Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.110 n.9-10 Pretoria Oct. 2014
http://dx.doi.org/10.1590/sajs.2014/20130339
RESEARCH ARTICLE
Trace element composition of two wild vegetables in response to soil-applied micronutrients
Sydney MavengahamaI; Willem P de ClercqII; Milla McLachlanIII
IDepartment of Agriculture, Faculty of Science and Agriculture, University of Zululand, Empangeni, South Africa
IIDepartment of Soil Science, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
IIIDivision of Human Nutrition, Faculty of Medicine and Health Sciences, Stellenbosch University, Tygerberg, Cape Town, South Africa
ABSTRACT
Wild vegetables are an important commodity in the subsistence farming sector. They are considered to be rich in micronutrients and can therefore be used to overcome inadequate nutrition. However, research on micronutrients in wild vegetables remains limited and sporadic. In this study, we evaluated the responses of two wild vegetables - Corchorus olitorius and Amaranthus cruentus var. Arusha - to micronutrients added to the soil in comparison with a reference crop, Swiss chard (Beta vulgaris var. cicla). Swiss chard concentrated significantly (o<0.01) higher amounts of Cu, Zn and Mn in the leaves than did the wild vegetables. Variations in micronutrients among the vegetables were greater for Zn (72-363 mg/kg) and Mn (97.9-285.9 mg/kg) than for Cu (8.8-14 mg/kg). C. olitorius had the least capacity to concentrate Mn and Zn in the leaves. However, C. olitorius concentrated significantly more Fe (327 mg/kg) in the leaves than did A. cruentus (223 mg/kg) or B. vulgaris (295 mg/kg). The mean per cent S concentration in the leaves ranged from 0.26% in C. olitorius to 0.34% in A. cruentus and B. vulgaris. We conclude that the different vegetables had different abilities to concentrate Cu and Zn in the order B. vulgaris > A. cruentus > C. olitorius. These results seem to contradict the belief that wild vegetables have an inherent ability to concentrate mineral micronutrients in their tissues.
Keywords: wild vegetables; soil; fertiliser; micronutrient concentration; fortification.
Introduction
A review of micronutrients in diets in South Africa and other developing countries by Steyn and Herselman1 revealed widespread shortages. They concluded that the poor micronutrient status among the poor is caused by insufficient and undiversified diets and compounded by soils of low micronutrient status. There are three main ways in which micronutrients can be added to foods: (1) food fortification or supplementation, (2) genetic bio-fortification and (3) agronomic biofortification.2 Food fortification is widespread and efficient for those who can afford fortified foods, but is not applicable to most rural populations in Africa, which produce most of their staple crops, vegetables and meat at their farms and therefore do not generally purchase micronutrient fortified foods. Biofortification through conventional breeding shows promise, but research is currently limited to a small range of staple crops.3 Genetic biofortification is still mostly at the experimental stage, and even when it becomes viable, it might be some time before the genotypes that result are widely available and affordable to subsistence farmers. Agronomic biofortification, which is the increase in plant tissue micronutrient content through soil or foliar application of fertilisers, also involves the purchase of these mineral fertilisers and is only viable in commercial agriculture. Another strategy, which is the long-term goal of our present study, is to use locally available wild, semidomesticated and domesticated vegetable species that have the ability to concentrate the mineral micronutrients Zn, Cu and Fe, even from depleted soils. The possibility of exploiting the variability in shoot mineral content among different plant species in combating micronutrient under-nutrition has been discussed in detail by Broadley et al.45
Our targeting of wild vegetables is based on the knowledge that they are widely available and relatively easily accessible to poor subsistence farmers. These wild vegetables (also known in South Africa as African leafy vegetables, indigenous vegetables, morogo and imfino) are variously reported to be superior to conventionally cultivated vegetables such as Swiss chard (Beta vulgaris var. cicla) and cabbage (Brassica oleracea var. capitata)67 in micronutrients, including beta-carotene and minerals such as Zn, Fe and Cu. These vegetables are important to subsistence farmers, especially the poor, some of whom rely on wild vegetables as a major form of relish used to complement and accompany staple meals such as phutu, pap, ugali and sadza.8
A recent (2012) report on the importance of African leafy vegetables reaffirmed the potential of these vegetables in the eradication of micronutrient under-nutrition.7 The report particularly highlighted the prevalence of vitamin A and Fe deficiencies in rural parts of South Africa. Although Zn deficiency was reported as not being well documented in developing countries9, including South Africa7, the World Health Organization has identified Zn as one of the most serious deficiencies in the past decade.10 The consumption of fruits and micronutrient-rich vegetables has been reported to be low, leading to monotonous and micronutrient-poor diets in several provinces of South Africa.7,11 It is against such a background that promotional and research efforts for wild vegetables need to be taken seriously.
Wild vegetables are widely reported to have superior nutritional properties with respect to micronutrients compared to conventional vegetables such as cabbage (Brassica oleracea var. capitata) and Beta vulgaris var. cicla.12-16 There is wide variability in the nutritional composition reported for these vegetables in different studies. The claim that uncultivated indigenous vegetables could have superior micronutrient levels to cultivated conventional vegetables suggests that there is the possibility of increasing their micronutrient content if micronutrients are added to the soil.17 For most conventional crops, research has established critical points for deficiency or excess (toxicity).18 Such information is important in crop production if correct amounts of fertilisers are to be applied. The toxicity risks associated with applying excessive amounts of micronutrients to the soil were extensively reviewed by White et al.18 The levels of nutrients applied in crop production also determine the ability of a food crop to supply nutrients to humans. Deficiencies of these micronutrients in the soil also lead to reduced crop yields, thereby presenting a double problem: reduced amounts of food of poor nutritional quality.17 Some studies that have reported the superior nutrient composition of wild vegetables in South Africa12-16 were not controlled experiments and involved collection from the wild or purchasing from the market and conducting tests. The aim of the present study was to compare, under well-defined conditions, the accumulation of Zn, Cu and Fe in the leaves of two leafy wild vegetable species commonly consumed in the rural areas of South Africa - wild okra (Corchorus olitorius) and pigweed (Amaranthus cruentus var. Arusha) - and a reference vegetable crop that is also widely eaten - Swiss chard (Beta vulgaris var. cicla).
Materials and methods
The experiment was conducted on potted plants in a greenhouse at the University of Zululand (28o51'S; 31o51'E). The growing medium used was soil collected from the university farm. The soil has been classified as Glenrosa soil form.19 Prior to filling the pots, the soil was sieved through a 13-mm diamond mesh wire mounted on a wooden frame, to homogenise it and to remove large stones, clods, sticks and grass.
Treatments
There were two factors in the study - vegetable species and micronutrient fertiliser. Vegetable species were of three types: Corchorus olitorius, Amaranthus cruentus var. Arusha and Beta vulgaris var. cicla. Micronutrient fertiliser had four levels: 0 kg/ha (control), 5 kg/ha, 10 kg/ha and 15 kg/ha each of Cu, Fe and Zn mixed and applied in the form of CuSO4.5H2O, FeSO4.7H2O and ZnSO4.7H2O (Table 1). The combination of the two factors resulted in a 3x4 factorial experiment with 12 treatments arranged in a randomised complete block design with three replications. The micronutrient, basal fertiliser and nitrogen top dressing rates were calculated per plant based on a standardised plant density of 100 000 plants per hectare for the three vegetable species. Because basal fertiliser was not a treatment in this study, a general basal fertiliser of 5 g per plant of NPK 2:3:2 (14) was added pre-plant to all the pots resulting in 0.2 g N, 0.3 g P and 0.2 g K applied per plant. Lime ammonium nitrate (LAN, 28% N) top dressing fertiliser was also applied 10 days after transplanting to all pots at a rate of 3 g per plant resulting in 0.84 g of N applied per plant. Each pot contained 2.4 kg of air-dried soil. The micronutrient treatments were added 15 days after transplanting.
Plant management
Seedlings of each of the three test species were germinated and grown for 38 days in the commercial growth medium Hygromix. (Hygrotech Sustainable Solutions, Pretoria, South Africa) in polystyrene trays. Seedlings of uniform size were selected and transplanted into moist soil in which basal fertiliser had been applied pre-plant as described above. One seedling was planted per pot and there were four pots for each treatment. Thereafter, plants were watered in a way that avoided leaching of the nutrients from the soil. There were no plant mortalities such that at harvesting time each treatment had four plants. Samples of the youngest fully expanded leaves (blade and petiole) were taken 26 days after the application of micronutrients for chemical analysis. Samples were harvested from all the four plants per treatment so as to collect enough samples for analysis. Leaf samples were washed in distilled water soon after harvesting. Excess water was allowed to drain off for 4 h before the samples were placed in new brown paper bags and dried in the oven at 60 0C until they had attained uniform mass.
Chemical analyses of youngest fully expanded leaves
Dried plant samples were milled in a Retsch ZM200. mill (Retsch GmbH, Haan, Germany) to pass through a 0.5-mm sieve. They were then submitted for analysis to the Fertiliser Advisory Services of the South African Sugar Research Institute, Mount Edgecombe, KwaZulu-Natal.
Measurement of leaf nutrients
All the analysed elements were determined according to methods described by Wood et al.20 For Zn, Cu, Fe, Mn and S, a 1-g dried leaf sample was digested in 15 mL nitric acid followed by 5 mL perchloric acid. After digestion, the resultant mixture was filtered and then made up to 50 mL using water. The elements were then determined using atomic absorption spectrophotometry. S was determined colorimetrically. For N, P K, Ca and Mg, a 0.25-g dried leaf sample was digested in 2 mL selenised sulphuric acid for 1.5 h in a Kjeldatherm block digester at a temperature of 370 0C. After digestion, the samples were diluted by adding water to a volume of 1 L and K, Ca and Mg were determined by atomic absorption spectrophotometry; N and P were determined colorimetrically.
Soil analyses
Information on physico-chemical attributes of the soil (Table 2) was obtained prior to fertiliser application. Soil pH was determined in 0.01 M CaCl2. Exchangeable acidity was determined by titration after extraction with 1 M KCl. The macronutrients P K, Ca, Mg and Ca and micronutrients Fe, Cu, Zn and Mn were determined using the method described by Van der Merwe et al.21 after extraction by the Ambic-2 extraction method using EDTA-di-ammonium solution. Truog-extractable P was determined colorimetrically after extraction with 0.02 N sulphuric acid. Si was determined as described by Miles et al.22 after overnight extraction with 0.01 M CaCl2.
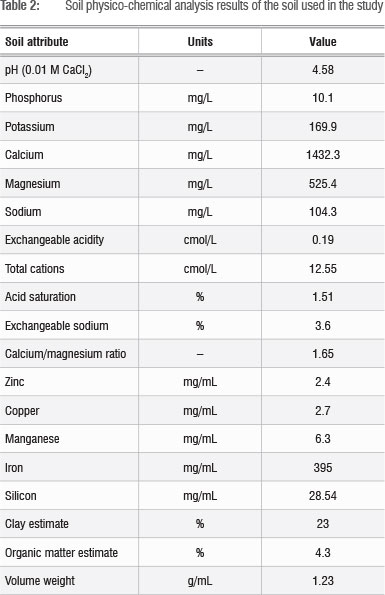
Data analysis
Analysis of variance was performed on the data using the Genstat 12 statistical package to test for significant treatment effects. Statistical significance was evaluated at p<0.05. Where the F-tests were significant, the treatment means were separated using the least significant difference test.23,24
Results and discussion
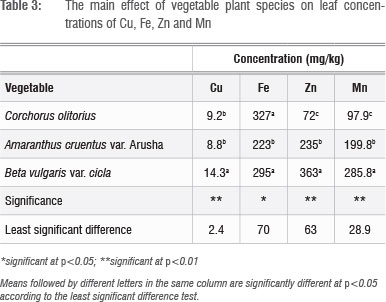
The effects of the micronutrient mixture on leaf nutrient concentration varied according to plant species and application rate. B. vulgaris concentrated Cu, Zn and Mn in the leaves significantly (p<0.01) more than did A. cruentus and C. olitorius (Table 3). The variations among the vegetables in these three micronutrients were greater for Zn (72-363 mg/kg) and Mn (98-286 mg/kg) than they were for Cu (9-14 mg/kg). In a previous study, in which Swiss chard was grown in several different types of soil in South Africa,1 wide variations in micronutrient concentrations (2.72-152.12 mg/kg for Cu and 11.9623.8 mg/kg for Zn) were reported. In that same study, Swiss chard samples from fruit and vegetable markets and shops had Cu levels of 9.4-111.7 mg/kg and Zn levels of 34.0-816.0 mg/kg.1 The large discrepancies between our study and that of Steyn and Herselman's1 in leaf Cu and Zn concentration ranges of Swiss chard are difficult to explain. Similar variations in Zn concentrations in plant tissues used for food were also reported in other studies.25 In the present study, C. olitorius had the least capacity to concentrate Mn and Zn in the leaf, which suggested that this vegetable is a less satisfactory candidate for agronomic biofortification of these micronutrients. However, C. olitorius leaves concentrated significantly more Fe (327 mg/kg) than did A. cruentus (223 mg/kg) or B. vulgaris (295 mg/kg).
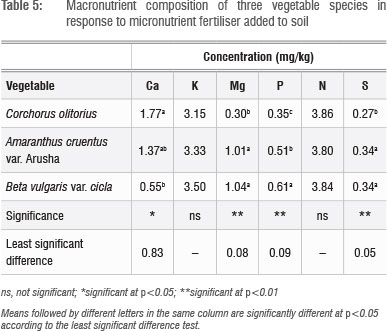
Leaf Cu, Zn and S concentrations increased with increasing application rate, whereas Fe concentrations did not show a defined pattern (Table 4). The application of micronutrient fertilisers did not affect the concentration of macronutrients Ca, K, Mg, P and N (not shown), but there were significant (p<0.05) differences in the concentrations of Ca, Mg, P and S among the plant species tested (Table 5). The general trend of B. vulgaris > A. cruentus > C. olitorius in terms of leaf micronutrient content was also observed for the macroelements Mg and P (Table 5). However, the trend was reversed for Ca concentration, with C. olitorius leaves having three times the Ca content of B. vulgaris. However, a regression analysis of this negative relationship showed that it was not significant. The concentration of shoot Ca and Mg and their variations across different plant families have been investigated in comprehensive studies.45 These studies revealed that the order Caryophyllales, to which Amaranthus and Swiss chard belong, has a tendency towards high shoot Mg levels, resulting in lower Ca:Mg ratios, as observed in B. vulgaris in the present study.
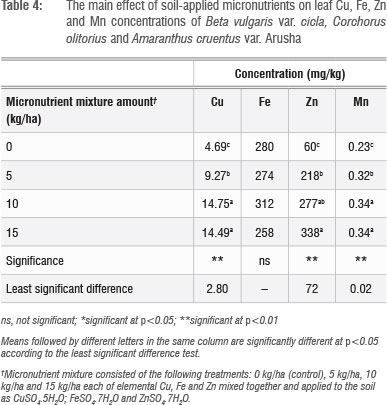
There were significant (p<0.05) interactions (Figure 1 and 2) among plant species and fertiliser rate in terms of leaf Zn and Cu. In all three vegetables, the leaf Cu concentration increased with Cu addition up to 10 kg/ha but declined at 15 kg/ha (Figure 1). The decline in leaf Cu concentration at 15 kg/ha Cu was more marked in C. olitorius than in Swiss chard and A. cruentus. The reason for the decline in Cu concentration in C. olitorius at high application rates in the current study is not known but could be a result of toxicity or simply the inherent inability of C. olitorius as a species to accumulate the microelement, as alluded to by several researchers.4,5,18,25-26 Swiss chard concentrated more Zn and Cu at all fertiliser application rates (Figures 1 and 2), which contradicted our hypothesis that wild vegetables have a greater inherent ability to accumulate micronutrients from the soil than the widely cultivated exotic vegetables. Nonetheless, the wild vegetables also responded positively to the incremental application of the two micronutrients, which supported our postulate that the addition of micronutrients would result in increased micronutrient concentrations in wild vegetables leaves. Thus, when the vegetables were grown in soil with augmented micronutrient content, they concentrated increased quantities from the soil.
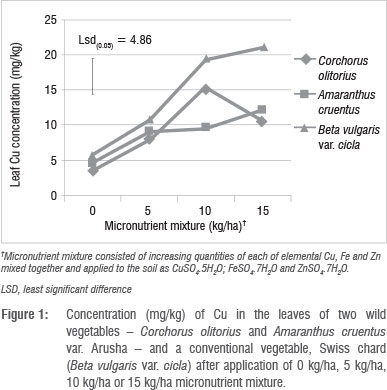
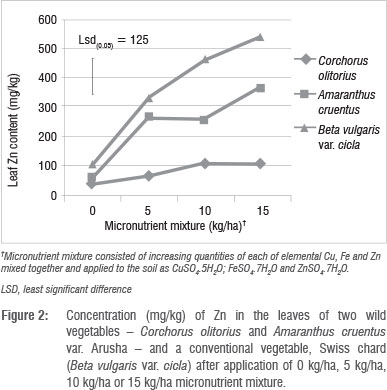
The concentrations of micronutrients tested in this study could be considered high, or even toxic to crops. We did not evaluate toxicity in this study, but observed no visible toxicity symptoms. The concentrations of micronutrients obtained from the leaf analysis results after applying micronutrient fertiliser were much higher than those reported for wild vegetables collected from the wild.12-16 However, not all micronutrients present in crops are available for uptake by humans. Some are complexed in unavailable forms by various biomolecules.2,25 It is therefore necessary to investigate if the increase in leaf micronutrient level has a positive effect on bioavailability of the micronutrients in wild vegetables. Micronutrients are generally applied in smaller quantities than macronutrients,27 but mostly in commercial agriculture. There is a danger of accumulation of micronutrients to hazardous levels in soils associated with high application rates of micronutrient fertilisers such that some countries have statutory maximum limits of micronutrients to be applied to soils to prevent accumulation to toxic levels.18 The nutrient content of most soils in the subsistence farming sector is generally not well known, yet there is a belief among agriculturalists that micronutrients occurring naturally in the soil are adequate for crop production. In contrast, in commercial agriculture, in which the nutrient status of soils is well known, farmers periodically apply micronutrients, either as foliar sprays or chelates to soils.28
Conclusions
The different vegetable species investigated demonstrated different abilities to take up Cu and Zn, in the order Swiss chard > A. cruentus > C. olitorius, and they responded to soil-applied micronutrients by taking up more from the soil, as more was supplied, but up to a certain point. The trend B. vulgaris > A. cruentus > C. olitorius was observed for the macroelements Mg and P but it was reversed for Ca concentration, with C. olitorius leaves containing three times the Ca content of B. vulgaris. Our results contradict the current claim that wild vegetables have superior micronutrient content to exotic vegetable species.
Acknowledgements
We thank the Research Committee of the University of Zululand for financial support and the Agricultural Research Council - Vegetable and Ornamental Plant Institute for supplying the Corchorus olitorius and Amaranthus cruentus seeds.
Authors' contributions
All authors contributed to the research and write-up. S.M. was responsible for the design and conduct of the experiments, and the data collection and analysis. W.Pd.C. and M.M. were responsible for overall supervision of the project.
References
1. Steyn CE, Herselman J. Trace elements in developing countries using South Africa as a case study. Comm Soil Sci Plant Anal. 2005;36:155-168. http://dx.doi.org/10.1081/CSS-200043017 [ Links ]
2. White PJ, Broadley MR. Biofortification of crops with seven mineral elements often lacking in human diets - iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49-84. http://dx.doi.org/10.1111/j.1469-8137.2008.02738.x [ Links ]
3. Nestlel M, Bouis HE, Meenakshi JV Pfeiffer WH. Biofortification of staple food crops. J Nutr. 2006;136:1064-1067. [ Links ]
4. Broadley MR, Bowen HC, Cotterill HC, Hammond JP Meacham MC, Mead A, et al. Phylogenetic variation in the shoot mineral concentration of angiosperms. J Exp Bot. 2004;55:321-336. http://dx.doi.org/10.1093/jxb/erh002 [ Links ]
5. Broadley MR, Hammond JP King GJ, Astley D, Bowen HC, Meacham MC, et al. Shoot calcium and magnesium concentrations differ between subtaxa, are highly heritable, and associate with potentially pleiotropic loci in Brassica oleracea. Plant Physiol. 2008;146:1707-1720. http://dx.doi.org/10.1104/pp.107.114645 [ Links ]
6. Flyman MV Afolayan AJ. The suitability of wild vegetables for alleviating human dietary deficiencies. S Afr J Bot. 2006;72:492-497. http://dx.doi.org/10.1016/j.sajb.2006.02.003 [ Links ]
7. Oelofse A, Van Averbeke W. Nutritional value and water use of African leafy vegetables for improved livelihoods. Report no. TT535/12. Pretoria: Water Research Commission; 2012. p. 332. [ Links ]
8. Mavengahama S, McLachlan M, De Clercq W. The role of wild vegetable species in household food security in maize based subsistence cropping systems. Food Security. 2013;5:227-333. http://dx.doi.org/10.1007/s12571-013-0243-2 [ Links ]
9. Rosado JL. Zinc and copper: Proposed fortification levels and recommended zinc compounds. J Nutr. 2003;133(9):2985-2989. [ Links ]
10. Food and Agriculture Organization of the United Nations (FAO). The state of food insecurity in the world. Economic crises - impacts and lessons learned. Rome: FAO; 2009. p. 61. Available from: http://www.fao.org/docrep/012/i0876e/i0876e00.htm [ Links ]
11. Labadarios D, Steyn NP Nel J. How diverse is the diet of adult South Africans? Nutr J. 2011;10:1-11. http://dx.doi.org/10.1186/1475-2891-10-33 [ Links ]
12. Ndlovu J, Afolayan AJ. Nutritional analysis of the South African wild vegetable Corchorus olitorius L. Asian J Plant Sci. 2008;7(6):615-618. http://dx.doi.org/10.3923/ajps.2008.615.618 [ Links ]
13. Nesamvuni C, Steyn NP Potgieter MJ. Nutritional value of wild, leafy vegetables consumed by the Vhavhenda. S Afr J Sci. 2001;97:51-54. [ Links ]
14. Odhav B, Beekrum S, Akula U, Baijnath H. Preliminary assessment of nutritional value of traditional leafy vegetables in KwaZulu-Natal, South Africa. J Food Comp Anal. 2007;20:430-435. http://dx.doi.org/10.1016/j.jfca.2006.04.015 [ Links ]
15. Steyn NP Olivier J, Winter P Burger S, Nesamvuni C. A survey of wild, green leafy vegetables and their potential in combating micronutrient deficiencies in rural populations. S Afr J Sci. 2001;97:276-278. [ Links ]
16. Van der Walt AM, Loots DT, Ibrahim MIM, Bezuidenhout CC. Minerals, trace elements and antioxidant phytochemicals in wild African dark-green leafy vegetables (morogo). S Afr J Sci. 2009;105:444-448. [ Links ]
17. Nube M, Voortman RL. Simultaneously addressing micronutrient deficiencies in soils, crops, animal and human nutrition: Opportunities for high yields and better health. Staff working paper WP-06-02. Amsterdam: Centre for World Food Studies; 2006. [ Links ]
18. White PJ, Broadley MR, Gregory PJ. Managing the nutrition of plants and people. Appl Environ Soil Sci. 2012;2012, Article ID 104826, 13 pages. [ Links ]
19. Mulder GJ. Subsurface flow response to rainfall on a hillslope plot within the Zululand coastal zone. In: Dardis GF, Moon BP, editors. Geomorphological studies in southern Africa. Brookfield/Rotterdam: AA Balkema; 1988. p. 445-456. [ Links ]
20. Wood RA, Meyer JH, Govender M. A rapid system of cane leaf analysis using X-ray spectrometry and infra-red reflectance. Proceedings of the South African Sugar Technologists Association. 1985;June:195-201. [ Links ]
21. Van der Merwe AJ, Johnson JC, Ras LSK. An NH4HCO3-NH4F-(NH4)2-EDTA method for the determination of extractable P K, Ca, Mg, Cu, Fe, Mn and Zn in soils. Pretoria: Soil and Irrigation Research Institute; 1984. [ Links ]
22. Miles N, Van Antwerpen R, Van Heerden PDR, Rhodes R, Weigel A, McFarlane SA. Extractable silicon in soils of the sugar industry and relationships with crop uptake. Proceedings of the South African Sugar Technology Association. 2011;84:189-192. [ Links ]
23. Gomez KA, Gomez AA. Statistical procedures for agricultural research. 2nd edition. New York: John Wiley and Sons; 1984. [ Links ]
24. Steel RG, Torrie JH, Dickey DA. Principles and procedures of statistics: A biometrical approach. New York: McGraw-Hill Book Co.; 1997. [ Links ]
25. White PJ, Broadley MR. Physiological limits to zinc biofortification of edible crops. Frontiers Plant Sci. 2011;2, 11 pages. [ Links ]
26. Broadley MR, White PJ, Hammond JP Zecko I, Lux A. Zinc in plants. New Phytol. 2007;173:677-702. http://dx.doi.org/10.1111/j.1469-8137.2007.01996.x [ Links ]
27. Fertilizer Society of South Africa. Fertilizer handbook. Pretoria: Fertilizer Society of South Africa; 2007. [ Links ]
28. Van der Waals JH, Laker MC. Micronutrient deficiencies in crops in Africa with emphasis on southern Africa. In: Alloway BJ, editor. Micronutrient deficiencies in global crop production. Dordrecht: Springer Science+Business Media B.V.: 2008. p. 201-224. [ Links ]
 Correspondence:
Correspondence:
Sydney Mavengahama
Department of Agriculture, Faculty of Science and Agriculture
University of Zululand
Private Bag X1001, KwaDlangezwa 3886, South Africa
EMAIL: sydmavengahama@yahoo.co.uk
Received: 31 Oct. 2013
Revised: 17 Jan. 2014
Accepted: 02 Mar. 2014














