 h to cause symptoms.
Fungicide bioassays
Most isolates under investigation demonstrated an ability to grow in the presence of bendazid at low concentrations. The ED50 values of bendazid against C. mycophilum isolates varied between 0.02 mg/L and 4.31 mg/L. The ED50 value of a few isolates could not be determined because they were too low. The highest resistance factor (RF) for bendazid was 215.5 mg/L for isolate US21. Isolate SA29 had the highest ED50 and RF values although values were not significantly different when compared with other isolates from South Africa.
All isolates tested were highly sensitive to prochloraz manganese and ED50 values ranged from 0.00001-0.55 mg/L. Isolates IR23, SA13 and US40 had ED50 values less than 0.00001 mg/L and could thus not be determined. Isolate SA12 had the highest RF of 55 000. Only four isolates (AU1, US36, US41 and SA27) had RFs of less than 100. There were significant differences among the isolates under examination. However, none of the isolates showed resistance to prochloraz manganese when their ED50 values were compared.
Clusfer analysis
Cluster analysis of the isolates based on their response to fungicide treatment tests resulted in 100% classification of six, four and three clusters under prochloraz manganese and carbendazim low and high concentration categories, respectively. Clustering within each treatment was analysed for significant differences using discriminate analysis. For all three treatments, there were significant differences between but not within clusters. Not all variables (test concentrations) contributed to the variations observed.
Cluster analyses of prochloraz manganese treatment results grouped the 32 isolates tested into four clusters (Figure 1). Clustering was done a priori. The largest cluster (IV) included 12 isolates with an average ED50 value of 0.33 mg/L. The isolate with the highest ED50 value was SA12 (0.55 mg/L) followed by US18 (0.54 mg/L), with RF values of 55 000 and 54 000, respectively (Table 2). There were no significant differences among the members of this group. The range in ED50 values was between 0.05-0.55 mg/L. South African isolates made up 50% of this cluster, followed by 25% USA; the rest of the isolates were from Australia, Ireland and New Zealand.
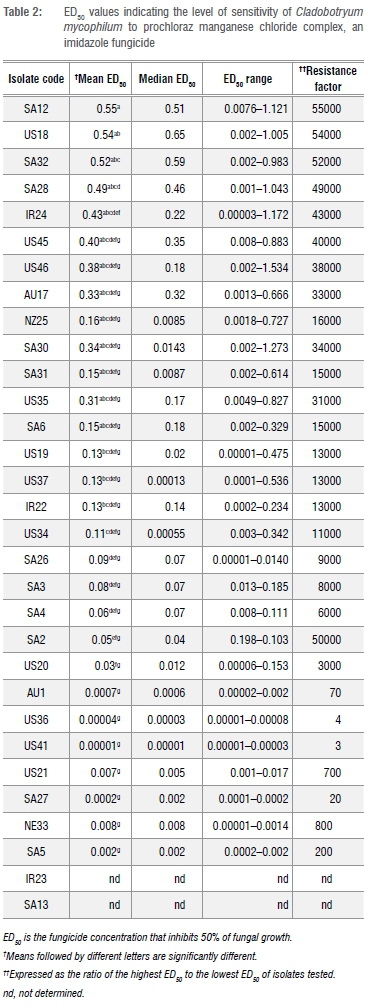
Cluster I was the second largest, represented by eight members (IR22, IR23, NR33, US20, US21, US36, US40 and US41) from Ireland, Netherlands and the USA with a mean ED50 value of 0.047 mg/L. The highest ED50 value in the cluster was 0.13 mg/L for IR22, an Irish isolate. Cluster II comprised 71.4% isolates from South Africa (SA26, SA2, SA3, SA4 and SA6) and 28.6% from the USA (US45 and US46). The average ED50 value for Cluster II was 0.23 mg/L with a range of 0.06-0.4 mg/L. The ED50 values of some of the individual members of this group were not significantly different from each other despite the wide range of ED50 values observed (Table 2.). In Cluster III, 60% of the isolates were from the USA, 20% were from Australia and the rest were from South Africa. Cluster III had an average ED50 value of 0.074 mg/L with a range of 0.0002-0.13 mg/L. The highest ED50 value in the group was for isolates from the USA (US19 and US34). The lowest ED50 value was from SA27.
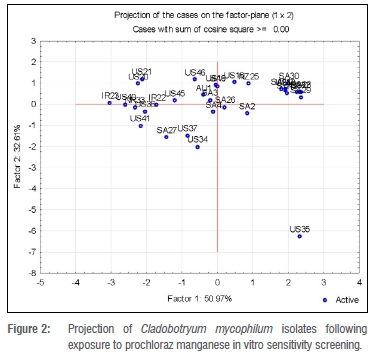
The projection of isolates exposed to prochloraz manganese on the plane were for Factors 1 and 2. In Figure 2, eight of the South African isolates are clustered in the higher region of the plane. This shows a tendency of those isolates to tolerate higher concentrations of prochloraz manganese than the rest of the isolates under study.
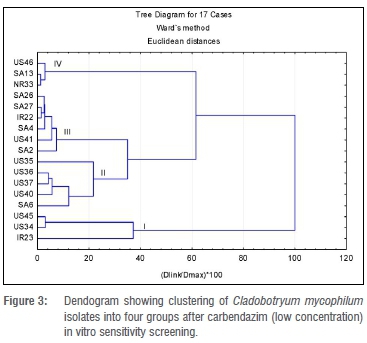
Cluster analysis for the carbendazim fungicide in vitro test was carried out separately for the low concentrations (ranging from 0.0-0.75 mg/L) and the high concentrations (ranging from 0.0-20.0 mg/L). For easier reference, these are called Analysis 1 and Analysis 2. For Analysis 1, four clusters were formed a priori, with the largest (Cluster III) consisting of six isolates (Figure 3). Four of the isolates in Cluster III were from South Africa and one each was from Ireland and the USA. The average ED50 value for Cluster III was 0.058 mg/L. Isolate US41 was significantly different from the rest of the group. Cluster I had an average ED50 value of less than 0.02 mg/L, which was the lowest of all clusters formed in Analysis 1. Three isolates (US45, IR23 and US34) that were included in this lower concentration category had EC50 values below 0.02 mg/L, the lowest determined value in the category. Cluster II consisted of four US isolates (US35, US36, US37 and US40) and one South African isolate (SA6). Isolates US40 and US37 were significantly different from each other but not from the rest of the cluster. Overall, ED50 values for the isolates exposed to the low carbendazim concentrations ranged from 2.5-10.5 mg/L (Table 3).
For Analysis 2 (carbendazim high concentration), three clusters were also determined a priori (Figure 4). Cluster II with nine isolates had the most members and had an average ED50 value of 3.0-4.31 mg/L.
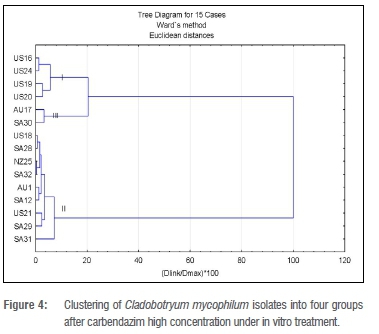
The two isolates (US21 and SA32) with the highest overall RF values (215.5 mg/L and 207.5 mg/L respectively) belonged to Cluster II. There was no significant difference in the ED50 values of the isolates in Cluster II. Cluster I consisted of all US isolates: US16, US19, US20 and US24. Apart from US16, with an ED50 value of 0.58 mg/L, the ED50 values of the isolates could not be determined, but they were all lower than that for US16. The average ED50 value for this cluster was 0.14 mg/L. Cluster III had only two isolates, AU17 and SA30, for which the ED50 values was 2.03 mg/L and 3.95 mg/L, respectively, with an average of 3.0 mg/L.
Discussion and conclusion
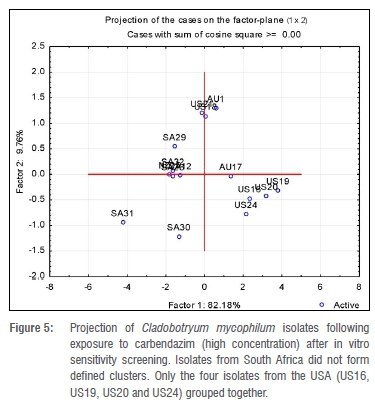
A total of 32 Cladobofryum isolates was assessed for their sensitivity to prochloraz manganese and carbendazim (Figure 5). Isolates from the USA, where prochloraz manganese is not registered for use in the mushroom industry,31 were used as a baseline reference culture for this study. All isolates were sensitive to both carbendazim (0.02-4.31 mg/L) and prochloraz manganese (0.00001-0.55 mg/L). The highest RFs observed with bendazid and prochloraz manganese were 215 (US210) and 5 5000 (SA13), respectively. According to the criteria used by Delp et al.32 and Gouot33 to define resistance (in which an RF value of more than 2 is considered resistant), both isolates would have been considered highly resistant to the tested fungicides. The RF provides an indication of the potential for resistance development in a given population. According to the definition by Gea et al.9, the higher the RF the greater the difference in sensitivity between the least sensitive individuals in a population relative to the mean sensitivity of the individuals in the entire population and therefore, the greater the potential for resistance to develop.
However, a higher resistance factor value does not imply that resistance will be rapidly expressed in the isolates under study because the ED50 values obtained were very low. In this instance, RF values were much higher for both fungicides tested, indicating the likelihood of resistance development for the isolates under investigation.32 ED50 and RF values are used as an index to determine resistance or sensitivity of a fungal pathogen to fungicide treatment.18 According to Potocnik et al.17 and Gea et al.21 a wide range in ED50 values indicates a wider range of sensitivity for isolates under investigation. Both Potocnik et al.17 and Gea et al.21 reported that an increase in the range of sensitivity increased the risk of individuals within a population becoming less sensitive to the test fungicide. The gradual selection pressure exerted on the pathogen population can therefore contribute to triggering resistance build-up in C. mycophilum. In this study, resistance was not observed in any of the isolates challenged with the two fungicides. However, production practices, such as strict farm hygiene to reduce the incidence of disease, should be maintained to minimise the potential risk of build-up of pathogen resistance.21
Among the isolates from the USA, the lowest and highest RFs were 1 and 54 000 with ED50 values of 0.00001 mg/L and 0.54 mg/L, respectively. This observation corresponds with the report by Erickson et al.22 indicating that sensitivity to a DMI fungicide among individuals not yet exposed to such a fungicide varies from highly sensitive to more resistant phenotypes. However, in this study, no isolate had an ED50 value of less than 5 mg/L or more than 50 mg/L to render them, respectively, moderately or highly resistant according to the criteria set by Russell et al.34 cited by Gea et al.24
The 12 isolates studied from the South African mushroom industry were highly sensitive to prochloraz manganese (ED50=0.0002-0.55 mg/L) and there were significant differences among the isolates under study. However, none of the isolates was resistant to prochloraz manganese. Prochloraz manganese was therefore more toxic than carbendazim to all isolates investigated in the study.
According to Bonnen et al.31, the presence of moderately resistant isolates prevalent prior to the benomyl era indicates the existence of a certain level of resistant isolates within the natural population. A number of studies has been conducted to determine sensitivity of mushroom pathogens to prochloraz and benomyl (carbendazim). In the mid-1970s, British researchers conducted a survey in the UK of Cladobotryum spp. sensitivity to benomyl.18 These researchers showed that none of the isolates grew at a benomyl concentration of 5 mg/L. Benomyl has since been replaced by carbendazim; a primary breakdown fungitoxic product of benomyl.35 In their cropping experiment, Fletcher et al.1 observed that benomyl, thiabendazole and prochloraz manganese controlled Cladobotryum spp. satisfactorily. In the Netherlands, it was reported that prochloraz manganese complex was effective against Hypomyces rosellus, Lecanicillium fungicola, Mycogone perniciosa (Magnus) in the cultivation of A. bisporus and A. bitorquis (Quel.) Sacc.20
In the 1994/1995 outbreak of cobweb disease in the UK, it was reported that Cladobotryum spp. isolates were inhibited at a 10 mg/L concentration of carbendazim.35 Resistance of Cladobotryum spp. to a benzimidazole fungicide was reported in Ireland in 1992. Further investigations by McKay et al.19 confirmed that these isolates were resistant to the benzimidazole fungicides benomyl and carbendazim with an ED50 of up to 10 mg/L.19 Grogan and Gaze36 also observed that the majority of the Cladobotryum spp. isolated in the UK was strongly resistant to thiabendazole (ED50>200 mg/L) and weakly resistant to carbendazim (3.5-4.4 mg/L).35
Among the isolates in our study, only a third of the isolates were moderately resistant and none were highly resistant. In accordance with the definition by Gea et al.21, an ED50 of 0-5 mg/L indicates sensitivity, 5-50 mg/L moderate resistance and >50 mg/L resistance - most isolates were sensitive to carbendazim (ED50=0.002-2 mg/L). Of all isolates moderately resistant to carbendazim, 60% were isolates from the South African mushroom farms. Based on Gouot's standard,33 isolate response to carbendazim exposure (ED50=0.05-4.15 mg/L) ranged from extremely sensitive to moderately resistant among the South African isolates.
Research findings by Grogan et al.36 showed that isolates in the UK exhibited a range of reactions to prochloraz manganese with ED50 values of 0.19-7.80 mg/L. Within their study population, the duo stated that a total of 75% were weakly resistant (1.1-3.7 mg/L) to prochloraz manganese. In another report, Potocnik et al.29 showed that Serbian isolates of Cladobotryum dendroides were highly sensitive, with ED50 values ranging from 0.01 mg/L to 0.09 mg/L for prochloraz manganese and 0.24-2.92 mg/L for carbendazim.29 In our investigation, none of the isolates showed reduced sensitivity to prochloraz manganese and the chemical remained highly toxic to the isolates under investigation (ED50=0.00001-0.55 mg/L).
From our findings, clustering of isolates using a multivariate analysis did not discriminate between isolates never exposed to prochloraz manganese and those that had been exposed. The earliest (2000) collected isolates received from the USA did not form one cluster, nor was such clustering obtained from the South African isolates. This finding is probably in accordance to observations made by McGath23 and Erickson et al.22 who proposed that in a population of isolates that have never been exposed to a DMI fungicide, there is a continuously varying range from highly sensitive to highly resistant phenotypes. However, in our study, no isolate had an ED50 value of 5 mg/L or more; therefore, no isolate investigated was highly resistant.
Even though the risk of resistance development in the Cladobotryum spp. under investigation seemed low, continuous fungicide usage selects for pathogen resistance; thus farm hygiene measures should be managed to reduce incidence of the disease and minimise the risk of build-up of resistance.26 Emergence of benzimidazole resistance reduces the value of these fungicides available for disease control. In addition, a lack of effective alternatives makes it important to manage fungicides with a holistic strategy to retain efficacy. Regular monitoring therefore becomes even more important.14 In conclusion, mushroom growers can no longer rely exclusively on prochloraz manganese as a primary management tool for fungal diseases.21
Acknowledgements
We are grateful to Frikkie Calitz and Liesl Morey (Biometry Unit, Agricultural Research Council, South Africa) for their statistical advice. We acknowledge the South African Mushroom Association (SAMFA) research team and the University of Pretoria for supporting the research. We also express our gratitude to Sida through the Organisation of Women in Science for the Developing World (OWSD) for a postgraduate fellowship.
Authors' contributions
L.K. was the project leader and promoter and was involved in the experimental design. E.J.v.d.L. was the co-promoter and was responsible for the experimental set-up and for arranging the statistical analyses. Both promoters were also responsible for editing the manuscript. A.C. was responsible for performing most of the experimental work and writing the manuscript.
References
1. Fletcher JT, Hims MJ, Hall PJ. The control of bubble disease and cobweb disease of mushrooms with prochloraz. Plant Pathol. 1983;32:123-131. http://dx.doi.org/10.1111/j.1365-3059.1983.tb01310.x [ Links ]
2. Fletcher JT, Gaze RH. Mushroom pest and disease control - A colour handbook. London: Manson Publishing Ltd; 2008. [ Links ]
3. McKay GJ, Egan D, Morris E, Scott C, Brown AE. Genetic and morphological characterization of Cladobotryum species causing cobweb disease of mushrooms. Appl Environ Microbiol. 1999;65(2):606-610. [ Links ]
4. Eicker A, Van Greuning M. Fungi in the cultivation of the Agaricus bisporus - An updated list of species. In: Van Greuning M, Van Griensvenled, editors. Genetics and breeding of Agaricus. Proceedings of the first International Seminar on Mushroom Science; 1991 May 14-17; Wageningen, the Netherlands. Wageningen: Centre for Agriculture Publishing and Documentation; 1991. p. 89-96. [ Links ]
5. Adie B, Grogan H, Archer S, Mills P. Temporal and spatial dispersal of Cladobotryum conidia in the controlled environment of a mushroom growing room. Appl Environ Microbiol. 2006;72:7212-7217. http://dx.doi.org/10.1128/AEM.01369-06 [ Links ]
6. Potocnik I, Recanovick E, Milijašević S, Todorović B, Stepanović M. Morphological and pathogenic characteristics of the fungus Cladobotryum dendroides, the causative agent of cobweb disease of the cultivated mushroom Agaricus bisporus in Serbia. Pestic Phytomed. 2008;23:175-181. http://dx.doi.org/10.2298/PIF0803175P [ Links ]
7. Back C-G, Kim Y-H, Jo W-S, Chung H, Jung H-Y Cobweb disease on Agaricus bisporus caused by Cladobotryum mycophilum in Korea. J Gen Plant Pathol. 2010;76:232-235. http://dx.doi.org/10.1007/s10327-010-0236-3 [ Links ]
8. Back C-G, Lee C-Y, Seo G-S, Jung H-C. Characterisation of species of Cladobotryum which cause cobweb disease of edible mushrooms grown in Korea. Mycobiology. 2012;40(3):189-194. http://dx.doi.org/10.5941/MYCO.2012.40.3.189 [ Links ]
9. Gea FT, Tello JC, Navarro M-J. Efficacy and effects on yield of different fungicides for the control of wet bubble disease of mushroom caused by the mycoparasite Mycogone perniciosa. Crop Prot. 2010;29:1021-1025. http://dx.doi.org/10.1016/j.cropro.2010.06.006 [ Links ]
10. Mehrparvar M, Goltapeh ME, Safaei N. Resistance of Iranian Lecanicillium fungicola to benzimidazole and ergosterol demethylation inhibition fungicides. J Agricult Sci Technol. 2013;15:389-395. [ Links ]
11. Chrysay-Tokousbalides M, Kastanias MA, Philippoussis A, Diamantopoulou P. Selective fungitoxicity of famoxadone, tebuconazole and trifloxystrobin between Verticillium fungicola and Agaricus bisporus. Crop Prot. 2007;26:469-475. http://dx.doi.org/10.1016/j.cropro.2006.02.016 [ Links ]
12. Stoddart H, Garthwaite DG, Thomas MR. Pesticide usage survey report 197. Mushroom crops in Great Britain 2003. Department of Environment, Food and Rural Affairs and Scottish Executive Environment and Rural Affairs Department. London: Defra; 2004. [ Links ]
13. Association of Veterinary and Crop Associations of South Africa (AVCASA). Fungicides [document on the Internet]. [ Links ] c2015 [cited 2015 Oct 29]. Available from: http://www.croplife.co.za/Portals/13/Croplife_Documents/Fungicides.pdf
14. Grogan HM. Fungicide control of mushroom cobweb disease caused by Cladobotryum strains with different benzimidazole resistance profiles. Pest Manag Sci. 2006;62:153-161. http://dx.doi.org/10.1002/ps.1133 [ Links ]
15. Staub T, Sozzi D. Fungicide resistance. Plant Dis. 1984;68(12):1026-1031. http://dx.doi.org/10.1094/PD-69-1026 [ Links ]
16. Samuels GJ, Johnston PR. Benomyl and the Verticillium diseases of cultivated mushrooms. New Zeal J Agr Res. 1980;23:155-157. http://dx.doi.org/10.1080/00288233.1980.10417861 [ Links ]
17. Potočnik I, Milijaešvić S, Rekanović E, Todorovic B, Stepanović M. Sensitivity of Cladobotryum spp., a pathogen of the button mushroom (Agaricus bisporus), to some fungicides. Pestic Phytomed. 2007;22:233-240. [ Links ]
18. Fletcher JT, Yarham DJ. The incidence of Benomyl tolerance in Verticillium fungicola, Mycogone perniciosa and Hypomyces rosellus in mushroom crops. Ann Appl Biol. 1976;84:343-353. http://dx.doi.org/10.1111/j.1744-7348.1976.tb01777.x [ Links ]
19. McKay GJ, Egan D, Morris E, Brown AE. Identification of benzimidazole resistance in Cladobotryum dendroides using a PCR-based method. Mycol Res. 1998;102:671-676. http://dx.doi.org/10.1017/S095375629700542X [ Links ]
20. Van Zaayen A, Van Adrichem JCJ. Prochloraz for control of fungal pathogens of cultivated mushrooms. Neth J Plant Pathol. 1982;88:203-213. http://dx.doi.org/10.1007/BF02140883 [ Links ]
21. Gea FT, Navarro MJ, Tello JC. Reduced sensitivity of mushroom pathogen Verticillium fungicola to prochloraz-manganese in vitro. Mycol Res. 2005;109:741-745. http://dx.doi.org/10.1017/S095375620500242X [ Links ]
22. Erickson EO, Wilcox W. Distribution of sensitivities to three sterol demethylation inhibitor fungicides among populations of Uncinula necator sensitive and resistant to triadimefon. Phytopathology. 1997;87:784-791. http://dx.doi.org/10.1094/PHYTO.1997.87.8.784 [ Links ]
23. McGrath MT. Fungicide resistance in cucurbit powdery mildew: Experiences and challenges. Plant Dis. 2001;85:236-245. http://dx.doi.org/10.1094/PDIS.2001.85.3.236 [ Links ]
24. Gea FJ, Tello JC, Honrubia M. In vitro sensitivity of Verticillium fungicola to selected fungicides. Mycopathologia. 1996;136:133-137. http://dx.doi.org/10.1007/BF00438918 [ Links ]
25. Albertini C, Gredt M, Lerous P Polymorphism of 14a-demethylase gene (CYP51) in cereal eyespot fungi Tapesia acuformis and Tapesia yallundae. Eur J Plant Pathol. 2003;109:117-128. http://dx.doi.org/10.1023/A:1022584822191 [ Links ]
26. Dyer PS, Hansen J, Delaney A, Lucas JA. Genetic control of resistance to the sterol 14a-demethylase inhibitor fungicide prochloraz in the cereal eyespot pathogen Tapesia yallundae. Appl Environ Microbiol. 2000;66(11):4599-4604. http://dx.doi.org/10.1128/AEM.66.11.4599-4604.2000 [ Links ]
27. Guarnaccia V Aiello D, Giancarlo P Emergence of prochloraz-resistant population of Calonectria pauciramosa and Calonectria polizzi in ornamental nurseries in Southern Italy. Plant Dis. 2014;98(3):344-350. http://dx.doi.org/10.1094/PDIS-04-13-0425-RE [ Links ]
28. Dekker J. The fungicide resistance problem: Will it grow worse? Bulletin OEPP/ EPPO Bulletin. 1985;15:337-344.http://dx.doi.org/10.1111/j.1365-2338.1985.tb00238.x [ Links ]
29. Potočnik I, Vukojević J, Stajić M, Rekanovic E, Milijaešvić S, Todorović B, et al. In vitro toxicity of selected fungicides from the groups benzimidazole and demethylation inhibitors to Cladobotryum dendroides and Agaricus bisporus. J Environ Sci Health B. 2009;44:364-370. http://dx.doi.org/10.1080/03601230902801059 [ Links ]
30. Tamm H, Põldmaa K. Diversity, host associations, and phylogeography of temperate aurofusarin-producing Hypomyces/Cladobotryum including causal agents of cobweb disease of cultivated mushrooms. Fungal Biol. 2013;117:348-367. http://dx.doi.org/10.1016/j.funbio.2013.03.005 [ Links ]
31. Bonnen A, Hopkins C. Fungicide resistance and population variation in Verticillium fungicola, a pathogen of the button mushroom, Agaricus bisporus. Mycol Res. 1997;101:89-96. http://dx.doi.org/10.1017/S0953756296002237 [ Links ]
32. Delp CJ, Dekker J. Fungicide resistance: Definitions and use of terms. Bulletin OEPP/EPPO Bulletin. 1985;15:333-335. http://dx.doi.org/10.1111/j.1365-2338.1985.tb00237.x [ Links ]
33. Gouot J-M. Characteristics and population dynamics of Botrytis cinerea and other pathogens resistant to dicarboximides. In: Delp CJ, editor. Fungicide resistance in North America. St Paul, MN: APS Press; 1988. p. 53-55. [ Links ]
34. Gea FJ, Tello JC, Honrubia M. In vitro sensitivity of Verticillium fungicola to selected fungicides. Mycopathologia. 1996;136:133-137. http://dx.doi.org/10.1007/BF00438918 [ Links ]
35. Hassall KA. The biochemistry and uses of pesticides. 2nd revised ed. New York: VCH Weinheim; 1990. [ Links ]
36. Grogan HM, Gaze RH. Fungicide resistance among Cladobotryum spp. - Causal agents of cobweb disease of the edible mushroom Agaricus bisporus. Mycol Res. 2000;104:741-745. http://dx.doi.org/10.1017/S0953756299001197 [ Links ]
 Correspondence:
Correspondence:
Lise Korsten
Department of Microbiology and Plant Pathology, University of Pretoria
Private Bag X20, Hatfield 0028
Pretoria, South Africa
Email: Lise.Korsten@up.ac.za
Received: 21 Nov. 2014
Revised: 16 Feb. 2015
Accepted: 24 Mar. 2015














