Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.111 n.7-8 Pretoria Jul./Aug. 2015
http://dx.doi.org/10.17159/SAJS.2015/20140280
RESEARCH ARTICLE
Evidence for climate-induced range shift in Brachystegia (miombo) woodland
Brenden PienaarI; Dave I. ThompsonII, III; Barend F.N. ErasmusI, *; Trevor R. HillIV; Ed T.F. WitkowskiI
ISchool of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa
IISouth African Environmental Observation Network, Phalaborwa, South Africa
IIISchool of Geography, Archaeology and Environmental Studies, University of the Witwatersrand, Johannesburg, South Africa
IVSchool of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
Brachystegia spiciformis Benth. is the dominant component of miombo, the sub-tropical woodlands which cover 2.7 million km2 of south-central Africa and which is coincident with the largest regional centre of endemism in Africa. However, pollen records from the genus Brachystegia suggest that miombo has experienced rapid range retraction (~450 km) from its southernmost distributional limit over the past 6000 years. This abrupt biological response created an isolated (by ~200 km) and incomparable relict at the trailing population edge in northeast South Africa. These changes in miombo population dynamics may have been triggered by minor natural shifts in temperature and moisture regimes. If so, B. spiciformis is likely to be especially responsive to present and future anthropogenic climate change. This rare situation offers a unique opportunity to investigate climatic determinants of range shift at the trailing edge of a savanna species. A niche modelling approach was used to produce present-day and select future B. spiciformis woodland ecological niche models. In keeping with recent historical range shifts, further ecological niche retraction of between 30.6% and 47.3% of the continuous miombo woodland in Zimbabwe and southern Mozambique is predicted by 2050. Persistence of the existing relict under future climate change is plausible, but range expansion to fragmented refugia in northeast South Africa is unlikely. As Brachystegia woodland and associated biota form crucial socio-economic and biodiversity components of savannas in southern Africa, their predicted further range retraction is of concern.
Keywords: Brachystegia spiciformis; climate change; ecological niche model; MaxEnt; refugia
Introduction
At regional and global scales, climate broadly limits the distribution of plant taxa,1,2 and the response of species to changing environments is consequently likely to be largely determined by population responses at range margins.3 However, few studies have examined climatic determinants of range shift at the trailing margin of a savanna species in the southern hemisphere. Understanding the historical and present-day spatial dynamics of vegetation plays a crucial role in our ability to predict the likely community responses and biodiversity consequences of future global change.4
Brachystegia spiciformis Benth. is the dominant component of miombo, the colloquial term used to describe sub-tropical woodlands dominated by Brachystegia, Julbernardia and Isoberlinia - three closely related genera in the family Fabaceae and subfamily Caesalpinioideae.5 Miombo encompasses the woodland-dominated savanna ecosystems6,7 which cover (Figure 1) an estimated 2.7 million km2 of south-central Africa8 and is coincident with White's9 Zambezian Phytochorion, the largest regional centre of endemism in Africa10. The dynamics of miombo woodland are largely determined by the woody component which, apart from climate, is predominantly influenced by people and fire. An estimated 75 million people inhabit areas covered by, or formerly covered by, miombo woodland; an additional 25 million urban dwellers rely on miombo wood or charcoal as a source of energy.11 Much of the woodland has been, and continues to be, modified by people. Changes in vegetation structure occur both directly as a result of woodland cover removal, and indirectly from changes in fire regime brought about by higher grass production under a more open tree canopy.12 Most of the dominant miombo canopy species, including B. spiciformis, are considered to be fire-tender species, which decline in abundance under regular burning and increase under complete fire protection.5

Mean annual precipitation and mean annual temperature throughout the miombo region range from 650 mm to 1400 mm and 15 °C to 25 °C, respectively. The majority (>95%) of precipitation falls during the summer season which prevails from October to March.5 However, sporadic dry periods during the onset of the precipitation season may cause large fluctuations in soil moisture and temperature. The resulting water stress during this concurrent and limited germination phase accounts for high seedling mortality in miombo woodlands.13 Once established, the influence of climate, and of precipitation in particular, on B. spiciformis growth performance is strongest during the core of the precipitation season (December to February).14
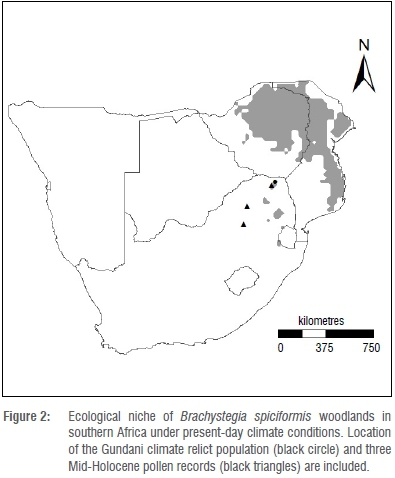
Brachystegia are likely under-represented in pollen spectra because of the relatively low pollen production typical of entomophily.5 Nevertheless, sediment cores containing pollen from the genus, dated to 38 000 years BP have been recovered from the south-central African plateau.5,15 Evidence suggests that the central plateau vegetation has undergone significant flux over this period, ranging from cool upland grassland during the Last Glacial Maximum (LGM, 19 000 years BP), to Brachystegia-dominated savanna during the warm interglacial period of the Mid-Holocene (6000 years BP).5,16 Although literature regarding climate-induced range shifts at the genus level is limited for the subcontinent, Eeley et al.17 concluded that the distribution of the forest biome in KwaZulu-Natal, South Africa, receded during the colder and drier conditions of the LGM, while warmer and wetter conditions during the Mid-Holocene were conducive to forest expansion. Correspondingly, Brachystegia woodland likely expanded to occupy a widespread historical range across south-central Africa prior to the LGM and during the warmer and wetter conditions of the Mid-Holocene.15,18,19 Sediment cores containing pollen from the genus Brachystegia have been recovered from Pretoria, Mookgophong and Tate Vondo (Figure 2) in South Africa,15 the dates of which stand as evidence of a much more widespread distribution during the recent past (~6000 to less than 1000 years BP). These records support the presence of the genus at least up to 450 km southwest of the present-day distribution limit. This most recent and abrupt range retraction suggests either a sudden change in climate, for which there is no evidence,19 or that minor shifts in temperature and moisture regimes have triggered marked changes in Brachystegia population dynamics.5
A change in geographical distribution from the simultaneous migration of populations throughout their range is unlikely. Instead, change is generated by the establishment of new, often discontinuous, populations at the leading edge of a species distribution and the coincident death of individuals and extirpation of populations at the trailing edge.20 These retractions are often not complete, but instead leave behind fragmented populations that persist as relicts, in isolated enclaves of favourable environmental conditions within an inhospitable regional climate.21 Globally, numerous relict populations have resulted from species range shifts experienced after the LGM.21 For example, the present-day distribution of Neotropical seasonal dry forest formations is considered to comprise fragmentary remnants of the once extensive forests that characterised the dry climatic maxima of the Pleistocene.22
Rutherford et al.23 suggested that B. spiciformis, which at the time was known only from north of the South African border, could find a suitable ecological niche under future global climate change conditions along the high rainfall savanna-grassland biome interface of northeast South Africa. However, they concluded that range expansion through long-distance dispersal of the species into South Africa, across the Limpopo River Valley, was unlikely to occur unassisted given that seed dispersal distance is limited to less than 6 m,13 and that regeneration takes place predominantly through coppice regrowth and root suckers, rather than seed.24 Startlingly, the discovery of the isolated B. spiciformis woodland (~15 ha, Figure 2) in the eastern Soutpansberg of South Africa25 placed the species within the region predicted by Rutherford et al.23 This isolated population, occurring some 200 km south of the continuous miombo woodlands of the subcontinent, suggests a trailing edge refugium persisting from a previously wider historical range dating prior to the LGM or during the Mid-Holocene. This woodland relict (known locally as Gundani) shares characteristics of the extensive B. spiciformis woodlands of Zimbabwe and southern Mozambique, including the open structure, medium canopy height, poorly developed lower strata and high dependence on root suckering and coppicing for regeneration.26 The presence of a second Brachystegia species within the Gundani woodland, limited to a single B. utilis individual, further suggests that this is likely a climate relict of a vegetation type from a time when, at least, the genus Brachystegia dominated the Soutpansberg massif in northeastern South Africa.27
Trailing range edge studies reflect a bias (86%) towards high-latitude range margins and temperate vegetation communities,3 which have minimum temperature-related constraints. Conversely, this rare situation provides a unique opportunity to explore climatic constraint at low-latitude range margins for an ecologically significant savanna species.
In this climate specific study, we used a predictive modelling approach to determine (1) the likely geographical footprint of the B. spiciformis woodland ecological niche in southern Africa under present-day and selected future (2050) global climate change scenarios, (2) the specific ecological niche dimensions of B. spiciformis woodland in southern Africa and (3) whether bioclimatic variables at palaeohistorical distribution sites for the genus could have supported B. spiciformis, the dominant component of present-day miombo woodland in southern Africa, during the Mid-Holocene.
Methods
Species data
A total of 914 mature B. spiciformis specimens were considered (717 in-situ observations and 197 from regional and online herbaria). Their locations were subsequently converted to quarter-degree square (QDS) centroid points to accommodate numerous herbaria records. After duplicates were excluded, a total of 76 QDS centroid points (n=76) remained. QDS is a commonly used format for general species distribution mapping in South Africa.28 These species data include the vicariant B. spiciformis population from South Africa, as the inclusion of relict populations has been shown to improve the performance of model-based projections.20
Predictor modelling
MaxEnt v.3.3.3k29 was used to project suitable ecological niche models (ENMs) for B. spiciformis woodland in southern Africa as it has been found to perform best among many different modelling approaches30. Functionality relies on a list of presence-only locations and a set of environmental predictors across a user-defined landscape, which is divided into grid cells, to generate species presence probability.
Predictor variables
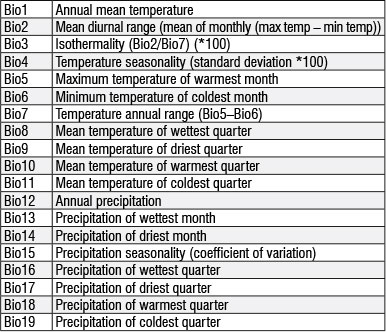
Nineteen regional bioclimatic variables and altitude ('BioClim') grid data were downloaded from the 'WorldClim'31 database. The bioclimatic variables are derived from monthly temperature and rainfall recorded worldwide (period 1950-2000) and are often used in ecological niche modelling (for examples see Blach-Overgaard et al.32 and Penman et al.33). A soil variable (dominant soil group) was added from the Harmonised World Soil Database.34 ENM Tools v.1.335 was used to produce pairwise Pearson correlation coefficients to eliminate spatially correlated variables (r>0.85) from the modelling process, with preference given to variables with higher influence on species presence probability. Summer precipitation variables were also prioritised during the elimination process as Trouet et al.14 suggest they have a strong influence on B. spiciformis growth performance. The 14 variables used in all models were Bio2-6, Bio8-9, Bio15-19, altitude and soil (see the Appendix for a description of the variables).
There are currently at least 24 coupled atmosphere-ocean general circulation models (AOGCMs) used to project climatic changes for more than 10 different greenhouse-gas emission scenarios.36 We selected three commonly used AOGCMs37,38 - HadCM3, CGCM2 and CSIRO-MK2 - to forecast the impact of climate change on B. spiciformis distribution. The A2a emission scenario, an intermediate scenario representing regional development and slow economic growth, was used across all three AOGCMs.
Model settings
The spatial resolution of all variables and landscape grid cells was resampled to QDS resolution. MaxEnt iterations were set to 5000 and accuracy was evaluated by constructing the model using 75% of presence records as training points, with the remaining 25% used in validation. The accuracy of the present-day ecological niche model was inferred from the area under the receiving operating characteristic curve (AUC) score that varies from 0 to 1, with 0 being the lowest and 1 being the highest probability of matching the species distribution.31 Subsequently, the present-day ecological niche model was applied to the three selected future (2050) AOGCMs. The increase in AUC score was used as a test metric to determine the most important bioclimatic variables explaining B. spiciformis woodland distribution, when each variable was used in isolation.
Ecological niche comparison
The fundamental niche39 was represented by a box plot, with the interquartile range (box with median present) representing 50% of the total data values and the upper and lower whiskers representing 25% of the values, respectively. The interquartile space is interpreted as the 'most suitable' range of climatic or environmental conditions for the species, while the lower and upper whiskers span less suitable conditions, reflecting lower and upper tolerance limits, respectively. Extreme values, beyond the 1.5 coefficient value, were plotted as individual open circles.
Geospatial Modelling Environment v.0.7.2.140 was used to intersect B. spiciformis distribution records (n=76) from selected (highest AUC scores and limiting factors) bioclimatic layers (Bio4, 5, 6, 15 and 16). The grid value at each point was used to create box plots representing the present-day ecological niche (Figure 3).
Seasonal data (precipitation and temperature) from Mid-Holocene ocean-atmosphere-vegetation models were used to intersect three palaeohistorical Brachystegia distribution points using Geospatial Modelling Environment. These grid values were subsequently used to construct Bio16 (precipitation of the wettest quarter) and Bio4 (temperature seasonality, the standard deviation of mean monthly temperature in degrees Celsius) as per BioClim. These two bioclimatic variables were selected as they achieved the highest increase in AUC score. This methodology was repeated across all available Mid-Holocene ocean-atmosphere-vegetation models (ECBILTCLIOVECODE, ECHAM53-MPIOM127-LPJ, FOAM, MRI-CGCM2.3.4fa, MRI-CGCM2.3.4nfa and UBRIS-HadCM3M2) from the Palaeoclimate Modelling Intercomparison Project Phase 2 database41 (http://www-lscedods.cea.fr/pmip2_dbext/pmip2_6k_oav/atm/se/). Mid-Holocene Bio16 and Bio4 values, averaged across all six models, were evaluated against the present-day ecological niche.
Limiting factors map
A limiting factor map (Figure 4) was produced as per Elith et al.30 Using Geospatial Modelling Environment and implementing the method described above, box plots were created from randomly selected QDS centroid points (n=76) within the area immediately adjacent to the current distribution of the species, for which a specific bioclimatic variable was shown as limiting. Box plots and paired sample t-tests were used to compare B. spiciformis ecological niche and limiting factor dimensions per selected bioclimatic variable (Bio4, 5, 6, and 16). Comparison of means was done using R v.3.0.3.42

Results
Model performance and variable contribution
The present-day ecological niche model for B. spiciformis achieved an AUC value of 0.923, which is considered very good.43 The model was subsequently applied to three future AOGCMs. Precipitation of the wettest quarter (Bio16) and temperature seasonality (Bio4) were identified as the two most important bioclimatic variables explaining B. spiciformis woodland distribution in southern Africa (Table 1). This result was consistent across the present-day and three future models.
Present-day ecological niche
The ENM (Figure 2) predicts the presence of isolated B. spiciformis populations in South Africa, one of which is coincident with the location of the Gundani relict discovered in 2001. The model further indicates suitable ecological conditions for the species elsewhere at disjunct locations along the high rainfall savanna-grassland interface of northeastern South Africa. However, the species does not presently occur outside of the relict population.44 Besides the fact that White's map of African vegetation9 does not indicate miombo woodland presence in South Africa, the distribution is coincident with the present-day ENM projection for B. spiciformis in southern Africa.
Precipitation of the wettest quarter (Figure 3) suggests a relatively narrow range of optimal suitability of 422-576 mm, with a median of 507 mm. Similarly, the 'most suitable' range for temperature seasonality (Figure 3) is constrained between 2.6 °C and 3.0 °C, with a median of 2.8 °C. The value of the former variable at the Gundani relict population (401 mm) falls within the lower tolerance limit of conditions experienced by the species, whereas the value for the latter variable at the relict population (2.6 °C) falls within the 'most suitable' range for B. spiciformis in southern Africa.
Past ecological niche
A crude palaeohistoric distribution of Brachystegia, as inferred from three Mid-Holocene sediment core pollen records from South Africa (Figure 2), supports past average climate values of 529 mm and 591 mm (two records) for precipitation of the wettest quarter, and temperature seasonality averages of 4.3 °C and 4.8 °C (two records) for that period. For precipitation (Figure 3), these averages fall within (or nearly within) the 'most suitable' range identified under present-day conditions (422-576 mm). Alternatively, the historical temperature seasonality values exceed even the upper tolerance limit (a maximum of 3.4 °C) for this species across its present-day southern African distribution (Figure 3).
Future ecological niche
The future ecological niches for all three models (Figure 5) are consistent with a predicted decrease in the continuous B. spiciformis woodland distribution of Zimbabwe and southern Mozambique (30.6% to 47.3%) and a decrease in the overall ecological niche (including South Africa) of between 16.9% and 32.2% by 2050 (Table 2). These figures reflect net change, with a decreased niche potentially resulting from larger retraction at present-day distributions combined with comparatively smaller range increases at newly favourable distributions elsewhere.
Two models (HadCM3 and CGCM2) suggest extensive southward ecological niche expansion in South Africa by approximately 200% and 400%, respectively, at the savanna-grassland biome interface, into areas south (29°S) of the present-day actual (22.5°S) and predicted (~27°S) ranges. Models HadCM3 and CSIRO-MK2 indicate that a suitable ecological niche at the location of the South African B. spiciformis woodland relict will not persist. Under no climatic scenario does the predicted future distribution mirror the southwestern range historically occupied by the species during the Mid-Holocene.
Present-day limiting factors
Climate values falling outside of the present-day fundamental niche or suitability range of B. spiciformis woodland in southern Africa place a climatic constraint on the species, and can therefore be considered as limiting factors to its geographical footprint. Three bioclimatic variables were identified as potential limiting factors for B. spiciformis.
Temperature seasonality (Bio4) and maximum temperature of the warmest month (Bio5) are suggested to be responsible for restricting distribution of the continuous B. spiciformis woodlands of Zimbabwe and southern Mozambique (Figure 4). The temperature seasonality range in areas to the southwest of the present-day distribution in Zimbabwe is broad (3.9-1.7 °C), but exceeds the maximum 'most suitable' temperature seasonality of 3.0 °C experienced by the species across its present-day range (Figure 3). Although there is overlap between the lower quartile of the limiting factor range and the upper tolerance limit of the current distribution, the mean of the limiting factor range (4.2 °C) is significantly higher (p<0.01) than the mean of the current distribution (2.8 °C).
Maximum temperature of the warmest month in areas to the north and northwest of the Zimbabwean population, and to the south and east of the Mozambican population (Figure 4), is tightly constrained between 35.9 °C and 37.2 °C (Figure 3). This maximum exceeds the upper limit of the 'most suitable' range of 35.2 °C for this variable established from the present-day distribution of the species. Despite substantial overlap between the upper tolerance of the current distribution range and the upper- and even the interquartiles for the limiting factor range, the means (36.6 °C versus 33.1 °C) differed significantly (p<0.01), with temperatures too high to support B. spiciformis woodland.
Correspondingly, precipitation seasonality (Bio15) and minimum temperature of the coldest month (Bio6) may restrict present-day distribution of the fragmented B. spiciformis ecological niche in South Africa (Figure 4). However, there is no significant difference (p=0.5499) between the limiting factor mean of adjacent minimum temperature of the coldest month (8.4 °C) and that of the 'most suitable' range (8.7 °C) for B. spiciformis (Figure 3). Consequently we rejected minimum temperature of the coldest month as a valid limiting factor for this species. Alternatively, precipitation seasonality's range is tightly constrained (70-78 mm) and falls short of the minimum precipitation seasonality value of 87.8 mm which bounds the present-day 'most suitable' range of the species (Figure 3). Although there is overlap between the interquartile range of the limiting factor and the lower tolerance limit of the current distribution range, the mean of the limiting factor range (74.3 mm) is significantly lower (p<0.01) than that of the current distribution (98.2 mm).
Discussion
Present-day ecological niche
The fragmented ecological niche suggested for B. spiciformis in South Africa is a refinement of the Rutherford et al.23 model, which predicted a similar distribution for this species under future global change conditions. Moreover, the presence of a population within one of the modelled ecological niche fragments suggests a refugium. Defined on climatic grounds, refugia are physiographical settings that can support a population once prevalent regional climates have been lost (or are being lost) as a result of climate shifts.45 The isolated B. spiciformis woodland relict at Gundani has therefore likely persisted in a refugium created by the varied topography of the Soutpansberg massif in South Africa. This topography has led to the formation of an isolated pocket of habitat that experiences the suitable climatic conditions (precipitation of the wettest quarter and temperature seasonality) that define the distribution of the species across the remainder of its current southern African range, and which prevailed at sites where Brachystegia occurred prior to the LGM and during the warmer and wetter conditions of the Mid-Holocene in the recent past.
Precipitation seasonality values in areas immediately adjacent to this isolated refugium are significantly lower than those for the ecological niche dimension of B. spiciformis woodland in the rest of southern Africa (Figure 3). Consequently, this bioclimatic variable reflects climatic constraints on the present-day ecological niche and isolated relict population in South Africa, potentially preventing the distribution of the species outside of the relict population. Similarly, high temperature seasonality and maximum temperature of the warmest month (Figure 3) place climatic constraints on the extremities (southwest and northeast, respectively) of the continuous B. spiciformis woodlands of Zimbabwe and southern Mozambique (Figure 4). Although most savanna plants can tolerate maximum temperature extremes, Chidumayo46 showed that some savanna trees are sensitive to seasonal highs.
Past ecological niche
According to the precipitation of the wettest quarter bioclimatic variable, conditions were considered suitable for the presence of B. spiciformis at the three Brachystegia pollen sites during the Mid-Holocene. However, temperature seasonality 6000 years BP was unfavourably high according to the present-day ecological niche dimensions of B. spiciformis woodland in southern Africa, casting doubt on the value of this climatic metric in controlling the distribution of B. spiciformis in its current range (from ENM), and in limiting its distribution north of South Africa's border (from limiting factors). However, two assumptions underlie most analyses of past climate using proxies and models. The first is that climate sets the boundaries to vegetation types, and therefore vegetation types are in equilibrium with climate except during the most rapid periods of climate change. Under this assumption, pollen-climate transfer functions can therefore provide reliable estimates of past climate. The second assumption is that, on long time scales, climate changes are driven by solar insolation changes.47
It should therefore be considered that precessional insolation in the southern hemisphere reached a minimum during the Early Holocene ~9000 years BP48 Hence, there were reduced seasonal cycles and lower temperature seasonality. Subsequently, a steady increase in summer insolation, and therefore temperature seasonality, between 9000 and 6000 years BP may already have reached an unfavourable range for B. spiciformis as the Mid-Holocene bioclimatic layer would suggest. The presence of pollen at these sites indicates that vegetation type boundaries are not in equilibrium with climate during rapid response phases to climate change. Adult trees may persist in an area of previously suitable climate during extended periods of climatic constraint (the storage effect), particularly if populations are capable of adaptive dynamics such as clonal regeneration. Brachystegia may therefore be subject to an extensive period of persistence between vegetation-climate equilibrium and local extirpation.
Future ecological niche
In keeping with the approximate northeastward retraction of the species distribution over the past 6000 years, a further ecological niche retraction of between 30.6% and 47.3% of the continuous B. spiciformis woodland in Zimbabwe and southern Mozambique is predicted by 2050. The decreasing suitability of habitat at the periphery of the continuous miombo population is supported by climate projections for the subcontinent.
It has been suggested that southern Africa will become hotter (temperature will increase by up to 3 °C) and drier (precipitation will decrease by ~10%) by 2060 and 2100, respectively.49,50 The projected increase in heat waves and the number of days above 35 °C50 over the continuous miombo woodland region will subsequently amplify variability in mean monthly temperatures. This temperature variability is likely to stress the tolerance of B. spiciformis further by pushing maximum temperature of the warmest month and temperature seasonality into the upper tolerance limit of the species, and closer to the limiting factor mean (Figure 3).
The future predicted decline in rainfall indicates a relatively strong drying signal for Zimbabwe and central Mozambique.50-52 The associated decrease in precipitation of the wettest quarter over central Zimbabwe50 may therefore place climatic constraint on the future ecological niche as this variable, which is considered the most important in determining B. spiciformis distribution, will become increasingly unsuitable.
In contrast, the suitable B. spiciformis niche in South Africa is modelled to experience very large and inconsistent spatial shifts by 2050, varying between range retractions of 70.6% to a range expansion of 376.5% (Table 2). The large discrepancy between models likely relates to the coincidence with a topographical heterogenous area on the northeastern escarpment, where the central highlands of South Africa abruptly give way to low-lying coastal plains. Fine-scale topographical effects on climate, which seem necessary for refugia, are not well captured within downscaled AOGCMs, contributing to model uncertainty.
Nevertheless, future climate change projections for northeast South Africa, which include the B. spiciformis woodland relict, suggest hotter (temperature increase of ~0.9 °C) and wetter (precipitation increase of conditions by 2100.53 Although subcontinental projections indicate increases in temperature seasonality,50 we suggest that future change will be buffered by the varied topography of the Soutpansberg massif, as has occurred historically. We suggest that the subsequent increase in precipitation of the wettest quarter (~8%53) will shift local climatic conditions at Gundani, from the present-day lower tolerance limit, into the 'most suitable' range identified for the species (Figure 3). However, the concurrent regional decrease (~6%53) in precipitation seasonality may cause conditions to become less tolerable as rainfall shifts away from the 'most suitable' range of B. spiciformis woodland (Figure 3).
Considering that there may be greater uncertainty with regard to the impact of climate on the ecological niche of B. spiciformis at the distribution edge, as compared to the continuous range, we should consider all three possible responses of populations to climate change: migration, adaptation and extinction.
Migration
It is unlikely that B. spiciformis woodland is capable of unaided range expansion through long-distance dispersal events, particularly given the very limited dispersal distances reported for the genus. There are limited untransformed or protected areas that could facilitate the establishment of new populations within the suggested ecological niche. In addition, current land-use practices have led to large-scale fragmentation which does not allow natural corridors for population expansion through clonal regeneration. Subsequently, any predicted range expansion of B. spiciformis woodland in South Africa along the eastern escarpment is considered unlikely as a result of anthropogenic constraints.
Adaptation
The persistence of populations as relicts may result from pockets of environmental suitability within the landscape, or may be the consequence of some adaptive dynamics by which species manage to overcome the climatic constraints posed by a changing climate. For example, plant ecology studies indicate climatic constraints on seed production.54 Consequently, rather than relying on pollination and seed production, many climate relicts rely more strongly on vegetative or clonal reproduction. The well-documented reliance of B. spiciformis on coppice regrowth and root suckering, rather than seed germination, both at the Gundani relict27 and in the continuous woodland6 suggests that even in the event of failed recruitment through climatic intolerance, the populations would persist though vegetative reproduction and the longevity of genets. Relict populations can furthermore improve their survival prospects by enlarging their climatic tolerance through phenotypic plasticity or micro-evolutionary adaptation.55 Once the capacity for phenotypic adjustment is exceeded, genetic adaptation remains the only option.22
However, several characteristics of relict populations imply that their potential for micro-evolutionary adaptation may be limited. Firstly, strong phenotypic divergence, compared with conspecifics from other parts of the range, does not appear to be a common phenomenon despite the presumed long-term exposure of relict populations to relatively strong selective pressures.56 Secondly, within-population genetic variation tends to be low in many climate relicts as a consequence of small population size, past bottlenecks3 and the occurrence of clonality. Thirdly, the small size of many relict populations, in combination with strong selection pressure, is likely to substantially elevate their risk of extinction as a result of demographic or environmental stochasticity before they can effectively adapt.56
Extinction
Although broadscale changes in species distribution can reasonably be forecast,21 our understanding of the environment and ecology of most climate relicts remains too poor to adequately anticipate their persistence or demise.22 The historical resilience of such populations demonstrates that we cannot simply assume that they will be extirpated rapidly after climate change.22 The storage effect suggests that individuals of the species may be present in an area long after the climate has become marginally tolerable, or completely unsuitable, depending on the life stage on which the climate control may act. The isolated B. spiciformis woodland plays an important role in the natural heritage of the local people and has been placed under a traditional woodland management regime. The tree is not currently used ethnobotanically and the direct threat of anthropogenic extinction is considered unlikely.
With future climate-induced migration ruled out and opportunities for adaptation beyond phenotypic adjustment limited, what then is the fate of South Africa's only miombo woodland? We suggest that the medium-term persistence of the relict is plausible based on (1) future climate change projections (2100), which suggest that local climatic variables will remain within (or nearly so) the 'most suitable' range of B. spiciformis, (2) the longevity of genets and the species' ability to regenerate vegetatively and (3) its historical resilience.
The suggested ecological niche retraction of the continuous B. spiciformis woodland in Zimbabwe and southern Mozambique may, however, be less exaggerated. Bioclimatic models are limited in their ability to determine the full extent of ecological interactions, especially in savannas in which the direct impact of climate on species distribution may be variable. One such example is that of future increases in atmospheric CO2. Species with large below-ground carbon sinks, like miombo, benefit from CO2 fertilisation57 which increases tree water use efficiency.58 This tree water use efficiency is consequently likely to contribute towards a greater tolerance of suggested decreased precipitation of the wettest quarter.
Conclusion
Through this unique study, we have identified the likely climatic determinants responsible for range shift at the trailing distribution margin of B. spiciformis in southern Africa.
Given the suggested divergent future responses of the continuous woodlands of Zimbabwe and Mozambique relative to that of the South African relict, an understanding of population dynamics and demographics across the subcontinent will prove critical in validating our predictions concerning the climate response phases (migration, adaptation, persistence) of B. spiciformis and elucidating the life-history stages during which climatic constraints may be imposed. Understanding both historical and contemporary climatic determinants of a species range is the first step towards assessing the vulnerability of extant climate relicts under global climate change. Theoretical responses must then be supported by in-situ monitoring of the populations.
Climate relicts have value as instructive models and natural laboratories for investigating how populations react to ongoing climatic change.59 Beyond the scope of this study exists an opportunity to explore genetic variation within the B. spiciformis relict population and investigate potential micro-evolutionary adaptation since isolation. Furthermore, development of long B. spiciformis and B. utilis tree-ring chronologies from the relict would allow for the investigation of temporal El Nino Southern Oscillation (ENSO) variability and predictions of future regional effect. Considering that ENSO effects on precipitation variability are strongest in southern Africa during the wettest quarter,14 the investigation may assist to adequately anticipate the persistence or demise of the relict population.
Acknowledgements
B.P. received funding from the Open Society Foundation through the Global Change and Sustainability Research Institute at the University of the Witwatersrand. The South African Environmental Observation Network provided partial financial support.
Authors' contributions
B.P. and D.I.T. conceived the study and the other authors helped to develop it; B.P and B.F.N.E. performed the analyses; B.P and D.I.T. drafted the first complete version of the manuscript; and all co-authors read and improved the manuscript.
References
1. Woodward FI. Stomatal numbers are sensitive to increases in CO2 from pre- industrial levels. Nature. 1987;327:617-618. http://dx.doi.org/10.1038/327617a0 [ Links ]
2. Prentice C, Cramer W, Harrison SP Leemans R, Monserud RA, Solomon AM. A global biome model based on plant physiology and dominance, soil properties and climate. J Biogeogr. 1992;19:117-134. http://dx.doi.org/10.2307/2845499 [ Links ]
3. Hampe A, Petit RJ. Conserving biodiversity under climate change: The rear edge matters. Ecol Lett. 2005;8:461-467. http://dx.doi.org/10.1111/j.1461-0248.2005.00739.x [ Links ]
4. Graham CH, Moritz C, Williams SE. Habitat history improves prediction of biodiversity in rainforest fauna. Proc Natl Acad Sci USA. 2006;103:632-636. http://dx.doi.org/10.1073/pnas.0505754103 [ Links ]
5. Campbell BM. The miombo in transition: Woodlands and welfare in Africa. Bogor: Centre for International Forestry Research; 1996. [ Links ]
6. Walker BH. Is succession a viable concept in African savanna ecosystems? In: West DC, Shugart HH, Botkin DB, editors. Forest succession: Concepts and applications. New York: Springer-Verlag; 1981. p. 431-447. http://dx.doi.org/10.1007/978-1-4612-5950-3_25 [ Links ]
7. Huntley BJ. Southern African savannas. In: Huntley BJ, Walker BH, editors. Ecology of tropical savannas. Berlin: Springer-Verlag; 1982. p. 101-109. http://dx.doi.org/10.1007/978-3-642-68786-0_6 [ Links ]
8. Millington AC, Critchley RW, Douglas TD, Ryan P. Estimating woody biomass in sub-Saharan Africa. Washington DC: The World Bank; 1994. [ Links ]
9. White F. Vegetation of Africa - A descriptive memoir to accompany the Unesco/AETFAT/UNSO vegetation map of Africa, Natural Resources Research Report XX. Paris: UN Educational, Scientific and Cultural Organization; 1983. [ Links ]
10. Chidumayo EN. Miombo ecology and management: An introduction. London: Southampton Raw; 1997. [ Links ]
11. Campbell BM, Angelson A, Cunningham A, Katerere Y, Sitoe A, Wunder S. Miombo woodland - Opportunities and barriers to sustainable forest management. Bogor: Centre for International Forestry Research; 2007. [ Links ]
12. Chidumayo EN. Responses of miombo to harvesting: Ecology and management. Stockholm: Stockholm Environmental Institute; 1993. [ Links ]
13. Ernst W. Seed and seedling ecology of Brachystegia spiciformis, a predominant tree component in miombo woodland in south central Africa. Forest Ecol Manag. 1988;25:195-210. http://dx.doi.org/10.1016/0378-1127(88)90087-4 [ Links ]
14. Trouet V, Esper J, Beeckman H. Climate/growth relationships of Brachystegia spiciformis from the miombo woodland in south central Africa. Dendrochronologia. 2010;28:161-171. http://dx.doi.org/10.1016/j.dendro.2009.10.002 [ Links ]
15. Scott L. A late Quaternary pollen record from the Transvaal Bushveld, South Africa. Quaternary Res. 1982;17:339-370. http://dx.doi.org/10.1016/0033-5894(82)90028-X [ Links ]
16. Cowling RM, Richardson DM, Pierce SM. Vegetation of southern Africa. Cambridge: Cambridge University Press; 2004. [ Links ]
17. Eeley HAC, Lawes MJ, Piper SE. The influence of climate change on the distribution of indigenous forest in KwaZulu-Natal, South Africa. J Biogeogr. 1999;26:595-617. http://dx.doi.org/10.1046/j.1365-2699.1999.00307.x [ Links ]
18. Scott L. Late Quaternary palaeoenvironments in the Transvaal on the basis of palynological evidence. In: Vogel JC, editor. Late Cainozoic palaeoclimates of the southern hemisphere. Rotterdam: Balkema; 1984. p. 317-327. [ Links ]
19. Scott L. Palynological evidence for Quaternary palaeoenvironments in southern Africa. In: Klein RG, editor. Southern African prehistory and palaeoenvironments. Rotterdam: Balkema; 1984. [ Links ]
20. Thuiller W, Albert C, Araujo MB, Berry PM, Cabeza M. Predicting global change impacts on plant species' distributions: Future challenges. Perspect Plant Ecol. 2008;9:137-152. http://dx.doi.org/10.1016/j.ppees.2007.09.004 [ Links ]
21. Hampe A, Jump AS. Climate relicts: Past, present, future. Annu Rev Ecol Evol S. 2011;42:313-333. http://dx.doi.org/10.1146/annurev-ecolsys-102710-145015 [ Links ]
22. Prado DE, Gibbs PE. Patterns of species' distributions in the dry seasonal forests of South America. Ann Mo Bot Gard. 1993;80(4):902-927. http://dx.doi.org/10.2307/2399937 [ Links ]
23. Rutherford MC, Midgley GF, Bond WJ, Powrie LW, Roberts R, Allsopp J. Plant biodiversity. In: Kiker G. Climate change impacts in southern Africa. Report to the National Climate Change Committee, Department of Environmental Affairs and Tourism. Pretoria: Department of Environmental Affairs and Tourism; 2000. [ Links ]
24. Luoga EJ, Witkowski ETF, Balkwill K. Regeneration and coppicing of miombo trees in relation to land use. Forest Ecol Manag. 2004;189:23-35. http://dx.doi.org/10.1016/j.foreco.2003.02.001 [ Links ]
25. Hurter PJH, Van Wyk E. First distribution record for Brachystegia spiciformis in South Africa. Bothalia. 2001;31:43-44. [ Links ]
26. Saidi TA, Tshipala-Ramatshimbila TV. Ecology and management of a remnant Brachystegia spiciformis (miombo) woodland in north eastern Soutpansberg, Limpopo Province. S Afr Geogr J. 2006;88(2):205-212. http://dx.doi.org/10.1080/03736245.2006.9713862 [ Links ]
27. Burrows J, Lutter M, Hahn N. A checklist of the plants found in the Venda Brachystegia sites and some comments on their future. PlantLife. 2003;28:5-10. [ Links ]
28. Erasmus BFN, Van Jaarsveld AS, Chown SL, Kshatriya M, Wessels K. Vulnerability of South African animal taxa to climate change. Global Change Biol. 2002;8:679-693 http://dx.doi.org/10.1046/j.1365-2486.2002.00502.x [ Links ]
29. Phillips SJ, Anderson RIP Schapire RE. Maximum entropy modelling of species' geographic distributions. Ecol Model. 2006;190:231-259. http://dx.doi.org/10.1016/j.ecolmodel.2005.03.026 [ Links ]
30. Elith J, Graham CH, Anderson RP Dudil M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129-151. http://dx.doi.org/10.1111/j.2006.0906-7590.04596.x [ Links ]
31. Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965-1978. http://dx.doi.org/10.1002/joc.1276 [ Links ]
32. Blach-Overgaard A, Svenning JC, Dransfield J, Greve M, Balslev H. Determinants of palm species distributions across Africa: The relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography. 2010;33;380-391. http://dx.doi.org/10.1111/j.1600-0587.2010.06273.x [ Links ]
33. Penman TD, Pike DA, Webb JK, Shine R. Predicting the impact of climate change on Australia's most endangered snake, Hoplocephalus bungaroides. Divers Distrib. 2010;16:109-118. http://dx.doi.org/10.1111/j.1472-4642.2009.00619.x [ Links ]
34. FAO / IIASA / ISRIC / ISSCAS / JRC. Harmonized World Soil Database (version 1.0). Rome and Luxemburg: FAO & IIASA; 2008. [ Links ]
35. Warren DL, Glor RE, Turelli M. ENM Tools: A toolbox for comparative studies of environmental niche models. Ecography. 2010;33:607-611. [ Links ]
36. PCMDI (Program for Climate Model Diagnosis and Intercomparison) [software]. [ Links ] c2007 [cited 2013 May 04]. Available from: http://www.pcmdi.llnl.gov/ipcc/about_ipcc.php.
37. Mika AM, Weiss RM, Olfert O, Hallett RH, Newman JA. Will climate change be beneficial or detrimental to the invasive swede midge in North America? Contrasting predictions using climate projections from different general circulation models. Global Change Biol. 2008;14:1721-1733. http://dx.doi.org/10.1111/j.1365-2486.2008.01620.x [ Links ]
38. Buisson L, Thuiller W, Casajus N, Lek S, Grenouillet G. Uncertainty in ensemble forecasting of species distribution. Global Change Biol. 2010;16:1145-1157. http://dx.doi.org/10.1111/j.1365-2486.2009.02000.x [ Links ]
39. Peterson AT, Soberón J, Anderson RP Pearson RG, Martinez-Meyer E, Nakamura M, et al. Ecological niches and geographic distributions: A modelling perspective. Princeton, NJ: Princeton University Press; 2012. [ Links ]
40. Beyer HL. Geospatial Modelling Environment 0.7.2.1 [software]. c2012 [cited 2013 Apr 24]. [ Links ] Available from: http://www.spatialecology.com/gme.
41. Braconnot P Otto-Bliesner B, Harrison S, Joussaume S, Peterchmitt JY, Abe-Ouche A, et al. Results of PMIP2 coupled simulations of the Mid-Holocene and Last Glacial Maximum - Part 1: Experiments and large-scale features. Clim Past. 2007;3:261-277. http://dx.doi.org/10.5194/cp-3-261-2007 [ Links ]
42. R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2012. Available from: http://www.R-project.org/ [ Links ]
43. Baldwin RA. Use of maximum entropy modelling in wildlife research. Entropy. 2009;11:854-866. http://dx.doi.org/10.3390/e11040854 [ Links ]
44. Kamundi DA, Victor JE. Brachystegia spiciformis Benth. National assessment: Red list of South African plants version 2014.1 [homepage on the Internet]. c2005 [cited 2015 June 17]. [ Links ] Available from: http://redlist.sanbi.org/species.php?species=240-36
45. Dobrowski SZ. A climatic basis for microrefugia: The influence of terrain on climate. Global Change Biol. 2011;17:1022-1035. http://dx.doi.org/10.1111/j.1365-2486.2010.02263.x [ Links ]
46. Chidumayo EN. Climate and phenology of savanna vegetation in southern Africa. J Veg Sci. 2001;12:347-354. http://dx.doi.org/10.2307/3236848 [ Links ]
47. Wassen RJ, Claussen M. Earth system models: A test using the mid-Holocene in the southern hemisphere. Quaternary Sci Rev. 2001;21:819-824. http://dx.doi.org/10.1016/S0277-3791(01)00130-5 [ Links ]
48. Wright HE. Global climates since the last glacial maximum. Minneapolis, MN: University of Minnesota Press; 1993. [ Links ]
49. Engelbrecht FA, McGregor JL, Engelbrecht CJ. Dynamics of the Conformal-Cubic Atmospheric Model projected climate-change signal over southern Africa. Int J Climatol. 2009;29:1013-1033. http://dx.doi.org/10.1002/joc.1742 [ Links ]
50. Tadross M, Davis C, Engelbrecht F, Joubert A, Archer van Garderen E. Regional scenarios of future climate change over southern Africa. In: Davis C, editor. Climate risk and vulnerability, a handbook for southern Africa. Pretoria: Council for Scientific and Industrial Research; 2011. [ Links ]
51. Hulme M, Doherty R, Ngara T, New M, Lister D. African climate change: 1900-2100. Climate Res. 2001;17:145-168. http://dx.doi.org/10.3354/cr017145 [ Links ]
52. Engelbrecht FA, Landman WA, Engelbrecht CJ, Landman S, Bopape MM, Roux B, et al. Multi-scale climate modelling over southern Africa using a variable-resolution global model. Water SA. 2011;37:5. http://dx.doi.org/10.4314/wsa.v37i5.2 [ Links ]
53. Davis CL. A climate change handbook for north-eastern South Africa. Pretoria: Council for Scientific and Industrial Research; 2010. [ Links ]
54. Montesinos D, Garcia-Fayos P Verdu M. Relictual distribution reaches the top: Elevation constrains fertility and leaf longevity in Juniperus thurifera. Acta Oecol. 2010;36:120-125. http://dx.doi.org/10.1016/j.actao.2009.10.010 [ Links ]
55. Reed TE, Schindler DE, Waples RS. Interacting effects of phenotypic plasticity and evolution on population persistence in a changing climate. Conserv Biol. 2011;25:56-63. http://dx.doi.org/10.1111/j.1523-1739.2010.01552.x [ Links ]
56. Pfenning DW, Wund MA, Snell-Rood EC, Cruickshank T, Schlichting CD, Moczek AP. Phenotypic plasticity's impacts on diversification and speciation. Trends Ecol Evol. 2010;25:459-467. http://dx.doi.org/10.1016/j.tree.2010.05.006 [ Links ]
57. Hoffmann WA. Post-establishment seedling success in the Brazilian cerrado: A comparison of savanna and forest species. Biotropica. 2000;32:62-69. http://dx.doi.org/10.1111/j.1744-7429.2000.tb00448.x [ Links ]
58. Polley HW, Tischler CW, Johnson HB. Growth, water relations, and survival of drought-exposed seedlings from six maternal families of honey mesquite (Prosopis glandulosa): Response to CO2 enrichment. Tree Physiol. 1999;19:359-366. http://dx.doi.org/10.1093/treephys/19.6.359 [ Links ]
59. Petit RJ, Hampe A, Cheddadi R. Climate changes and tree phylogeography in the Mediterranean. Taxon. 2005;54(4):877-885. [ Links ]
 Correspondence:
Correspondence:
Ed Witkowski
School of Animal, Plant and
Environmental Sciences,
University of the Witwatersrand,
Private Bag 3, Wits 2050,
South Africa
Email: Ed.witkowski@wits.ac.za
Received: 13 Aug. 2014
Revised: 06 Oct. 2014
Accepted: 16 Oct. 2014
* Current address: Global Change and Sustainability Research Institute, University of the Witwatersrand, Johannesburg, South Africa
Appendix:
Key to bioclimatic variables