Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.111 no.3-4 Pretoria Mar./Abr. 2015
http://dx.doi.org/10.17159/sajs.2015/20130255
REVIEW ARTICLE
The precautionary principle: making managerial decisions on GMOs is difficult
Fredrika W. Jansen van RijssenI; Jacobus N. EloffI; E. Jane MorrisII
IParaclinical Sciences, University of Pretoria, Pretoria, South Africa
IISchool of Biology, University of Leeds, Leeds, United Kingdom
ABSTRACT
The precautionary approach of the Cartagena Protocol on Biosafety, if incorporated into legislation of countries as a precautionary principle (PP), could cause great difficulty in decision-making on genetically modified organisms. No consensus seems to be possible on the interpretation of the PP, as responsibility often is passed on to political decision-making and, eventually, to court rulings. A case study on the assessment of possible unintended effects of endogenous allergens illustrates the complexity decision-makers may experience. We review the descriptions of the PP and the debate on the interpretation and conclusions that a number of authors have come to, as a step closer to a solution in decision-making. South Africa may have to consider the PP in the broader context of its food security needs, which would require improved communication as an additional step in the process of risk analysis.
Keywords: genetically modified organisms; South Africa; risk assessment; endogenous allergens
Introduction
A lack of coherence is observed in decisions made by governments for control of genetically modified (GM) crops. Examples include rejection by some African countries of donor GM maize; increased regulatory requirements; indecisiveness regarding new applications for permits with many delays and negative consequences to producers and consumers; and creation of negative perceptions towards genetically modified organisms (GMOs). There are many reasons for this situation, one of which is the variable application of precaution in decision-making, in particular different interpretations of the precautionary principle (PP). The control of GMOs by legislation and international interventions in this respect jointly speak of a cautious approach to risks from new technologies. However, genetic modification is no longer a new technology. Although international agreement exists on the general approach to risk and safety assessment of food from genetic modification, and many countries follow the international guidelines, debates on matters such as possible unintended effects from this technology are currently prominent. The difficulty that decision-makers often experience is illustrated by issues of possible unintended effects of the genetic modification on endogenous allergens. An understanding of the PP could give perspective to the burning issues, such as food security, with which decision-makers are confronted.
Description of terms and concepts
Risk, risk assessment, uncertainty
Risk analysis describes a dynamic iterative process composed of risk assessment, risk management and risk communication.1 The term 'risk' describes the probability of an adverse (health, environmental) effect (leading to harm or undesired consequence) and the severity of that effect, consequential to a hazard(s) or threat(s).1,2 In scientific terms, zero risk is non-existent.3 Some uncertainty is always present4 and forms an inherent and integral element of scientific analysis and risk assessment5-7.
Evolution of crop plants
Plant breeding
The assessment of the safety of food from GM crops should be placed in the context of the evolution of crop plants which started thousands of years ago when plants were first domesticated. A recent example is the Chinese gooseberry, which is not edible, but with breeding has become palatable, and is now called kiwi fruit. Today every crop plant that is grown is related to a wild species that occurred naturally in its centre of origin. Dramatic phenotypic changes occurred through new mutations and natural hybridisation that farmers selected for and then maintained as landraces. Scientific developments in agriculture, such as knowledge of genetics, contribute to improved plant-breeding practices. An array of scientific tools is now used to increase existing genetic variation, for example: hybrid embryo rescue; application of colchicine, a chemical employed to induce polyploidy; ionising irradiation; mutagenic chemicals and somaclonal variation (cell culture). Gene transfer techniques to develop GM crops are considered a logical extension of the continuum in the scientific development to improve plant breeding.8
Interesting new developments
Recent molecular techniques have shown that the techniques used in traditional (non-transgenic) plant breeding are associated with genetic changes such as mutations, deletions, insertions and rearrangements.9 These changes occur in addition to the movement of mobile genetic elements such as transposons (jumping genes) that are responsible for most genome plasticity.9 Many of these genetic alterations occur in nature.10 Plant breeders traditionally eliminate observed off-types during the evaluation process. Despite the dynamic nature of the genomes, and the effect of traditional breeding on the genome, only a few safety concerns from traditional plant breeding have been recorded over years. Several recent articles have shown that traditional breeding causes more inherent variability than GM.11,12
Arguments for less stringent requirements or exemption from regulation are heard frequently.13,14 There may also be a need for policy reform to take into account the new developments.15
Food safety assessment of GM crops
The Codex Alimentarius Commission, a body under the joint auspices of the Food and Agriculture Organization and the World Health Organization, played a prominent role in the development of guidelines for the risk and safety assessment of food products from GMOs.16 These guidelines outline the safety and risk assessment of food from GMOs in a precautionary way by proposing the steps to be taken in the assessment. Substantial equivalence, a concept mentioned in the Codex guidelines, was previously mistaken as the endpoint of the assessment. The concept has been replaced by an improved description of the approach for safety and risk assessment, which involves a comparative analysis of the composition and of the phenotype.17,18 This approach is preferred as animal toxicity studies would be difficult because of the complex nature of food compared with chemical molecules such as pesticides. Molecular characterisation is included in this starting point of the assessment to identify hazards. The composition of the edible parts of the genetically modified crop is compared with those of its near-isoline with a history of safe use. A broad range of parameters (macro- and micronutrients, antinutrients, toxicants and secondary compounds) is considered in the comparative analysis. Safety assessments of intended changes and of unintended significant differences are the next step in the safety assessment. Differences need not necessarily be unintended effects of genetic modification, but can be caused by slightly different genetic backgrounds or environmental effects. Compositional safety is considered in the context of the normal composition of the crop by including a number of commercial non-GM crops in the trials that are conducted across several environments. Safety is informed by considering the normal array of compound levels present in crops that have a history of safe consumption19 including the antinutrients, toxicants and endogenous allergens of the crop. The nutritional value of the crops is an important consideration in the assessment. Endogenous allergens had not received serious attention from regulatory authorities until recently.20
South African precautionary approach to GMOs
The establishment in South Africa of SAGENE (South African Committee for Genetic Experimentation)21 in 1978 is evidence of the environmental and human health concerns of scientists when progressing with a new technology such as genetic engineering (also known as genetic modification or modern biotechnology). The need for a precautionary approach to possible environmental threats and concern for human health is illustrated by several South African laws. A precautionary approach in managing risks is included, for example, in the two South African environmental management Acts,22,23 which provide for 'a cautious approach which takes into account the limits of current knowledge about the consequences of decisions and actions'. The Genetically Modified Organisms Act of 1997, as amended, incorporates the requirements of the Cartagena Protocol on Biosafety (CPB), and, in the regulations to the Act, requirements are described for the protection of human health and the environment against possible risk from GMOs.24 No mention is made of cost/benefit or risk/benefit, or proportionality of risk in applying the PP,24(p.3) although the GMO Act does refer to 'socio-economic impact', with the implication that an impact could be positive or negative.
South Africa has published a number of guiding documents. However, different South African government departments represented on the GMO Council apparently hold different positions. The absence of specific policies is obvious in the recent mandatory GMO labelling requirements in which regulations were promulgated by the Department of Trade and Industry25 without consideration of existing GMO labelling regulations of the Department of Health. The Department of Environmental Affairs, in its 'framework'26, refers to 'null risk', 'avoid' and 'prevent', which describe precaution at its extreme, whereas other government departments do not seem to have any specific interpretation of the GMO Act in terms of their mandate.
There seems to be a need for policy and guidance on matters such as the PP, new breeding technologies and dealing with possible unintended effects from endogenous allergens. The new strategy on bio-economy is a step in the right direction to address national policies.27
The precautionary principle
Cartagena Protocol on Biosafety
Against the background to risk assessment and the decisions with which regulatory authorities are confronted when dealing with genetic modification of crops, an understanding of the PP is important.
A precautionary approach was originally developed to provide risk managers with a tool for making decisions on environmental threats from processes or substances that had not undergone safety evaluation or regulatory approval.28 Cooney29 has summarised the history of the development that resulted in a number of international agreements.
The CPB30 is one of a number of important agreements among nations to consider possible harm to the environment and human health. It requires countries to introduce measures to safely manage transboundary movement of living modified organisms. Countries that became signatories to the CPB were expected to incorporate the CPB into legislation and to adhere to the requirements for environmental safety and human health. A precautionary approach in consideration of risks, articulated in the CPB as well as in other international agreements and environmental law, is the cause of ongoing debates on the interpretation and implementation of precaution.29
The PP was first incorporated into the World Trade Organization's (WTO) Agreement on Sanitary and Phytosanitary Measures (SPS Agreement) in 1994.31 Article 5.7 makes it possible to obtain additional information within 'a reasonable period of time'31(p.72) when existing information is inadequate, whereas Article 3.3 allows for more stringent protection than relevant international standards, if there is 'scientific justification'31(p.70). The Codex standards are accepted by WTO as references.
Many debates seem to have ignored the fact that the point of departure in assessing biosafety of living modified organisms is determined in Article 4 of the CPB. The focus of the Protocol is on LMOs [living modified organisms] that may have adverse effects on biodiversity as well as risks to human health (Article 1)30(p.3) and 'risk assessments shall be carried out in a scientifically sound manner' (Annex III, para 3)30(p.28). Furthermore, the directive for application of a precautionary approach has been set in Principle 15 of the Rio Declaration,32 namely, 'Where there are threats of serious or irreversible damage, lack of full scientific certainty shall not be used as a reason for postponing cost-effective measures to prevent environmental degradation'32(p.2). Neither of these approaches demands that all applications of biotechnology or of genetic modification must undergo extensive assessments to comply with the precautionary approach and neither implies that biotechnologies are inherently unsafe. The interpretation of the requirements of the CPB in many aspects has been debated for a number of years. Some of the implementation procedures seem not to be in proportion to the risk or a cost/benefit analysis; for example, the need for milling GMO commodities such as maize in the Southern Africa Development Community.
A principle or an approach?
Legislating GMOs by pre-market regulatory requirements for risk assessment and by managing risks at the different steps of the development and production of GM crops are precautionary measures. On the other hand, the 'precautionary approach' as applied according to the CPB, intends to address uncertainties that occur in risk assessment. 'Precaution' is generally recognised - not as a hypothesis, theory or methodological rule - but as a normative principle for making practical decisions under conditions of scientific uncertainty.33 A normative principle implies obligations to 'anticipate harm and moral obligations in judging the adequacy of available knowledge'34(p.263). 'Normative' is defined in the Collins English Dictionary as (1) Implying, creating, or prescribing a norm or standard, as in language: normative grammar; (2) Expressing value judgements or prescriptions as contrasted with stating facts.35 In teaching of religion, distinction is very broadly made between the 'regulative principle' of worship meaning binding in exact accordance to the Holy Scripture, whereas 'normative principle' of worship in general means nonbinding.36
The implementation of the PP 'requires different normative commitments and choices'37(p.2). Ahteensuu describes the PP as a principle of 'practical decision-making which may be justified on the basis of ethical and socio-political grounds and/or as a form of rational action'37(p.2). The obligatory nature of this normative principle has resulted in more than policy design criteria, but becomes a 'regulatory philosophy'38(p.23) when included in legislation, which, in turn, has to be interpreted by regulators. Von Schomberg39 explains the normative challenges for application and implementation of the PP The scope of PP deliberations stretches across broad political debate, policy level (political and societal), science-policy interface and risk management.
Authors such as Recuerda40 (p.5) analysed the legal interpretations of the US versus the European system. The conclusion was that 'principle' had the connotation of legal language, of law, a 'principle of law', which is the status of the PP in Europe, whereas the USA considers it an approach with no legal connotation. The English language version of the CPB30 uses the word 'approach', French 'l'approche de precaution', German 'Vorsichtprinzip' and Spanish 'principio'. It seems that the words 'approach' and 'principle' are used without clear distinction in different languages.
The precautionary approach is recognised as a precautionary principle when included in legislation with obligations, as explained by authors such as Levidow et al.34 and Lofstedt38. Cooney29 reasoned that the PP would not determine a specific outcome or decision, unless a specific formulation required it. Therefore, the terms 'precautionary approach' and 'precautionary principle' were used interchangeably. The PP nomenclature is followed in this paper.
Definitions
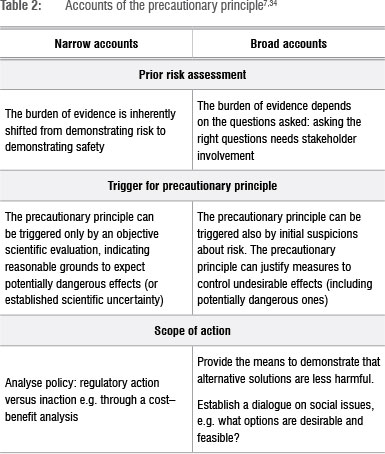
A normative principle may be interpreted in various ways. This multi-interpretation is illustrated by about 19 definitions of the PP (Table 1). Central to the PP is the obligation of action to reduce harm to the environment and human health, and the moral obligation that action be taken even if scientific evidence is inconclusive. These obligations are formulated in different ways - strong 'obligatory' versions and weak 'optional' versions (Table 2). The strong form of the PP, for example the Wingspread Statement (1998), is advocated by Greenpeace41 and UNESCO-COMEST42, while an example of a weak form is included in the Rio Declaration. The difference between weak and strong precaution lies mainly in the greater emphasis on risk avoidance, provision of safety and the obligation to take safety measures. Variations in the scope of 'precaution' from narrow to broader accounts are reflected in (1) prior risk assessment, (2) what triggers the use of the PP and (3) the scope of action.7,34,43

Core of the debate on the precautionary principle
As threats to health and the environment become more complex, uncertain and global in nature, the PP is increasingly being debated.45 Cognisance has to be taken of the debate. At the core of the debate on the PP is the degree of scientific uncertainty in risk assessment and what decisions should be made by managers in the face of uncertainty, when to apply precaution, and what precautionary measures should be taken to achieve certain levels of protection.46
Klinke and Renn47 identified five major noteworthy themes in this debate. These themes can be grouped into two very closely related issues: how risks are perceived by different people and how regulatory authorities deal with these risks.
Perception of risks and evaluation of uncertainties
There are two camps on the perception and evaluation of risks. One claims that risks are mental constructs that originate in human minds and are only real within a specific group of people. The opposing camp argues that technical estimates of risks are true representations of observable hazards and that the effect is predictable, regardless of the analyst's beliefs. In between these two viewpoints are those who believe that a combination of the two is more realistic.48
The concept of 'sound science' that is included in international agreements and guidelines16,30,49 is being challenged. It is questioned7,34,43,50 whether scientists can conduct objective analyses of risks because they interpret information according to their scientific knowledge and values. Anti-commercial sentiment is also often observed in literature on the PP, by remarks on the integrity and independence of scientists, the regulators' public accountability and those with 'financial stake in scientific development'51(p.376).
Charnley52, former president of the Society for Risk Analysis, has it that risk analysis is 'threatened by a serious, growing, anti-risk analysis sentiment that is challenging the legitimacy of science in general, and risk analysis in particular'52(p.3). Scientists and managers receive blame for many 'risky' incidents, although there is perhaps an implication here that the PP replaces risk assessment. Berry53(p.7) responded to the accusations:
Evaluation of data obtained from scientific investigations is not easy and the process often seems counter-intuitive to the uninformed. Some hold the conviction that ideological motives colour all deliberations - this makes it easy to suggest that in any scientific debate an opponent's reason for holding a particular viewpoint or belief depends on his or her motives rather than their knowledge base. This position may be useful in providing the grounds on which to mount a polemic against any perceived threat (drugs in modern medicine, pesticides in intensive agriculture or genetically modified organisms). The conviction that opinions cannot be based on independent thought, has led to a disregard of professionalism and the development of the view that who pays you determines your opinion - not your science.
The debate also includes evaluation of uncertainty in risk assessment, the validity of animal models54, variability in data19 and lack of sufficient knowledge47. Approaches to assessment of GMOs, for example the substantial equivalence, and concepts of familiarity and 'history of safe use' have been criticised as pseudoscience.55 As an alternative, a 'holistic' approach is advocated by some.55-57
Instead of gaining more knowledge about uncertainties, alternative management strategies could be proposed - for example, human interventions that are manageable.47 Additional and more stringent control to the point of embargos or refusal to avoid any risk as a precautionary measure might be detrimental to progress. Steering direction is difficult in these situations without clear policies at every level of decision-making.
There is no well-established classification of uncertainty in risk assessment.47 Renn and Klinke48 have described six groups of risks named from Greek mythology. They grouped GMO technology with disintegrating polar ice sheets because of uncertainty in both probability of occurrence and extent of damage.48 Vlek58 grouped GMOs with risks such as the AIDS epidemic as a 'diffuse source' with the potential risk of long-term and extensive effects. Risks associated with conventional agricultural plant breeding are not mentioned; neither are far less precise techniques such as induced-mutation breeding in which plants or seeds are exposed to ionising radiation for which regulatory control does not exist or is more lenient than that for GMOs.
Public interest
Public perception on how uncertainty in risk assessment is handled is a valid issue to some47 and engagement of interested and affected parties in appraisal is also a matter of scientific rigour7. In a survey on people's opinions on some scientific issues in the UK, some responded that most scientists are poor communicators,59 resulting in a gap in knowledge transfer to the public at large, while some sensational media contributions have led to misguided public perceptions. The debate also focuses on the legitimate role of public deliberations in risk analysis and management. The International Risk Governance Council's position on values is that all dimensions of risk, both the factual and the socio-cultural60(p.12), need to be considered.
The 'contextual variables of risk' as they affect perceptions of consumers are important.47(p1077) One of the many issues is trust in regulatory agencies and risk handling, often described as credibility.38
The debate in perspective
Risk is a societal construct as well as a physical reality.49 Results from the continued debate are observed in changes in the process of risk analysis, the critical assessment of approaches to risk assessment and proposals for improved structured communication.61 Some valid arguments have been raised. Inclusion of public concern/social criteria needs further research. The inclusion of sociological issues in decision-making is anything but simple. There are many aspects - such as cultural differences, country needs, human nature, philosophies, religions and political issues - to take into account.
Analysis of the precautionary principle and its application
Critique of the precautionary principle
Vlek58 groups the multiple criticisms of the PP into ten objections. Some of the objections are that: the PP is vague and broadly ambitious62; serious or irreversible harm is ill defined62,63; it is dependent on plausibility reasoning64; it is a policy of risk avoidance65; it is too absolute and obligatory, thereby blocking or slowing down technology innovation and progress66; it demands 'impossible' proof of safety; identifying the nature and likelihood of possible serious harm may yield high costs of safety tests and long delays in relevant policy decisions67; and it can be misused by powerful interest groups51.
Vlek's58(p.533) conclusion was that the PP has 'an unusually protective inclination towards foregoing an activity or imposing strict|er) safety measures upon it, both of which are induced by large uncertainty about possible disastrous consequences'.
Peterson63 rejected the use of the PP as a basis for decision-making, citing examples of decisions on conducting clinical trials, mobile phones and GM-derived foods. He said, 'the precautionary principle therefore replaces the balancing of risks and benefits with what might best be described as pure pessimism' 63(p.306). He argued:
We need a principle that tells what to do and what not to do for each possible input of qualitative information...no generally accepted formulation will ever emerge as the PP is not a single well defined idea...it makes more sense to describe it as a cluster of vague related intentions about risk aversion, burden of proof, irreversible damage and normative obligations [and] any reasonable formulation of the PP will imply a value judgment that no rational decision maker would be prepared to accept.63'1'306'307
With respect to the burden of proof, Petersen claimed, 'It rests with anyone who makes a claim, regardless of what is being claimed' and concluded, 'There is nothing wrong with the precautionary principle - as long as it is not used for decision-making'63(p.308).
Berry53(p.7) commented that 'convictions with ideological motives colour all deliberations'. He mentioned the PP as a good example of only considering results that fit a preconceived viewpoint. He asserted:
But it should be made clear when political or socio-economic judgments are being made and the pretence that they are scientific judgment, should be eschewed. It is comforting to pretend that we know more than we think, but damaging to pretend too much.53(p.7)
In summary, Vlek58 said that the PP is mostly derogated for its general inclination and motivation, its dependence on plausibility reasoning, its lack of comparative risk evaluation, its lack of explicit decision-making considerations, its openness with regard to legal obligations, and its implied shift of the burden of proof of safety.
Decisions from arbitration
Proof of the difficulty in interpretation of the meaning of the PP concept lies in the opinions of jurists who are grappling with it because of its 'philosophical characteristic, inherent uncertainty, and ambiguous and arbitrary nature'66(p10). The PP is open ended and undefined, which 'gives regulators almost unlimited discretion to impose restrictions'66(p.32). Ultimately, the courts will have to flesh out the principle.51,66,68,69 The reality is that prevailing social and political values influence to some degree the trend in case law. In legal formulation, UNESCO-COMEST42(p.22) advises: 'first, the recognition of a value by a society is worthy of protection, and, second, the provision of a legislative tool [is] in order to protect this new recognised value'.
A WTO31 ruling on GMOs illustrates the application of the PP in international trade. A long-standing dispute existed between the USA and Europe over the European Commission and several European member states' de facto moratorium on approval of GMOs. The moratorium lasted from 1998 to 2004. In 2003, the USA, Canada and Argentina sought legal recourse at the WTO under WTO SPS (Sanitary and Phytosanitary) law based on unjustified and illegal denial of access to European markets (EC Biotech Products case) that resulted in financial losses to US farmers. The WTO based its final decision in 2006 on failure of the defendant to conduct 'adequate' risk assessments (SPS Article 5.1 and Annex A (4)) by not taking into account risk assessment techniques (protocols) of relevant international organisations. Although their scientists' conclusions were based on scientific methods, the WTO panel found that legislators often based decisions on 'unverifiable facts and public fears'70(p.2). The European Commission's arguments apparently rested on concerns by regulators on 'scientific uncertainty', thereby ignoring their own risk assessments. The WTO panel rejected the defendant's arguments (Articles 5.1 and 2.2). The argument that there was 'insufficient scientific evidence' (Article 5.7) was also rejected as the European Commissions' scientific committees indeed reviewed the relevant information and did not question their previous conclusions. Therefore, additional information in this case was not an issue. 'Scientific uncertainty' and 'insufficient scientific evidence' are not the same (SPS Article 5.7). The WTO also concluded that the European Commission had acted inconsistently with its obligation under Annex C (1) (a) and Article 8 because of the undue delay. The European Commission accepted the ruling.
Europe introduced legislation to improve the framework for assessing the application of GM plants and introduced strict labelling and traceability requirements for GMOs in 2003 to accommodate public perception and address fears. An assessment of the WTO panel's decision is not pursued further in this study.
The interpretation of uncertainty, and perhaps consumer perceptions, is further illustrated by the November 2011 ruling of the two highest courts in the European Union - the European Court of Justice and the Conseild'Etat of France - against the French ban on planting of GM Bacillus thuringiensis maize (Bt maize). The ban was based on a European Union 'safeguard clause' and legal provision for 'emergency measures' in case of evidence of serious hazards to human health and the environment. The courts ruled that France did not present any such new evidence to substantiate their ban on Bt maize. France responded by stating that it will reinstate the ban.71
In South Africa, appeals72 against several decisions made by the GMO Executive Council on the general release of Bt11 maize, the use of biofortified sorghum for greenhouse studies and planting of cassava field trials, resulted in the Appeal Board ruling in favour of the applicants, although in the latter two cases certain conditions which require more stringent management were added.72 In the case of an appeal by Biowatch against the decision to grant general release of Bt11 maize, the appeal board ruled against the appellant.73 Valuable lessons were learned from this case, one of which was that demands for additional data, as a matter of 'nice to know', illustrating the interpretation of 'precaution' by some groups in the society could result in costly delays to the applicant, as well as the complainant and government.
Acceptable solutions in the precautionary principle debate?
Key issues in the precautionary principle
Having summarised the issues in the debate, the reality is that clear guidance is needed to facilitate regulatory decisions based on an even-handed approach to precaution. In order to come to acceptable solutions, the key issues at this stage are:
- Key inherent problems with the application of the PP and the corresponding precautionary approach were identified by Vlek58; for example, (1) substantive issues such as determining the plausibility, nature and seriousness of possible harm or damage and (2) procedural issues, for instance optional versus obligatory precaution, and the need for further research and policy development. These problems are also described as factors triggering recourse, which is the decision to act or not to act, and the measures on how to act.
- The PP applies to serious uncertain risks or threats; it is inclined to be unusually protective or even preventative; the proponent has a large burden of demonstrating the likelihood of safety; and there is the tendency to delay risk-taking until sufficient new information becomes available.58,74
- A number of authors have described models for decision-making based on assessment of risks in general.58,74,75 These risks rest upon axioms and assumptions that are not always valid in practice, such as perceptions of cultural differences.
In trying to find a way forward, the following comments on the application of the Pp by Feintuck51 are noteworthy: 'The PP is currently applied as a procedural rather than a substantive device' and 'substantive content and value-orientation' are necessary. Feintuck51 contended that if the 'PP is devoid of intrinsic values, these may simply be filled by the values of dominant groups'. His conclusion, after studying the development and implementation of the PP was that it is a 'complex picture of interaction between science, economics, public policy and law'51(p.377,392).
Risk governance of GMOs
The European Commission46 places the burden of determining an acceptable level of risk for society as a judgement of an eminently political responsibility: 'Decision-makers faced with an unacceptable risk, scientific uncertainty and public concerns have a duty to find answers'46(p4). Guidance from the European Commission perspective is followed by, for example, the South African regulatory authorities for GMO governance (Table 1).44
The International Risk Governance Council - a private, independent, not-for-profit foundation - was established in 2003 to support governments, industries, non-governmental organisations and other organisations to deal with major and global risks and to foster public confidence in risk governance. Debates within the PP protagonist circles focus on the relative importance of substance versus procedure. At the very least, it is important to be in agreement on the importance of procedural steps in instances of great uncertainty about the available evidence, possible consequences, feasible options, long-term effects and minority views. The International Risk Governance Council has developed a framework to assist governments in decision-making on all kinds of risks.60
The designers of the International Risk Governance Council's framework60 emphasise the importance of stakeholder participation. This is also elaborated on by a number of proponents of the PP37,43,76 One can conclude that interaction at different levels is required, but it would have specific challenges.
Vlek58 suggested that the parties involved might do well to attend carefully to the kind of participants, structure, content and process making up the relevant assessment and management strategy. Vlek58 also warned against 'individual judgements and social decision-making, for example allowing room for prior beliefs and biases, selective information processing, authoritative dominance and groupthink at the cost of minority views'58(p.535). In participative, multi-stakeholder situations, these could lead to disputable judgements, decisions and actions.51
In a democratic political situation, and to improve credibility of risk governance, improved interaction with stakeholders (for example the public, scientists and the owners of the technology) has to be considered. Much more thought will have to go into defining the nature and substance of such interactions. Participation has to be correctly defined, as accountability remains with the regulatory authority. Codex1 describes an interphase for determination of 'risk assessment policy' as a specific component of risk management interaction among risk managers, risk assessors and stakeholders that governments could consider to improve communication.
Case study: Assessment of endogenous allergens
This case study illustrates some of the complexities with which decision-makers could be confronted in the governance of risks.
Codex's guidance for risk assessment, as a precautionary approach, describes the case-by-case process to be followed in the safety/risk assessment of GMO products (see section on safety assessment of GM crops). Keeping in mind the conclusions from the molecular characterisation, phenotypic and agronomic comparative studies as well as comparative analyses of the nutrients, toxicants and antinutrients would follow. Codex16 considers that endogenous allergens should be included in the compositional comparative safety assessment. The safety/risk assessments of possible unintended effects of endogenous allergens pose problems, as described in the sections following.
Step: Risk assessment framing
Policy development
A precautionary/risk assessment debate regarding inclusion of endogenous allergens in the safety and risk assessment may proceed as follows.
GM-derived foods are assessed according to regulatory requirements and, if approved for human consumption, different laws of a country may have additional requirements (e.g. labelling of food). Labelling in many countries includes information on the eight food allergens (Box 1). A possible question is: Would it be necessary to include endogenous allergens in the compositional analysis of those eight foods when derived from GMOs when allergenicity labelling is a standard required? Another question could be: What about possible unintended increased levels of the endogenous allergen in these eight allergenic foods? It is difficult to determine the prevalence of allergenicity, as consumers tend to avoid foods to which they are allergic. Although allergenicity to some foods, such as peanuts and tree nuts, could affect up to 1% of the population, none of these foods has been withdrawn from the market. However, some countries do require analysis of these allergenic foods.20

Another question could be: What about possible unintended increased levels of endogenous allergens in GM-derived food in addition to the eight allergenic foods? This question provokes a number of issues; hypothetically, it is possible for someone to be allergic to any food, processed or raw. A question on concentration levels would be: What level would trigger a tolerance level that could serve as a point for decision-making by regulators? Information that could stimulate more questions is given in Box 1.
Uncertainty in answering these questions because of a lack of sufficient scientific information is illustrated in the following discussion in the case of maize.
Maize - a staple food for many people - is a crop that has been genetically modified to introduce a number of new traits. To better understand the complexity encountered in decision-making, a hypothetical case is made for endogenous allergens of maize. Known allergen information is given in Box 2. A general conclusion from this information is that there are a number of issues that would make it difficult to make a decision of absolute safety unless more information is generated. The shortage of serum donors would be critical in most cases.

There are more questions and issues. It would only be possible to consider the tolerance levels once the range of endogenous allergen levels has been determined for each crop plant. How sensitive are the tests and what serum sample size is required? What percentage (or concentration) increase above the range of natural biological variation is acceptable? What percentage of the population should be protected?
What levels would cause reactions in patients, from mild to severe? What percentage of severe reactions such as anaphylactic shocks has been documented for the population?
There is also a question on the labelling regime for those GM-derived foods which are not one of the mentioned eight allergenic foods. And should elevated levels of the endogenous allergens be detected, could it be shown that they were explicitly caused by the genetic modification and not by normal variability? Would an Identification Preservation System be feasible, practical and affordable?
More recently, the results from information accumulated over more than 20 years, as described in this paper, showed no significant differences in the composition of tested components (excluding endogenous allergens) between GM crops and the near-isolines. Furthermore, consumers have been exposed to GM-derived foods through a number of different crop species and traits - all assessed case-by-case - with no adverse effects recorded. These are important observations that regulators should take into consideration.
The final question that a managerial team could ask might be: Does the case under consideration qualify as a situation of serious uncertainty and an irreversible risk? This question may not be easy to answer.
Regulating the assessment of endogenous allergens of GMOs?
Decision-makers are confronted with a number of challenges. A study of the literature on natural allergens and GM crop plant endogenous allergens shows that many questions remain unanswered. It seems that some regulatory authorities are overreacting by asking for more and more information to confirm possible unintended differences between the endogenous allergens of the GMO and its non-GMO near-isoline. Adequacy of the assessment of allergenicity is debated. A school of allergen specialists81 commenting on the validation of the tests, and particularly availability of serum for testing, contended that the 'extreme precautionary position is not scientifically defensible'81. They opined that 'we need to know more about endogenous allergen levels and natural variation and have not seen data that demonstrate an enhanced risk to the consumer, based on the observed variation'. Upregulation of allergen levels is contested by Herman and Ladics91. There is no evidence tabled on consumers showing adverse reactions owing to allergens from eating approved GMO products. Therefore, the postulated risk remains a hypothetical one.
Regulatory authorities have to make decisions, while scientists continue to debate at technical level. Before requesting additional studies, policies on risk to consumers should be placed in the broader context of the country's needs. The example shows the need for proactively considering the approach to be followed. These should be included in a risk assessment policy interface that does not exist in many risk governance situations. Consequences for additional precautionary requirements that are not well thought through are far reaching. Knowledge gathered over many years and now assessed, brings new perspectives on the effect of different plant-breeding practices, including genetic modification. The need for assessment of endogenous allergens is under debate. The case study with endogenous allergens illustrates the difficulty decision-makers may experience in implementing Codex guidelines. These guidelines are not compulsory regulatory instruments, but are significant in international trade. New knowledge of the genome and the place of genetic modification compared to traditional plant-breeding technologies, with respect to unintended effects, could influence the outcome of potential international trade disputes.
Conclusions and recommendations
The debate on the PP illustrates the diverse opinions on safety requirements for GM crop plants. Some consider GM crops irreversibly harmful, while others view them as representing only a continuum of existing knowledge and agricultural practices. The key problem with the PP is that it is a normative principle - ill-defined and vague. The rational way forward seems to be that a number of experts, including stakeholders, should be included in a structured way to contribute to policy development and to frame the risk assessment.
Recommendations for South Africa
- A new dispensation in South African risk governance of GMOs should be considered that requires benefits of modern biotechnology |including genetic modification) to be given adequate consideration and applied to the advantage of the population.
- The new Bio-Economy Strategy for South Africa27, published in 2013, addresses, inter alia, food security and economic growth as some of the key imperatives. The strategy specifically mentions: identifying 'areas of public policy that can remove barriers' and 'improve cooperation between stakeholders'27|p.7). These matters should be investigated with a view to establishing an interface between risk management |decision-making) by the GMO council member departments, the scientific GMO advisory committee and stakeholders. Stakeholders should include the relevant scientific communities, such as specialist agricultural scientists, as the most trusted parties for credibility of information. Socio-economic matters should be proactively considered during this phase of the iterative interactions of risk analysis.
- Development of policy and guidelines on issues of risk assessment is a matter of importance. These include, inter alia, the principle of precaution; consideration of dealing with possible unintended effects from genetic modification; and a policy on new plant-breeding techniques.
Relevance to other countries
A number of countries are in the process of finding a way forward in terms of the regulation of GM crops, and, in so doing, are determining their own approaches to decision-making and the application of the PP. The issues raised in this paper may be useful in their deliberations on the way forward.
Acknowledgements
The study was made possible by a grant from the National Research Fund. We thank the anonymous reviewers for their comments on the manuscript. The article was submitted in revised form as a chapter in the PhD thesis of F.W.J.v.R.
Authors' contributions
F.W.J.v.R authored the manuscript; E.J.M. and J.N.E co-supervised the study.
References
1. Codex Alimentarius Commission (CAC). Procedural manual. 23rd ed. Rome: FAO/WHO Food Standards Programme; 2015. Available from: ftp://ftp.fao.org/codex/Publications/ProcManuals/Manual_23e.pdf [ Links ]
2. United States Environmental Protection Agency (US-EPA). Risk assessment [document on the Internet]. No date [cited 2015 Feb 20]. Available from: http://www.epa.gov/risk_assessment/basicinformation.htm [ Links ]
3. Querci M, Kleter G, Malingreau JP Broll H, Van den Eede G. Scientific and technical contribution to the development of an overall health strategy in the area of GMOs. JRC-IHCP Reference Reports. EUR 23542. Luxembourg: European Committee; 2008. [ Links ]
4. De Bruijn J, Ten Heuvelhof E. Scientific expertise in complex decision-making processes. Sci Public Policy. 1999;26(3):179-184. http://dx.doi.org/10.3152/147154399781782428 [ Links ]
5. Renn O. Risk governance: Coping with uncertainty in a complex world. London: Earthscan; 2008. [ Links ]
6. Wolt JD. Understanding risk and safety assessment for genetically modified plants [document on the Internet]. c2008 [cited 2015 Feb 19]. Available from: http://agribiotecj.info/details/wolt-riskassessmentoweb02.pdf. [ Links ]
7. Stirling A. On 'precautionary' and 'science-based' approaches to risk assessment and environmental appraisal. In: Stirling A, editor. On science and precaution in the management of technological risk. JRC-IPTS Technical Report. EUR19056/EN/2.2001 [document on the Internet]. c2001 [cited 2015 Feb 20]. Available from: http://ftp.jrc.es/EURdoc/eur19056llen.pdf. [ Links ]
8. Prakash CS. The genetically modified crop debate in the context of agricultural evolution. Plant Physiol. 2001;126:8-15. http://dx.doi.org/10.1104/pp.126.1.8 [ Links ]
9. Weber N, Halpin C, Hannah LC, Jez JM, Kough J, Parrot W. Crop genome plasticity and its relevance to food and feed safety of genetically engineered breeding stacks. Plant Physiol. 2012;160:1842-1853. http://dx.doi. org/10.1104/pp.112.204271 [ Links ]
10. Lusser M, Parisi C, Plan D, Rodríguez-Cerezo E. New plant breeding techniques: State-of-the-art and prospects for commercial development. JRC-IHCP Scientific and Technical Reports. EUR 24760 EN. Luxembourg: European Committee; 2011. [ Links ]
11. Batista R, Saibi N, Lourenco T, Oliveira MM .Microarray analyses reveal that plant mutagenesis may induce more transcriptomic changes than transgene insertion. Proc Natl Acad Sci USA. 2008;105(9):3640-3645. http://dx.doi. org/10.1073/pnas.0707881105 [ Links ]
12. Kogel KH, Voll LM, Schafer P Jansen C, Wu Y Langen G, et al. Transcriptome and metabolome profiling of field-grown transgenic barley lack induced differences but show cultivar-specific variance. Proc Natl Acad Sci USA. 2010;107:6198-6203. http://dx.doi.org/10.1073/pnas.1001945107 [ Links ]
13. Herman RA, Chassy BM, Parrott W. Compositional assessment of transgenic crops: An idea whose time has passed. Trends Biotechnol. 2009;27(10):555-557. http://dx.doi.org/10.1016/j.tibtech.2009.07.003 [ Links ]
14. Parrott W, Chassy B, Ligon J, Meyer L, Petrick J, Zhou J, et al. Application of food and feed safety assessment principles to evaluate transgenic approaches to gene modulation in crops. Food Chem Toxicol. 2010;48:1773-1790. http://dx.doi.org/10.1016/j.fct.2010.04.017 [ Links ]
15. Durham T, Doucet J, Snyder LU. Risk of regulation or regulation of risk? A de minimus framework for genetically modified crops. Agbioforum. 2011;14(2):61-70. [ Links ]
16. Codex Alimentarius Commission (CAC). Food derived from modern biotechnology. 2nd ed. Rome: FAO/WHO Food Standards Programme [document on the Internet]. c2009 [2015 Feb 20]. Available from: ftp://ftp.fao.org/docrep/fao/011/a1554e/a1554e00.pdf. [ Links ]
17. Kuiper HA, Kleter GA, Noteborn HPJM, Kok EJ. Assessment of the food safety issues related to genetically modified foods. Plant J. 2001;27(6):503-528. http://dx.doi.org/10.1046/j.1365-313X.2001.01119.x [ Links ]
18. Kok EJ, Kuiper HA. Comparative safety assessment for biotech crops. Trends Biotechnol. 2003;21(10):439-444. http://dx.doi.org/10.1016/].tibtech.2003.08.003 [ Links ]
19. Herman RA, Price WD. Unintended compositional changes in genetically modified (GM) crops: 20 Years of research. Agric Food Chem. 2013;61:11695-11701. http://dx.doi.org/10.1021/jf400135r [ Links ]
20. European Food Safety Authority (EFSA). Scientific opinion on the assessment of allergenicity of GM plants and micro-organisms and derived food and feed. EFSA Journal. 2010;8(7):1700-1868. [ Links ]
21. South African Committee on Genetic Experimentation (SAGENE). GovernmentGazette 15420. 1994;48:17-18. [ Links ]
22. South Africa. National Environmental Management Act (Act No. 107 of 1998). Government Gazette 19519. 1998;401, Article 4(a)(vii) [online]. c1998 [cited 2015 Feb 20]. Available from: http://www.gov.za/sites/www.gov.za/files/a107-98.pdf [ Links ]
23. South Africa. National Environmental Management: Biodiversity Act (Act No.10 of 2004). Government Gazette 26436/467. 2004; Chapter 5 Part 3 Section78:62-64 [online]. c2004 [cited 2015 Feb 20]. Available from: https://www.environment.gov.za/sites/default/files/gazetted_notices/nemba_gypaetus_barbatus.pdf [ Links ]
24. South Africa. Genetically Modified Organisms (Act No. 15 of 1997). Government Gazette 18029, 1997;323; as amended (Act No. 23 of 2007), Government Gazette 29803, 2007;502; Regulations: Government Gazette 32966, 2010;120 [online] c2010 [cited 2015 Feb 16]. Available from: http://www.daff.gov.za/daffweb3/Branches/Agricultural-Production-Health-Food-Safety/Genetic-Resources/Biosafety/Legislation/ [ Links ]
25. South Africa. Consumer Protection Act (Act No. 68 of 2008). Government Gazette 32186. 2009;526; Regulations (R293, 1 April 2011), Government Gazette 34180. 2011;550 [online]. c2011 [cited 2015 Feb 20]. Available from: http://www.thedti.gov.za/business_regulation/acts/consumer_protection.PDF [ Links ]
26. Department of Environmental Affairs (DEA). Environmental risk assessment framework for genetically modified organisms: A guidance document. Pretoria: DEA; n.d. [ Links ]
27. Department of Science and Technology (DST). The Bio-economy strategy. Pretoria: DST; 2013. Available from: http://www.pub.ac.za/files/BioeconomyX20Strategy.pdf [ Links ]
28. Hathcock JN. The precautionary principle - An impossible burden of proof for new product. Agbioforum. 2001;3(4):255-258. [ Links ]
29. Cooney R. The precautionary principle in biodiversity conservation and natural resource management: An issues paper for policy-makers, researchers and practitioners. IUCN Policy and Global Change Series Issue 2. Gland: World Conservation Union; 2004. Available from: http://www.sehn.org/pdf/PrecautionaryPrincipleissuespaper.pdf [ Links ]
30. Cartagena Protocol on Biosafety to the Convention on Biological Diversity (CPB): Text and Annexes. Montreal, Canada: Secretariat of the Convention on Biological Diversity; 2000. Available from: http://www.cbd.int/doc/legal/cartagena-protocol-en.pdf [ Links ]
31. World Trade Organization (WTO). Uruguay Round Agreement: Agreement on the application of sanitary and phytosanitary measures. [document on the Internet]. c1995 [cited 2015 Feb 15]. Available from: http://www.wto.org/english/docs_e/legal_e)15sps_01_e.htm [ Links ]
32. United Nations Environmental Programme (UNEP). Rio Declaration on Environment and Development [document on the Internet]. c1992 [cited 2015 Feb 14]. Available from: http://www.unep.org/Documents.Multilingual/Default.asp?documentid=78&articleid=1163 [ Links ]
33. Cranor CF. Learning from the law to address uncertainty in the precautionary principle. Sci Eng Ethics. 2001;7:313-326. http://dx.doi.org/10.1007/s11948-001-0056-0 [ Links ]
34. Levidow L, Carr S, Wield D. European Union regulation of agri-biotechnology: Precautionary links between science, expertise and policy. Sci Public Policy. 2005;32(4):261-276. http://dx.doi.org/10.3152/147154305781779452 [ Links ]
35. Collins English Dictionary [online]. 10th ed. London: HarperCollins Publishers. Normative. [cited 2015 Feb 20]. Available from: http://www.collinsdictionary.com/dictionary/english/normative?showCookiePolicy=true [ Links ]
36. Masters P. Is the Bible always binding for today? [document on the Internet].c1995 [cited 2015 Feb 20]. Available from: http://www.reformedbaptist.co.uk/Normative.htm [ Links ]
37. Ahteensuu M. Rationale for taking precautions: Normative choices and commitments in the implementation of the precautionary principle. In: Risk & Rationalities Conference Proceedings; 2007 March 29-31; Cambridge, UK [online]. c2007 [cited 2015 Feb 20]. Available from: http://www.kent.ac.uk/scarr/events/ahteensuu.pdf [ Links ]
38. Lõfstedt R. The swing of the pendulum in Europe: From precautionary principle to (Regulatory) impact assessment. J Risk Uncertain. 2004;28(3):237-260. http://dx.doi.org/10.1023/B:RISK.0000026097.72268.8d [ Links ]
39. Von Schomberg R. The precautionary principle and its normative challenges. In: Fisher E, Jones J, Von Schomberg R, editors. Implementing the precautionary principle; perspectives and prospects. Cheltenham, UK: Edwin Elgar; 2006. p. 19-42 Available from: http://www.oecd.org/agriculture/agricultural-policies/43891297.pdf [ Links ]
40. Recuerda MA. Dangerous interpretations of the precautionary principle and the foundational values of European Union food law: Risk versus risk. J Food Law Policy. 2008;4(1):1-44. [ Links ]
41. Wingspread. Wingspread consensus statement on the precautionary principle [homepage on the Internet]. c1998 [cited 2015 Feb 16]. Available from: http://sehn.org/wingspread-conference-on-the-precautionary-principle/ [ Links ]
42. United Nations Educational Scientific and Cultural Organization (UNESCO) and World Commission on the Ethics of Scientific Knowledge and Technology (COMEST). The precautionary principle. Paris: UNESCO; 2005. Available from: http://unesdoc.unesco.org/images/0013/001395/139578e.pdf [ Links ]
43. Stirling A. Risk at a turning point? J Risk Res. 1998; 1(2):97-109. [ Links ]
44. South Africa Parliamentary Hearing on the GMO Amendment Bill; 2004 January 17-19. Response from the Department of Agriculture [unpublished]. [ Links ]
45. Martuzzi M, Tickner JA, editors. The precautionary principle: Protecting public health, the environment and the future of our children. Copenhagen: WHO Regional Office for Europe; 2004. Available from: http://www.euro.who.int/__data/assets/pdf_file/0003/91173/E83079.pdf. [ Links ]
46. Commission of the European Communities. Communication from the Commission on the precautionary principle. Brussels: Office for Official Publications of the European Communities; 2000. Available from: http://ec.europa.eu/dgs/health_consumer/library/pub/pub07_en.pdf [ Links ]
47. Klinke A, Renn O. A new approach to risk evaluation and management: Risk-based, precaution-based, and discourse-based strategies. Risk Anal. 2002;22(6):1071-1094. [ Links ]
48. Renn O, Klinke A. Risk evaluation and risk management for institutional and regulatory policy. In: Stirling A, editor. On science and precaution in the management of technological risk. JRC-IPTS Technical Report. EUR19056/ EN/2.2001 [document on the Internet]. c2001 [cited 2015 Feb 20]. Available from: http://ftp.jrc.es/EURdoc/eur19056llen.pdf. [ Links ]
49. Organization for Economic Cooperation and Development (OECD). Emerging systemic risks in the 21st century: An agenda for action [document on the Internet]. c2003 [cited 2015 Feb 15]. Available from: www.oecd.org/dataoecd/20/23/37944611.pdf [ Links ]
50. Wynne B. Creating public alienation: Expert cultures of risk and ethics on GMOs. Sci Cult (Lond). 2001;10(4):445-481. [ Links ]
51. Feintuck M. Precautionary maybe, but what's the principle? The precautionary principle, the regulation of risk, and the public domain. J Law Soc. 2005;32(3):371-398. [ Links ]
52. Charnley G. Annual meeting. Past president's message: Risk analysis under fire. Risk Newsletter. 2000;20(3):3. [ Links ]
53. Berry CL. Relativism, regulation and the dangers of indifferent science: The Sir Roy Cameron Lecture of the Royal College of Pathologists. Toxicology. 2010;267(1):7-13. [ Links ]
54. Bartolomeus A, Parrott W, Bondy G, Walker K. The use of whole food animal studies in the safety assessment of genetically modified crops: Limitations and recommendations. Crit Rev Toxicol. 2013;43(S2):1-24. [ Links ]
55. Millstone E, Brunner E, Mayer S. Beyond substantial equivalence. Nature. 1999;401(6753):525-526. [ Links ]
56. Traavik T, Ching LL. Biosafety first: Holistic approaches to risk and uncertainty in genetic engineering and genetically modified organisms. Trondheim: Tapir Academic Press; 2007. [ Links ]
57. Hilbeck A, Meier M, Rombke J, Jãnsch S, Teichmann H, Tappeser B. Environmental risk assessment of genetically modified plants - Concepts and controversies. Environmental Sciences Europe. 2011;23(1):1-13. Available from: http://www.enveurope.com/content/23/1/13 [ Links ]
58. Vlek C. Judicious management of uncertain risks I: Developments and criticisms of risk analysis and precautionary reasoning. J Risk Res. 2010;13(4):517-543. [ Links ]
59. Castell S, Charlton A, Clemence M, Pettigrew N, Pope S, Quigley A, et al. Public attitudes to science [document on the Internet]. c2014 [cited 2015 Feb 14]. Available from: http://www.ipsos-mori.com/Assets/Docs/Polls/pas-2014-main-report.pdf [ Links ]
60. International Risk Governance Council (IRGC). White paper: Risk governance towards an integrated approach [homepage on the Internet]. c2005 [cited 2015 Feb 14]. Available from: http://irgc.org/wp-content/uploads/2012/04/IRGC_WP_No_1_Risk_Governance_reprinted_version_3.pdf [ Links ]
61. Dreyer M, Renn O. Food safety governance: Integrating science, precaution and public involvement. Berlin: Springer-Verlag; 2009. [ Links ]
62. Majone G. What price safety? The precautionary principle and its policy implications. J Common Mark Stud. 2002;40(1):89-109. [ Links ]
63. Peterson M. The precautionary principle should not be taken as a basis for decision-making: Talking point on the precautionary principle. EMBO Rep. 2007;8(4):305-308. [ Links ]
64. Gray J. Statistics and the precautionary principle. Mar Pollut Bull. 1990;21(4):174-176. [ Links ]
65. Baker T. Liability and insurance after September 11: Embracing risks meets the precautionary principle. Geneva Papers on Risk and Insurance. 2002;27(3):349-357. [ Links ]
66. Marchant GE, Mossman KL. Arbitrary and capricious: The precautionary principle in the European Union courts. Washington DC: American Enterprise Institute; 2005. [ Links ]
67. Hanekamp J, Bast A. Food supplements and European regulation within a precautionary context: A critique and implications for nutritional, toxicological and regulatory consistency. Crit Rev Food Sci Nutr. 2007;47(3):267-285. [ Links ]
68. De Sadeleer N. The precautionary principle as a device for greater environmental protection: Lessons from EC courts. Rev Eur Comm Int Environ Law. 2009;18(1):3-10. [ Links ]
69. Peel J. Interpretation and application of the precautionary principle: Australia's contribution. Rev Eur Comm Int Environ Law. 2009;18(1):11-25. [ Links ]
70. Kogan LA. WTO ruling on biotech foods addresses 'precautionary principle'. Legal Backgrounder. 2006;21(38):1-4. [ Links ]
71. EuropaBio. Highest courts in France and EU confirm Frances ban on biotech crops is illegal [homepage on the Internet]. c2011 [cited 2015 Feb 14]. Available from: www.europabio/agricultural/press [ Links ]
72. South African Department of Agriculture, Forestry and Fisheries (DAFF). Minutes of the GMO Executive Council [document on the Internet]. c2006-2015 [cited 2015 Feb 20]. Available from: http://www.daff.gov.za/daffweb3/Branches/Agricultural-Production-Health-Food-Safety/Genetic-Resources/Biosafety/Information [ Links ]
73. Morris EJ, Van Rensburg JBJ, Hoffmann JH, Lazarus P. Good agricultural practices for developing countries - Lessons learned in South Africa from an appeal under the GMO Act. In: Ninth ICABR International Conference on Agricultural Biotechnology: Ten Years Later; 2005 Jul 6-10; Ravello, Italy. Available from: http://www.economia.uniroma2.it/conferenze/icabr2005/papers/Morris_Jane_paper.pdf [ Links ]
74. Resnik DB. Is the precautionary principle unscientific? Studies in history and philosophy of science. Part C: Stud Hist Phil Biol Biomed Sci. 2003;34:329-344. [ Links ]
75. Salo A. On the role of decision analytic modelling. In: Stirling A, editor. On science and precaution in the management of technological risk. JRC-IPTS Technical Report EUR19056/EN/2.2001 [document on the Internet]. c2001 [cited 2015 Feb 20]. Available from: http://ftp.jrc.es/EURdoc/eur19056llen.pdf. [ Links ]
76. De Marchi B. Public participation and risk governance. Sci Public Policy. 2003;30(3):171-176. [ Links ]
77. Bush RK, Hefle SL. Food allergens. Crit Rev Food Sci Nutr. 1996;36(S1):119-163. [ Links ]
78. Hefle SL, Nordlee JA, Taylor SL. Allergenic foods. Crit Rev Food Sci Nutr. 1996;36(S1):69-89. [ Links ]
79. Sampson H. Food allergy - Accurately identifying clinical reactivity. Allergy. 2005;60(suppl. 79):19-24. [ Links ]
80. Zuberbier T, Edenharter G, Worm M, Ehlers I, Reimann S, Hantke T, et al. Prevalence of adverse reactions to food in Germany - A population study. Allergy. 2004;59(3):338-345. [ Links ]
81. Goodman RE, Vieths S, Sampson HA, Hill D, Ebisawa M, Taylor SL, et al. Allergenicity assessment of genetically modified crops: What makes sense? Nat Biotechnol. 2008;26(1):73-81. [ Links ]
82. Goodman RE, Tetteh AO. Suggested improvements for the allergenicity assessment of genetically modified plants used in foods. Curr Allergy Asthma Rep. 2011;11(4):317-324. [ Links ]
83. Anderson JA. Allergic reactions to foods. Crit Rev Food Sci Nutr. 1996;36(S1):19-38. [ Links ]
84. Sten E, Skov PS, Andersen SB, Torp A, Olesen A, Bindslev-Jensen U, et al. A comparative study of the allergenic potency of wild-type and glyphosate-tolerant gene-modified soybean cultivars. APMIS. 2004;112(1):21-28. [ Links ]
85. Ladics GS, Knippels LM, Penninks AH, Bannon GA, Goodman RE, Herouet-Guicheney C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul Toxicol Pharmacol. 2010;56(2):212-224. [ Links ]
86. Pastorello EA, Farioli L, Pravettoni V Ispano M, Scibola E, Trambaioli C, et al. The maize major allergen, which is responsible for food-induced allergic reactions, is a lipid transfer protein. J Allergy Clin Immunol. 2000;106 (4):744-751. [ Links ]
87. Asero R, Mistrello G, Roncarolo D, De Vries SC, Gautier MF, Ciurana CLF, et al. Lipid transfer protein: A pan-allergen in plant-derived foods that is highly resistant to pepsin digestion. Int Arch Allergy Immunol. 2001;124(1-3):67-69. [ Links ]
88. Pastorello EA, Pompei C, Pravettoni V Farioli L, Calamari AM, Scibilia J, et al. Lipid-transfer protein is the major maize allergen maintaining IgE-binding activity after cooking at 100 degrees C, as demonstrated in anaphylactic patients and patients with positive double-blind, placebo-controlled food challenge results. J Allergy Clin Immunol. 2003;112(4):775-783. [ Links ]
89. Zavala MPV Robledo GBV Olivas MAS, Diaz RJD, Oviedo C. Maize (Zea mays): Allergen or toleragen? Participation of the cereal in allergic disease and positivity incidence in cutaneous tests. Rev Alerg Méx. 2006;53(6):207-211. [ Links ]
90. Goodman RE, Panda R, Ariyarathna H. Evaluation of endogenous allergens for the safety evaluation of genetically engineered food crops: Review of potential risks, test methods, examples and relevance. J Agric Food Chem. 2013;61:8317-8332. [ Links ]
91. Herman RA, Ladics GS. Endogenous allergen upregulation: Transgenic vs. traditionally bred crops. Food Chem Toxicol. 2011;49:2667-2669. [ Links ]
 Correspondence:
Correspondence:
Fredrika Jansen van Rijssen
Paraclinical Sciences, University
of Pretoria, Private Bag X04
Onderstepoort 0110
South Africa
Email: wilnajvr@telkomsa.net
Received: 15 Aug. 2013
Revised: 07 Mar. 2014
Accepted: 08 July 2014














