Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.110 no.3-4 Pretoria feb. 2014
RESEARCH ARTICLE
High biomass yielding winter cover crops can improve phosphorus availability in soil
Ernest DubeI; Cornelius ChiduzaII; Pardon MuchaonyerwaIII
IAgricultural Research Council - Small Grain Institute Production Systems, Bethlehem, South Africa
IIDepartment of Agronomy, University of Fort Hare, Alice, South Africa
IIISoil Science, School of Agricultural, Earth and Environmental Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
We investigated the effects of high biomass yielding winter cover crops, namely grazing vetch [Vicia dasycarpa L.) and oats [Avena sativa L.), on soil phosphorus (P) availability in low fertiliser input maize-based conservation agriculture systems. Soil samples were collected from the 0-50-mm depth of experimental plots after 4 years of maize-winter cover crop rotations. A sequential fractionation scheme was used to separate total soil P into labile, moderately labile and non-labile organic P (Po) and inorganic (Pi) pools. Labile P pools included microbial biomass-P as well as Pi and Po pools extracted using 0.5 M NaHCO3 and 1.0 M HCl. The non-labile P pools were humic-P and 1.0 M H2SO4 extracted P Soil on the maize-winter cover crop rotations had higher HCl-Pi and total P than the soil on the maize-fallow rotation. The cover crops had no significant (p>0.05) effect on NaHCO3-Po, NaHCO3-Pi, HCl-Po, fulvic acid-P and recalcitrant H2SO4-P fractions. Non-application of fertiliser increased accumulation of humic-P on the maize-oats rotation. Cover crop biomass input explained 73% of the variations in microbial biomass-P and 33% of variations in total labile P. Phosphorus concentration of young maize plants was significantly increased by the cover crops, with a positive correlation to HCl-Pi (rs=0.90). This contribution from winter cover crops to P availability in the surface soil suggests that, in the long term, fertiliser P could be reduced in such systems.
Keywords: conservation agriculture; cover crop biomass; no-till; phosphorus fertiliser; soil organic matter
Introduction
Phosphorus (P) is an essential macronutrient required in almost every aspect of plant functions. It is a vital component of compounds required to build proteins, plant structure, seed yield and genetic transfer. Symptoms of P deficiency in maize include stunted growth, delayed maturity, purplish hues, poor root development and reduced yield potential. Phosphorus is the second most important fertiliser applied to soils for improving soil fertility after nitrogen (N) in maize production. Cultivated soils in South Africa are deficient in P and fertiliser compounds have to be supplied to soils in large quantity to meet maize P requirements. Meanwhile, it is reported that the world's P stocks are dwindling and P production will not be able to meet half the world's needs by the year 2050.1 Global P fertiliser prices are on the increase1,2 and eating into the profits of farmers. It is becoming increasingly imperative for maize farmers to adopt low-cost farming strategies that increase P availability in the soil, conserve soil P and optimise its use.
Conservation agriculture (CA) is an important farming practice for conserving soil resources, including P through retention of crop residues and minimising soil erosion.3 In CA, the practices of crop rotation, no till and permanent cover through crop residues and cover crops contribute immensely to soil quality and nutrient dynamics. A number of winter cover crops, which are legumes or small grains, can be grown between regular grain crop production periods for the purpose of protecting and improving the soil through their high biomass production.4 Through their extensive root systems, leguminous winter cover crops can explore subsoil nutrient pools, whereas grass species can increase P uptake by both the cover crop and the succeeding crop through enhancement of viable mycelia of mycorrhizal fungi in soils.5,6 Almost no P is added to the soil by winter cover crops; they only take up P from the soil solution and return it to the same soil. However, high biomass yielding winter cover crops can increase surface organic matter significantly.7
In warm temperate regions such as the Eastern Cape Province of South Africa, planting a winter cover crop before the summer maize crop is possible as an entry point into CA, provided irrigation water is available.7,8 Grazing vetch (Vicia dasycarpa) and oats (Avena sativa) are examples of fast-growing, winter hardy cover crops which can provide high biomass (> 6 t/ha) in this system.8 The maize is planted immediately after cover crop termination onto winter cover crop residue mulches. Decomposition of the winter cover crop residues and subsequent mineralisation of organic P (Po) plays an essential role in P-cycling and maintenance of plant-available P in this system.9 The major source of P in unfertilised low P soils is Po.9,10 When no winter cover crops are used, biomass production tends to be lower, reducing the size of the Po pool. However, where high amounts of organic matter are generated in situ from winter cover crops, P may be temporarily immobilised in the organic matter accumulated on the soil surface, especially if the C:P ratio is greater than 300:1.11 There is therefore a need to investigate the effects of winter cover crops on maize P nutrition, especially during the early crop growth stages. Adequate P nutrition at the seedling stage in maize is critical because deficiency at this stage cannot be remedied by side-dressing as a result of a lack of P mobility in soils.
The size of the Po pool that undergoes rapid mineralisation contributing to plant available P over at least one growing season, known as labile P, may be dependent on crop residue quality, soil and environmental characteristics, and the duration and type of the cropping system.12,13 Bicarbonate extractable P (NaHCO3-P) and microbial biomass-P fractions constitute labile P in the soil.10,14 The soil microbial biomass may be considered as a reservoir of potentially plant-available nutrients, including P14 It is important to understand the effects of winter cover crops on these P pools in low fertiliser input CA systems for the development of effective fertiliser management strategies that maximise maize yield and profit. In this paper, we report the effects of winter cover crops on soil P pools and maize P nutrition during early growth in a low fertiliser input CA system.
Materials and methods
A long-term field experiment was established in 2007 on a research farm to study the effects of winter cover crops and fertiliser on biomass input, soil organic matter and maize yield under no-till and irrigation conditions.8 The farm is located at 32°46' S 26°50' E and at 535 m above sea level in the Eastern Cape Province of South Africa. The soil type and climate of this research site has been described previously.8 The field trial was a split plot design and the winter cover crops oats (cv. Sederberg) and grazing vetch (cv. Max) were planted in the main plots. Control plots with no winter cover crops (fallow) were also included. Subsequent to winter cover crop termination, plots were split and summer maize was planted at two fertiliser levels (with and without fertiliser). Fertiliser was never applied to the cover crops or the fallow plots. There were thus two factors in this experiment - type of cover crop mulch and fertiliser application - resulting in a 3x2 split plot design which was replicated three times. The six treatment combinations and the amount of P fertiliser applied are presented in Table 1.

Detailed agronomic management of the field trial has been described previously.8 Twelve random soil samples were collected from the organic matter rich depths (0-50 mm) of the plots at the beginning of the fourth year using a precision auger (70 mm diameter) after cover crop termination and before maize planting. Soil samples were collected from the inner two thirds of each plot and the soil from each plot was bulked to form one sample. The samples were air dried and sieved (<2 mm) to remove coarse fragments and roots. At 6 weeks after maize planting, six maize plants per plot (two from each of rows 2, 7 and 8) were sampled by cutting near the soil surface. The plants were oven dried to a constant weight at 65 °C and dry matter was determined before the sample was ground (<2 mm).
Soil P was separated into labile, moderately labile and non-labile organic and inorganic pools following the sequential fractionation scheme.12,15 In this method, the labile P pool was extracted using 0.5 M NaHCO3 at pH 8.5, while the microbial biomass-P was determined through a chloroform (CHCl3) fumigation-extraction technique.8 The moderately labile pool was extracted with 1.0 M HCl, followed by 0.5 M NaOH. The NaOH extract was acidified with concentrated HCl to separate the non-labile humic acid P fraction from the moderately labile fulvic acid P fraction. Residue from the NaOH was ashed at 550 °C for 1 h and dissolved in 1.0 M H2SO4 to determine the highly resistant, non-labile P fraction. Total P in all extracts was measured after persulphate digestion.16 Organic P in the extracts was calculated as the difference between total P and inorganic P (Pi). Plant tissue P concentration of maize plants was extracted using a wet digestion procedure with H2SO4 and H2O2.17 Phosphorus concentrations in all extracts were analysed by continuous flow analysis using the molybdenum blue colourimetric method on a Skalar San Plus System (Breda, the Netherlands).18 All data were subjected to an analysis of variance as a split plot design to test the effects of the winter cover crops and the fertiliser using GenStat Release 12.1 statistics software. Separation of means was done by using the least significant difference at a 5% level of significance. Biomass inputs from the winter cover crops in this field trial have been reported previously.8 A Spearman's rank correlation was used in the current study to analyse the biomass data.
Results and discussion
Winter cover crop type x fertiliser interaction effect on total soil P was not significant (p>0.05). Cover crop type effects were significant (p<0.05), that is, total P in soils was higher on maize-oats (619±32 mg/kg) and maize-grazing vetch (634±41 mg/kg) rotations than in soils on maize-fallow rotation (524±27 mg/kg). This finding implies that winter cover crop residues can be a source of P in the low P fertiliser input no-till systems. This P is mined from larger soil volumes through their extensive root systems. When the cover crops are terminated, the crop residue P contributes to the total P levels in the surface soils of no-till systems. This soil P however, occurs in different pools which vary in the level of P availability to plants. Fertiliser effects on total soil P were not significant (p>0.05).
Winter cover crop type x fertiliser interaction effects on all the labile and moderately labile soil P pools were not significant (p>0.05) (Table 2). However, winter cover crop type had a significant (p<0.05) effect on microbial-P and HCl-Pi, but not on NaHCO3-Po, NaHCO3-Pi, HCl-Po and fulvic acid-P (Table 2). The soil on maize-oats rotation had higher microbial-P than the soil on either the maize-grazing vetch or maize-fallow rotation (Figure 1). Oats residues tend to have a slower decomposition rate than vetch residues.7 In this case, most mineral soil P in the soil under oats residues may be converted into microbial biomass.

Bicarbonate is thought to solubilise P that is adsorbed on surfaces of crystalline P-compounds, carbonates and oxides of Fe and Al.19 The lack of significant differences in bicarbonate-extractable P fractions (NaHCO3-Po, NaHCO3-Pi) among the cover crop treatments could be caused by low contents of crystalline minerals like carbonates and oxides of Fe and Al20 in the soil used for the study, which was a Haplic cambisol. NaHCO3-P also includes soluble P, which is often detected using ion-exchange resins.19
Soil on the maize-oats and maize-grazing vetch rotations had higher HCl-Pi than that on the maize-fallow rotation (Figure 1). This high level of the inorganic pool of HCl-P fraction in the cover crop treatments suggests that when the large biomass decomposes a greater proportion of the mineral P produced forms calcium phosphates,20 and a portion is taken up by soil microbial biomass. Dissolution of these calcium phosphates makes moderately labile P (HCl-P) available to crops.21
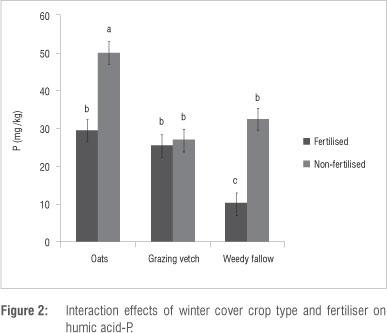
Fertiliser had no significant (p >0.05) effect on all the soil P pools except for NaHCO3-Po (Table 2) for which the fertilised maize rotations had a higher amount (6.78 mg/kg) than the non-fertilised ones (4.33 mg/kg). Winter cover crop x fertiliser interaction had a significant (p<0.05) effect on humic-P, but not on H2SO4-Po. Soil in the non-fertilised maize-oats rotation had the highest amount of humic acid-P while the fertilised maize-fallow had the lowest amount (Figure 2). Whereas humic-P in the maize-vetch rotation was not increased by fertiliser application, humic-P in the maize-oats and maize-fallow rotations was higher when unfertilised. Although humic-P and H2SO4-P are considered non-labile, their effects as a slow supply of plant available P over the long term could be significant. The high humic-P in the non-fertilised maize-oats rotation suggests an accumulation of humic acid fraction of organic matter which could be effectively locking up P over the short term. Decomposition of the humic fraction of soil organic matter proceeds at a slower rate than the material from which it was formed. A possible explanation for high humic-P under non-fertilised maize-oats rotation is the fact that N fertiliser application accelerates decomposition of the humic acid fraction to form HCl-Pi and/or microbial biomass-P. Application of fertiliser generally increases the soil organic matter mineralisation rate and reduces humic acid content.22
Winter cover crop x fertiliser interaction effect on maize plant P concentration was not significant (p>0.05). However, winter cover crop type effects were significant and the maize planted after fallow had a lower tissue P concentration (1138±72 mg/kg) than that planted after either oats (1650±88 mg/kg) or grazing vetch (1759±103 mg/kg). It is possible that cover crop residues release organic acids that compete for P sorption sites with orthophosphate, thereby resulting in increased amounts of P in the soil solution for crop uptake. Soluble plant litter P can also be released to the soil from cover crop residues as an initial flush of Pi with rainfall or irrigation for utilisation by the maize crop during early stages of growth.23 Decomposition of the cover crop residues can further increase P availability by releasing CO2, which forms H2CO3 in the soil solution, resulting in the dissolution of primary P-containing minerals.24 Fertiliser effects on maize plant tissue P concentration were not significant (p>0.05).
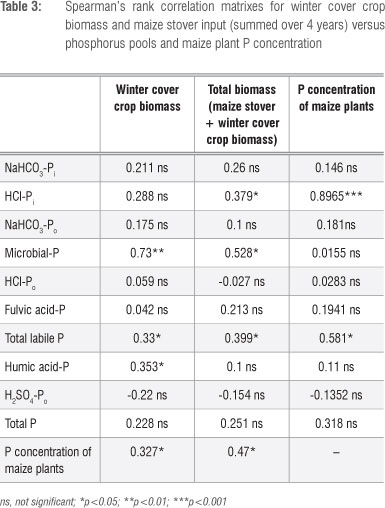
A Spearman's rank correlation analysis of the P data showed a strong positive relationship between HCl-Pi and the maize P concentration (rs=0.90) (Table 3). This result suggests that maize was dependent on these labile Pi pools for P nutrition during early growth. Correlations of maize tissue P concentration with microbial-P, HCl-Po, NaHCO3-Po, NaHCO3-Pi and fulvic acid-P were not significant (Table 3). Spearman's rank correlation coefficients between winter cover crop biomass input (summed over 4 years) and soil P pools are presented in Table 3. Total biomass accumulation (maize stover + winter cover crop biomass) significantly correlated with HCl-Pi, microbial P, total labile P and maize plant tissue P (Table 3). Winter cover crop biomass input was correlated with humic-P, whereas total biomass input was correlated with HCl-Pi (Table 3). This finding suggests that at least some component of the humic-P was coming from the cover crops. Winter cover crop biomass alone explained 73% of the variations in microbial P and 33% of total labile P (Table 3).
Under no-till, P tends to be stratified, which may partly explain these high values of NaHCO3-Pi (>40 mg/kg, Figure 1) in the surface soil. It is noted that elevated levels of plant available Pi in the soil can induce Zn and Cu deficiency.25 Further studies may therefore be needed to evaluate the effects of the winter cover crops on Zn and Cu availability.
Conclusions
The maize-winter cover rotations increased total P and some labile P pools in the surface soil when compared with the maize-fallow rotation, and this effect was positively correlated to cover crop biomass. The HCl-Pi pool was strongly correlated to maize seedling tissue P concentration and thus P supply for early maize growth. Non-application of fertiliser to maize, however, increased the accumulation of the recalcitrant humic-P fraction on the maize-oats rotation. Overall, the contribution from the winter cover crops to P availability in the surface soil suggests that, in the long term, fertiliser P could be reduced in low fertiliser input CA systems. Further work is recommended to evaluate the effects of winter cover crops on other soil nutrient pools such as Zn and Cu.
References
1. Cordell D, Drangert J, White S. The story of phosphorus, global food security and food for thought. Global Environ Chang. 2009;19:292-305. http://dx.doi.org/10.1016/j.gloenvcha.2008.10.009
2. Ward J. Peak phosphorus, quoted reserves vs. production history. Energy Bulletin [serial on the Internet]. 2008 Aug 26 [cited 2014 Feb 17]. Available from: http://www.resilience.org/stories/2008-08-26/peak-phosphorus-quoted-reserves-vs-production-history [ Links ]
3. Franzluebbers AJ. Introduction to special section - Supporting ecosystem services with conservation agricultural approaches. Renew Agr Food Syst. 2013;28:99-101. http://dx.doi.org/10.1017/S1742170513000021
4. Mannering Jy Griffith DR, Johnson KD. Winter cover crops - Their value and management. West Lafayette, IN: Department of Agronomy, Purdue University Cooperative Extension Service; 2007. Available from: http://www.agry.purdue.edu/ext/forages/rotational/articles/PDFs-pubs/winter-cover-crops.pdf [ Links ]
5. Gathumbi SM, Cadisch G, Buresh RJ, Giller KE. Subsoil nitrogen capture in mixed legume stands as assessed by deep nitrogen -15 placement. Soil Sci Soc Am. 2003;67:573-582. http://dx.doi.org/10.2136/sssaj2003.5730
6. Karasawa T, Kasahara Y Takebe M. Differences in growth responses of maize to preceding cropping caused by fluctuation in the population of indigenous arbuscular mycorrhizal fungi. Soil Biol Biochem. 2002;34:851-857. http://dx.doi.org/10.1016/S0038-0717(02)00017-2
7. Dube E, Chiduza C, Muchaonyerwa P. Conservation agriculture effects on soil organic matter on a Haplic Cambisol after four years of maize-oat and maize-grazing vetch rotations in South Africa. Soil Till Res. 2012; 123:21-28. http://dx.doi.org/10.1016/j.still.2012.02.008
8. Murungu FS, Chiduza C, Muchaonyerwa P, Mnkeni PNS. Mulch effects on soil moisture and nitrogen, weed growth and irrigated maize productivity in a warm-temperate climate of South Africa. Soil Till Res. 2011;112:58-65. http://dx.doi.org/10.1016/j.still.2010.11.005
9. Maroko JB, Buresh RJ, Smithson PC. Soil phosphorus fractions in unfertilized fallow-maize systems on two tropical soils. Soil Sci Soc Am. 1999;63:320-326. http://dx.doi.org/10.2136/sssaj1999.03615995006300020009x
10. Hedley MJ, Stewart JWB, Chauhan BS. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci Soc Am. 1982;46:970-976.
11. Tate KR. Soil phosphorus. In: Vaughan D, Malcolm RE, editors. Soil organic matter and biological activity, developments in plant and soil sciences. Vol 16. Dordrecht: Martinus Nijhoff/Dr W. Junk Publishers; 1985. p. 329-377. http://dx.doi.org/10.1007/978-94-009-5105-1_10
12. Bowman RA, Cole CV. An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978;125:95-101. http://dx.doi.org/10.1097/00010694-197802000-00006 [ Links ]
13. Lupwayi NZ, Clayton GW, O'Donovan JT, Harker KN, Turkington TK, Soon YK. Phosphorus release during decomposition of crop residues under conventional and zero tillage. Soil Till Res. 2007;95:231-239. http://dx.doi.org/10.1016/j.still.2007.01.007
14. Brookes PC, Powlson DS, Jenkinson DS. Phosphorus in the soil microbial biomass. Soil Biol Biochem. 1984;16:169-175. http://dx.doi.org/10.1016/0038-0717(84)90108-1
15. Kovar JL, Pierzynski GM. Methods of phosphorus analysis for soils, sediments, residuals, and waters - Revised edition. Southern Cooperative Series Bulletin No 408. Blacksburg, VA: Virginia Tech University; 2009. Available from: http://www.sera17.ext.vt.edu/Documents/P_Methods2ndEdition2009.pdf [ Links ]
16. Thien SJ, Myers R. Determination of bioavailable phosphorus in soil. Soil Sci Soc Am. 1992;56:814-818. http://dx.doi.org/10.2136/sssaj1992.03615995005600030023x
17. Okalebo JR, Gathua KW, Woomer PL. Laboratory methods of soil and plant analysis, a working manual. Nairobi: TSBF-KARI, SSEA, SACRED Africa; 2002. [ Links ]
18. Murphy J, Riley JP. A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. 1962;27:31-36. http://dx.doi.org/10.1016/S0003-2670(00)88444-5
19. Tiessen H, Moir JO. Characterization of available P by sequential extraction. In: Carter MR, editor. Soil sampling and methods of analysis. Boca Raton: CRC Press; 1993. [ Links ]
20. Pavinato PS, Merlin A, Rosolem CA. Phosphorus fractions in Brazilian Cerrado soils as affected by tillage. Soil Till Res. 2009;105:149-155. http://dx.doi.org/10.1016/j.still.2009.07.001
21. Agbenin JO, Tiessen H. Phosphorus forms in particle size fractions of a toposequence from Northeast Brazil. Soil Sci Soc Am. 1995;59:1687-1693. http://dx.doi.org/10.2136/sssaj1995.03615995005900060026x
22. Arlauskienè A, Maiksténiené S, Slepetienè A. Effect of cover crops and straw on the humic substances in the clay loam Cambisol. Agron Res. 2010;8:397-402.
23. Noack SR, McLaughlin MJ, Smernik RJ, McBeath TM, Armstrong RD. Crop residue phosphorus: Speciation and potential bio-availability. Plant Soil. 2012;359(1-2):375-385. http://dx.doi.org/10.1007/s11104-012-1216-5
24. Sharpley AN, Smith SJ. Fractionation of inorganic and organic phosphorus in virgin and cultivated soils. Soil Sci Soc Am. 1985;49:127-130. http://dx.doi.org/10.2136/sssaj1985.03615995004900010025x
25. Landon JR. Booker tropical soil manual: A handbook for soil survey and agricultural land evaluation in the tropics and subtropics. New York: Booker Agriculture International Ltd., Longman; 1984. [ Links ]
 Correspondence:
Correspondence:
Ernest Dube
Agricultural Research Council,
Small Grain Institute Production Systems, Lindley Road, Bethlehem 9700, South Africa
dubee@arc.agric.za
Received: 06 May 2013
Revised: 26 Oct. 2013
Accepted: 29 Oct. 2013














