Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.109 n.11-12 Pretoria Jan. 2013
REVIEW ARTICLES
A review of Kudoa-induced myoliquefaction of marine fish species in South Africa and other countries
Suné S. HenningI, II; Louwrens C. HoffmanIII; Marena ManleyI
IDepartment of Food Science, Stellenbosch University, Stellenbosch, South Africa
IIDepartment of Food Technology, Cape Peninsula University of Technology, Cape Town, South Africa department of Animal Sciences, Stellenbosch University, Stellenbosch, South Africa
IIIDepartment of Animal Sciences, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
Myoliquefaction of fish musculature results in customer quality complaints and in huge economic losses, especially with regard to Pacific hake (Merluccius productus), farm-reared Atlantic salmon (Salmo salar), South African pilchards (Sardinops ocellatus) and Cape snoek (Thyrsites atun). Myoliquefaction, or 'jelly flesh', is caused by proteolytic enzymes released by the marine myxosporean parasite, Kudoa thyrsites, after the death of the fish. Currently there are no fast methods of detection for this microscopic parasite, and because myoliquefaction is evident only after 38-56 h post-mortem, infected fish inevitably reach the processor and/or consumer. Several methods of detection have been investigated, but most of these methods are time-consuming and/or result in destruction of the fish, and are thus impractical for fishing vessels and fish processors. Limited research is available on possible means of destroying or inhibiting the post-mortem activity of the parasitic proteolytic enzyme. Means such as manipulating post-mortem pH and temperature control have been suggested; leaving opportunities for research into food technology applications such as cold-chain management and ionising radiation.
Keywords: myoliquefaction; marine fish species; myxosporean parasite; Kudoa infection; proteolytic enzyme; detection methods
Introduction
Post-mortem myoliquefaction of fish muscle, commonly known as 'milky flesh', 'soft flesh' or 'jelly flesh', is a phenomenon associated with parasitic infection by Kudoa thyrsites and/or K. paniformis. Kudoa infection has been researched extensively1 as a result of the economic implications to marine and aquaculture industries. Pacific hake (Merluccius productus)2 and farm-reared Atlantic salmon (Salmo salar)3-5 have been studied more extensively. Parasites from the family Kudoidae Maglitsch, 1960, result in post-mortem myoliquefaction, and, in certain fish species, in visible cysts in the musculature.6 The economic implications are of great concern for the salmon aquaculture industry, as infected fish show no external signs of infection. Even after death, myoliquefaction becomes apparent only after several hours (38-56 h) post-mortem.7 Currently there is no rapid method to identify and quantify Kudoa infection. A visual counting method, in which visible white pseudocysts are counted, has been suggested for Pacific hake. Although this method is time consuming,8 as it requires careful examination, it could be implemented as a means to remove heavily infected fillets with black streaks caused by black pseudocysts. White pseudocysts are only one of the few stages in the Kudoa life cycle. In some fish host species, black pseudocysts are present, while in other host species, such as salmonids, there is no formation of black pseudocysts. The muscle fibre can thus become completely infected and rupture without any macroscopic evidence of infection. While some kudoid species are responsible for post-mortem myoliquefaction and cyst formation, others seem to have relatively little effect in host species.9 Many kudoid species show low host specificity, for example, K. thyrsites has been recorded from 18 different fish families representing nine fish orders.10 Taxonomies of the class Myxosporea11,12 and the genus Kudoa11, have been elaborated in previous review articles, and are not the focus of this review. Information about host species-specific kudoids, level of infection and effects on host musculature, in commercially important fish species, is of great economic importance. However, limited information about Kudoa infections and the effects thereof on marine fish quality and the economic impact thereof in South Africa is available. Here we review some of the global research on K. thyrsites and K. paniformis in several marine fish species of economic importance, with reference, where applicable, to South African marine fish species.
Myoliquefaction in marine fish in South Africa and other countries
Myoliquefaction is a common phenomenon in South African Cape snoek (Thyrsites atun). The musculature of infected fish becomes completely soft and jelly-like (Figure 1) and is commonly known as 'pap snoek'. Soft flesh was described for the first time in T. atun in Australia and South Africa as early as 1910 and 1924, respectively.13 Myoliquefaction in Cape snoek is caused by the myxosporean parasite K. thyrsites. This parasite was shown to have infected up to 27 fish species worldwide11, including mahi-mahi (Coryphaena hippurus)14, Atlantic salmon and Pacific hake3,10,11. South African pilchard (Sardinops ocellatus), Cape hake (Merluccius capensis) and Cape dory (Zeus capensis) are also known to be infected by K. thyrsites (Table 1), with pilchards being among the most heavily infected fish species in South African waters.15
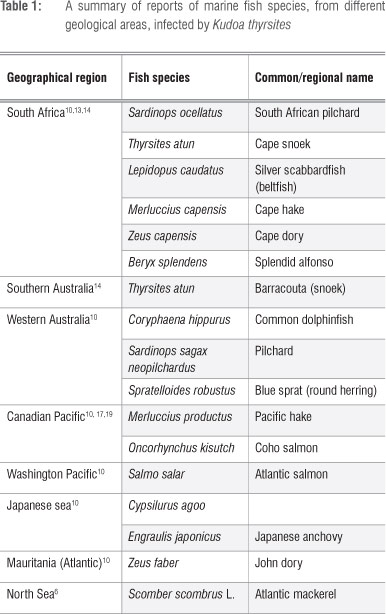
The presence of K. thyrsites, associated with myoliquefaction in dried fillets exported to Japan, was reported for the first time in 2005 in splendid alfonso (Beryx splendens) from South Africa.16 In the Northwest Pacific of North America, the occurrence of K. thyrsites is of great economic importance as it has a significant impact on farmed Atlantic salmon and, to a lesser extent, in coho salmon (Oncorhynchus kisutch).17
Kudoa paniformis often occurs simultaneously with K. thyrsites in Pacific hake.18 South African hake19 is believed to undergo accelerated myoliquefaction because K. paniformis produces both acidic and neutral proteolytic enzymes19; K. thyrsites only produces acidic enzymes1. Several studies2,20,21 have indicated that the optimum conditions for endogenous enzymes from K. paniformis in Pacific hake are a pH range of 5.25-5.50 and a temperature range of 52-55 °C.
The cytoplasmic cysteine proteases from K. thyrsites and K. paniformis have been identified and characterised in Pacific hake, and it was found that K. paniformis was the predominant parasitic species in the muscles.21 Pacific hake off the coast of Vancouver Island (British Columbia, Canada) was also mainly infected with K. paniformis, while only a small number of K. thyrsites spores was detected.2 In a study in which 322 Pacific hake from the west coast of British Columbia (offshore) were investigated for the presence of both K. thyrsites and K. paniformis, K. paniformis was found to be the predominant infection, with 32.3% and 38.8% of fish samples infected with either K. thyrsites or K. paniformis, respectively, and 18.3% infected with both parasitic species.18 Only 10.5% of the 322 samples was uninfected; however, the intensity of infection in the 89.5% infected samples was not uniform between samples or within a sample. Infection was generally more concentrated in the anterior part of the fillets and diminished toward the posterior area.
Mode of action of Kudoa thyrsites on fish tissue
Kudoa thyrsites releases pro-enzymes from the pre-sporogenic plasmodia1,20 that support its development by breaking down fish tissue without causing harm to the live fish. Ultra-structural evidence presented by Stehr and Whitaker1 suggested that while the fish is alive, neighbouring muscle fibres, and myofibrils of the infected muscle fibre that are not in direct contact with K. thyrsites plasmodia, are unaffected by the presence of the parasite. However, when these enzymes continue to be released by the parasite after the fish is killed, myoliquefaction of the musculature occurs. The level of proteolytic activity seems to be linked to the stage of parasitic infection19 as well as to the level of infection2. Proteolytic activity in hake muscle infected with the young parasite stage (white pseudocysts) was on average higher (7-fold) at an acidic pH range than at a neutral pH range. Muscle infected with older black pseudocysts showed a smaller difference in proteolytic activity between acidic and neutral pH ranges.19 However, fish muscle infected with black pseudocysts showed higher proteolytic activity, even though the correlation of black pseudocysts with proteolytic activity was low.8 A strong linear relationship was observed between spore counts and endogenous proteolytic activity in Pacific hake infected mainly by K. paniformis.2
Cathepsin L proteases are responsible for myoliquefaction in infected fish and it is clear this cysteine protease is derived from the parasite and not from a host response to the parasite.21 Cathepsin L from K. thyrsites differs from other cathepsin L proteases in that it contains only four of the six cysteine residues believed to be involved in disulphide bonds. K. thyrsites cathepsin L is translated as a pro-enzyme which is then chemically altered to an active enzyme by removal of the pro-region during limited proteolysis.20,21 This process is regulated by pH, where a decrease in pH results in the destabilisation of the pro-region, thus exposing the cleavage site. Cathepsin L protease from K. thyrsites shows maximum activity at pH 5.5, the iso-electric point of muscle proteins, and a marked loss of activity at pH 6.5.19 As the pH in fish muscle drops from 7.0 to 6.5 during early post-mortem storage,22,23 myoliquefaction is not evident early post-mortem. Reducing the amount of glycogen in Atlantic salmon muscle cells prior to harvesting might possibly reduce the amount of lactic acid generated in the muscle postmortem and consequently reduce the activity of cathepsin L proteases released from the parasite.2
Characteristics of Kudoa thyrsites infection
Kudoa thyrsites is a multivalvulid myxosporean parasite of marine fishes and has a complex life cycle with more than one host; very little is known about the life cycle of this parasite.11,19,24
Based on the life cycle of freshwater myxosporean species, a life cycle for K. thyrsites has been hypothesised.24,25 Parasitic plasmodia (cysts containing many spores) grow within the myocytes and sporulation of plasmodia within the somatic musculature of the host fish results in many myxospores inducing a chronic inflammatory response, but without any apparent harm to the health of the fish. The parasite penetrates the core of muscle fibre and spreads along myocytes without damaging the sarcolemma, resulting in individual muscle fibres being filled with spores without involving the connective tissue.19 This aggregate of spores within the somatic muscle fibre is referred to as the pseudocyst.26 The myxospores are released from the pseudocyst upon death of the host fish, and are then taken up by an intermediate host. Within the intermediate host, a second sporulation results in the development of actinospores. These actinospores are released to infect a new host fish and continue the parasite's life cycle. Whilst the parasite remains within the muscle fibre (where it contains both developing and mature spores), infected fibres appear white1; however, if the sarcolemma is destroyed by the parasite, there is a rapid development of a fibroblast layer around the parasite and the pseudocyst becomes black in appearance. In certain fish host species, such as salmonids, there is no formation of black pseudocysts26 and the muscle fibre can be completely infected and rupture without any macroscopic evidence of infection.
Although the stage of K. thyrsites that is infective to fish has not yet been identified,4 the presence of plasmodia in myocytes of Atlantic salmon has been observed after only 9 weeks of exposure to contaminated seawater27. K. thyrsites is different from other members of the myxozoan species in that it infects a wide variety of fish species around the world (Table 1). The route of infection is unknown; however, transmission of the parasite does not occur directly between fish. Infection by K. thyrsites occurs only in seawater and not in fresh water.6 South African snoek and pilchards appear to be major host reservoir species for K. thyrsites12,15 in South African waters. It cannot, however, be assumed that transmission of K. thyrsites occurs through ingestion of infected fish by other predatory fishes, firstly, because the life cycle and route of transmission and infection is unknown, and, secondly, because the Australian pilchard (Sardinops sagax neopilchardus), a planktivorous fish species, is also heavily infected with K. thyrsites and is suggested to be a major reservoir host in Southwest Australian waters. It is thus suggested that infection of plantivorous fish is through ingestion of infected invertebrate hosts.12
Factors affecting the level of Kudoa thyrsites infection
The degree of visible myoliquefaction from K. thyrsites infection may be influenced by several factors, such as time to evaluation post-mortem, the number of K. thyrsites present in the flesh, temperature during storage, and the inherent quality of the muscle.4 The latter is affected by factors such as growth and feeding condition of the fish, water temperature, and processing conditions. From a correlation between post-mortem myoliquefaction and intensity of K. thyrsites infection in Atlantic salmon, it was found that high intensity of infection resulted in severe autolysis of the somatic musculature.3 A mean plasmodia count of 0.3 mm-2 or a mean spore count of 4 x 105/g tissue is suggested to be indicative of severe myoliquefaction.4
The age and size5,7 of fish as factors affecting the degree of post-mortem myoliquefaction was demonstrated in 2 0 0 87, when large (>600 g) Atlantic mackerel had a significantly greater prevalence of myoliquefaction than medium-sized (400-600 g) mackerel. Pen-reared sexually mature
Atlantic salmon (Salmo salar) are also more likely to be infected with K. thyrsites than their sexually immature counterparts.5 A local snoek processor (Theron R 2012, oral communication, June) reported that large Cape snoek tend to develop myoliquefaction to a greater extent than do smaller fish. Levsen et al.7 hypothesised that mackerel become infected at a late stage of their lifespan, or, that the parasite develops relatively slowly. Previous studies19,27 have indicated that K. thyrsites has a slow plasmodial development in fish and develops into microscopically visible plasmodia in adult fish, although a histologically undetectable parasite stage may be present in younger fish. Early stages of the parasite may be detected by PCR11,28,29 and immunohistochemistry29 techniques. K. thyrsites was identified in young tube-snouts (Aulorhynchus flavidus) using PCR when visual or histological methods were negative.25 Similarly to results of an earlier study7, it was also found that the prevalence of infection was greater in adult tube-snouts (up to 100%) than in young tube-snouts (about 50%)25.
The prevalence of Kudoa infection is also affected by season, with the level of infection tending to be higher during summer than during winter months.5,6,11,30,31 The effect of season on K. thyrsites and K. paniformis infection levels in South African marine fish species, such as Cape snoek, Cape hake, Cape dory and South African pilchard, is not well documented.
Detection and identification of Kudoa thyrsites infection
Kudoa thyrsites myxospores (Figure 2) are the most characteristic stage of infection and are suggested to be the infective stage of invertebrate hosts or vertebrate predatory fish species.7,12,14,24K. thyrsites myxospores vary between 12.0 µηι32 and 16.7 µηι10,33 in diameter, and, in the absence of visible pseudocysts, infection with K. thyrsites is not macroscopically visible. Infection is thus not macroscopically detected until myoliquefaction, several hours post-mortem. Examination of both muscle and blood samples of Atlantic mackerel (Scomberscombrus L.) immediately after catch was unsuccessful in identifying infection by K. thyrsites.7 Several researchers8,34 have suggested visual counting of white pseudocysts as a potential method for sorting Pacific hake according to level of infection; a positive correlation between the number of white pseudocysts, protease activity and texture of Pacific hake has been found. The drawback of this method is that it requires careful and time-consuming examination, because white pseudocysts are not readily detectable, and is therefore not feasible for a production line.

Detection of the parasite by means of ultraviolet fluorescence has been investigated,15 but this method is unreliable because the parasitic cysts do not glow in dark-coloured muscles or in dead flesh that is more than a few hours old.
Identification of parasitic infections relies on traditional skills such as microscopy and taxonomy. Modern methods, such as DNA (PCR) technologies, have made important contributions to the screening, diagnosis and taxonomy of parasitic infections in fish species.33 Primers have been designed for amplification of the SSU rDNA region for detection of specific K. thyrsites where the primer pair Kt18S6f and Kt18S1r amplifies a 909-bp region of the K. thyrsites SSU rDNA and does not produce a PCR product from host DNA.29
The identification of infection by K. thyrsites is usually done by microscopy, using either wet mounts or histological sections of muscle tissue, which results in disfiguration of the fish and thus negatively influences its market value. Sampling from the hyohyoideus ventralis muscle (striated muscle) from under the operculum of Atlantic salmon, from which a wet mount is prepared and examined under phase contrast microscopy provides a non-destructive method for assessing fish for the presence or absence of K. thyrsites spores as only a small amount of fillet is needed.34 Counting the number of plasmodia in the opercular mandibular muscle of Atlantic salmon did not reflect the number of plasmodia in the fillets and was not a good indicator of fillet quality (i.e. it did not correlate with the number of pits in the fillet).4 The most reliable predictive parasite counts were those from somatic and opercular samples.4 Because sampling from somatic muscle tissue damages the fillet, this sampling method has been considered undesirable; however, as only a small sample (25 mg) is required, the sample could be removed from the fillet without any visual damage to the fillet.4 Because the number of plasmodia and spores vary significantly among individual fish and among tissue samples within a fish, it is recommended that multiple samples be taken from a single fish. To avoid false negatives and to improve detection when using the PCR test method, a large sample of tissue from several anatomical areas from a single fish should be included in the initial homogenate.34 An alternative quantitative method is an antigen-capture enzyme-linked immunosorbent assay36 which utilises uncharacterised soluble parasite antigens to estimate the severity of the infection. This assay is similar in sensitivity to PCR and may be used, with an appropriate sampling plan, as an early diagnostic tool.
In comparison with microscopic methods, in which only myxospores (Figure 2) are detected, the PCR method is useful for detecting less severe and early stage infections. Although PCR testing for K. thyrsites is specific and more sensitive than wet mount and histological examinations, it provides limited information on parasite locality in specific tissues. Although histological staining provides for localisation of recognisable mature infections, it does not allow characterisation of obscure developmental stages of the parasite. By combining methods of in-situ hybridisation and immunohistochemistry, previously undescribed developmental stages of K. thyrsites may be characterised.29 In-situ hybridisation can be used as a tool to localise K. thyrsites in specific tissues and to visualise cryptic stages, whereas immunohistochemistry can be used to detect early developmental stages, but not pre-sprogonic stages or mature spores of K. thyrsites.
A recent study36 investigated the efficacy of dietary nicarbazin, an equimolar complex of 4,4'-dinitrocarbanilide and 2-hydroxy-4,6-dimethylpyrimidine used in poultry feeds for the prevention of coccidiosis37, against K. thyrsites in seawater-reared Atlantic salmon post-smolts. Dietary nicarbazin was found to significantly reduce the prevalence and severity of K. thyrsites in fish. Effects on mortality of fish and of high residues of nicarbazin in skin, liver and muscle warrant further studies. Development of treatment regimes using medicated diets is possible for farmed-reared fish, but such strategies are not possible for wild fish.
Conclusions
Although the parasite K. thyrsites is considered harmless if consumed by humans38, there has been a report39 of an immunological response after consumption of fish infected with Kudoa. No conclusive relationship with any pathological disorder has, however, been found.
In relation to global food security issues, the most important concern with regard to Kudoa infections is that it results in waste of animal protein and economic losses. In the case of wild-caught fish, rapid methods of identifying infected fish as soon as possible after harvest or capture at sea will be of benefit to the fishery industry. If infected fish can be identified at an early stage post-mortem, the infected fish may be used for alternative processing. Alternatively, opportunities exist for investigating post-harvest technologies, such as ionising irradiation40 to inhibit the development of myoliquefaction, and, consequently, reduce waste and economic losses. Such possibilities for South African conditions will enable the fishing industry, especially the small-scale fishing industry, to minimise the wastage experienced currently.
Authors' contributions
All authors contributed to the writing of the manuscript.
References
1. Stehr C, Whitaker DJ. Host-parasite interaction of the myxosporeans Kudoa paniformis Kabata & Whitaker, 1981 and Kudoa thyrsites (Gilchrist, 1924) in the muscle of Pacific whiting, Merluccius productus (Ayres): An ultrastructural study. J Fish Dis. 1986;9:505-517. http://dx.doi.org/10.1111/j.1365-2761.1986.tb01047.x [ Links ]
2. Samaranayaka AGP Ho TCW, Li-Chan ECY Correlation of Kudoa spore counts with proteolytic activity and texture of fish mince from Pacific Hake (Merluccius productus). J Aquat Food Product Tech. 2006;15(4);75-93. http://dx.doi.org/10.1300/J030v15n04_06 [ Links ]
3. St-Hilaire S, Hill M, Kent ML, Whitaker DJ, Ribble C. A comparative study of muscle texture and intensity of Kudoa thyrsites infection in farm-reared Atlantic salmon Salmo salar on the Pacific coast of Canada. Dis Aquat Organ. 1997;31:221-225. http://dx.doi.org/10.3354/dao031221 [ Links ]
4. Dawson-Coates JA, Chase JC, Funk V Booy MH, Haines LR, Falkenberg CL, et al. The relationship between flesh quality and numbers of Kudoa thyrsites plasmodia and spores in farmed Atlantic salmon, Salmo salar L. J Fish Dis. 2003;26:451-459. http://dx.doi.org/10.1046/j.1365-2761.2003.00477.x [ Links ]
5. St-Hilaire S, Ribble C, Whitaker DJ, Kent M. Prevalence of Kudoa thyrsites in sexually mature and immature pen-reared Atlantic salmon (Salmo salar) in British Columbia, Canada. Aquaculture. 1998;162:69-77. http://dx.doi.org/10.1016/S0044-8486(98)00208-7 [ Links ]
6. Moran JDW, Whitaker DJ, Kent ML. Natural and laboratory transmission of the marine myxozoan parasite Kudoa thyrsites to Atlantic salmon. J Aquat Anim Health. 1999;11:110-115. http://dx.doi.org/10.1577/1548-8667(1999)011 <0110:NALTOT>2.0.CO;2 [ Links ]
7. Levsen A, J0rgensen A, Mo TA. Occurence of post-mortem myoliquefactive kudoosis in Atlantic mackerel, Scomber scombrus L., from the North Sea. J Fish Dis. 2008;31:601-611. http://dx.doi.org/10.1111/j.1365-2761.2008.00937.x [ Links ]
8. Morrissey MT, Hartley PS, An H. Proteolytic activity in Pacific whiting and effects of surimi processing. J Aquat Food Product Tech. 1995;4(4):6-18. [ Links ]
9. Whipps CM, Kent ML. Phylogeography of the cosmopolitan marine parasite Kudoa thyrsites (Myxozoa: Myxosporea). J Eukaryot Microbiol. 2006;53:364-373. http://dx.doi.org/10.1111/j.1550-7408.2006.00114.x [ Links ]
10. Lom J, Dyková I. Myxozoan genera: Definition and notes on taxonomy, life-cycle terminology and pathogenic species. Folia Parasitol. 2006;53:1-36. [ Links ]
11. Moran JDW, Whitaker DJ, Kent ML. A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture. 1999;172:163-196. http://dx.doi.org/10.1016/S0044-8486(98)00437-2 [ Links ]
12. Langdon JS, Thorne T, Fletcher WJ. Reservoir hosts and new clupeoid host records for the myoliquefactive myxosporean parasite Kudoa thyrsites (Gilchrist). J Fish Dis. 1992;15:459-471. http://dx.doi.org/10.1111/j.1365-2761.1992.tb00678.x [ Links ]
13. Gilchrist JDF. A protozoal parasite Chloromyxum thyrsites sp.n. of the Cape sea-fish the 'snoek' (Thyrsites atun, EUPHR). Trans Roy Soc S Afr. 1924;11:263-273. http://dx.doi.org/10.1080/00359192309519587 [ Links ]
14. Langdon JS. Myoliquefaction post-mortem ('milky flesh') due to Kudoa thyrsites (Gilchrist) (Myxosporea: Multivalvulida) in mahi mahi, Coryphaena hippurus L. J Fish Dis. 1991;14:45-54. http://dx.doi.org/10.1111/j.1365-2761.1991.tb00575.x [ Links ]
15. Webb SC. Pap pilchards and protozoa. South African Shipping News & Fishing Industry Review. 1990;45(4):35. [ Links ]
16. Yokoyama H, Itoh N. Two multivalvulid myxozoans causing post-mortem myoliquefaction: Kudoa megacapsula N. sp. from red barracuda (Sphyraena pinguis) and Kudoa thyrsites from splendid alfonso (Beryx splendens). J Parasitol. 2005;91(5):1132-1137. http://dx.doi.org/10.1645/GE-548R.1 [ Links ]
17. Whitaker DJ, Kent ML. Myxosporean Kudoa thyrsites: A cause of soft flesh disease in farm-reared Atlantic salmon. J Aquat Anim Health. 1991;3:291-294. http://dx.doi.org/10.1577/1548-8667(1991)003<0291:MKTACO>2.3.CO;2 [ Links ]
18. Morado JF, Sparks A. Observations on the host-parasite relations of the Pacific whiting, Merluccius productus (Ayres), and two myxosporean parasites, Kudoa thyrsites (Gilchrist, 1924) and K. paniformis Kabata & Whitaker, 1981. J Fish Dis. 1986;9:445-455. http://dx.doi.org/10.1111/j.1365-2761.1986.tb01038.x [ Links ]
19. Tsuyuki H, Williscroft SN, Kabata Z, Whitaker DJ. The relationship between acid and neutral protease activities and the incidence of soft cooked texture in the muscle tissue of Pacific hake Merluccius productus infected with Kudoa paniformis and/or K. thyrsites, and held for varying times under different pre-freeze chilled storage conditions. Canadian Technical Report of Fisheries and Aquatic Sciences No. 1130. Ottawa: Department of Fisheries and Oceans; 1982. p. 1-39. [ Links ]
20. An H, Seymour TA, Wu J, Morrissey MT. Assay systems and characterization of Pacific whiting (Merluccius productus) protease. J Food Sci. 1994;59(2):277-281. http://dx.doi.org/10.1111/j.1365-2621.1994.tb06947.x [ Links ]
21. Funk VA, Olafson RW, Raap M, Smith D, Aitken L, Haddow JD, et al. Identification, characterization and deduced amino acid sequence of the dominant protease from Kudoa paniformis and K. thyrsites: A unique cytoplasmic cysteine protease. Comp Biochem Physiol B. 2008;149:477-489. http://dx.doi.org/10.1016/j.cbpb.2007.11.011 [ Links ]
22. Chéret R, Delbarre-Ladrat C, De Lamballerie-Anton M, Verrez-Bagnis V. Calpain and cathepsin activities in post-mortem fish and meat muscles. Food Chem. 2007;101:1474-1479. http://dx.doi.org/10.1016/j.foodchem.2006.04.023 [ Links ]
23. Frangoise L. Occurrence and role of lactic acid bacteria in seafood products: A review. Food Microbiol. 2010;27:698-709. http://dx.doi.org/10.1016/j.fm.2010.05.016 [ Links ]
24. Young CA. An immunolocalization study of the life stages of Kudoa thyrsites in Atlantic salmon (Salmo salar) (research report). Nanaimo, British Columbia: Malaspina University-College; 2002. [ Links ]
25. Shaw RW, Hervio DML, Devlin RH, Adamson MI. Infection of Aulorhynchus flavidus (Gill) (Osteichthyes: Gasterosteiformes) by Kudoa thyrsites (Gilchrist) (Myxosporea: Multivalvulida). J Parasitol. 1997;83(5):810-814. http://dx.doi.org/10.2307/3284272 [ Links ]
26. Harrel LW, Scott TM. Kudoa thyrsites (Gilchrist) (Myxosporea: Multivalvulida) in Atlantic salmon, Salmo salar L. J Fish Dis. 1985;8:329-332. http://dx.doi.org/10.1111/j.1365-2761.1985.tb00950.x [ Links ]
27. Moran JDW, Margolis L, Webster JM, Kent ML. Development of Kudoa thyrsites (Myxozoa: Myxosporea) in netpen-reared Atlantic salmon determined by light microscopy and a polymerase chain reaction test. Dis Aquat Org. 1999;37:185-193. http://dx.doi.org/10.3354/dao037185 [ Links ]
28. Hervio DML, Kent ML, Khattra J, Sakanari J, Yokoyama H, Devlin RH. Taxonomy of Kudoa species (Myxosporea), using a small-subunit ribosomal DNA sequence. Can J Zool. 1997;75(12):2112-2119. http://dx.doi.org/10.1139/z97-846 [ Links ]
29. Young CA, Jones SRM. Epitopes associated with mature spores not recognized on Kudoa thyrsites from recently infected Atlantic salmon smolts. Dis Aquat Org. 2005;63:267-271. http://dx.doi.org/10.3354/dao063267 [ Links ]
30. Munday BL, Su X, Harshbarger JC. A survey of product defects in Tasmanian Atlantic salmon (Salmo salar). Aquaculture. 1998;169:297-302. http://dx.doi.org/10.1016/S0044-8486(98)00381-0 [ Links ]
31. Moran JDW, Kent ML, Whitaker DJ. Kudoa thyrsites (Myxozoa: Myxosporea) infections in pen-reared Atlantic salmon in the Northeast Pacific Ocean with a survey of potential nonsalmonid reservoir hosts. J Aquat Anim Health. 1999;11:101-109. http://dx.doi.org/10.1577/1548-8667(1999)011<0101:KTMMII>2.0.CO;2 [ Links ]
32. Kabata Z, Whitaker DJ. Two species of Kudoa (Myxosporea, Multivalvulida) parasite in the flesh of Merluccius productus (Ayres, 1855) (Pisces, Teleostei) in the Canadian Pacific. Can J Zool. 1981;59:2085-2091. http://dx.doi.org/10.1139/z81-285 [ Links ]
33. Cunningham CO. Molecular diagnosis of fish and shellfish diseases: Present status and potential use in disease control. Aquaculture. 2002;206:19-55. http://dx.doi.org/10.1016/S0044-8486(01)00864-X [ Links ]
34. St-Hilaire S, Ribble C, Whitaker DJ, Kent M. Evaluation of a non-destructive diagnostic test for Kudoa thyrsites in farmed Atlantic salmon (Salmo salar). Aquaculture. 1997;156:139-144. http://dx.doi.org/10.1016/S0044-8486(97)00081-1 [ Links ]
35. Taylor K, Jones S. An enzyme linked immunosorbent assay for the detection of Kudoa thyrsites in Atlantic salmon Salmo salar. Aquaculture. 2005;250:8-15. http://dx.doi.org/10.1016/j.aquaculture.2005.02.040 [ Links ]
36. Jones SRM, Forster I, Liao X, Ikonomou MG. Dietary nicarbazin reduces prevalence and severity of Kudoa thyrsites (Myxosporea: Multivalvulida) in Atlantic salmon Salmo salar post-smolts. Aquaculture. 2012;342-343:1-6. http://dx.doi.org/10.1016/j.aquaculture.2012.01.033 [ Links ]
37. Chapman HD. A review of the biological activity of the anticoccidial drug nicarbazin and its application for the control of coccidiosis in poultry. Poultry Sci Review. 1993;5:231-243. [ Links ]
38. Alvarez-Pellitero P, Sitja-Bobadilla A. Pathology of myxosporea in marine fish culture. Dis Aquat Org. 1993;17:229-238. http://dx.doi.org/10.3354/dao017229 [ Links ]
39. Martinez de Velasco G, Rodero M, Chivato T, Cuellar C. Seroprevalence of anti-Kudoa sp. (Myxosprea: Multivalvulida) antibodies in a Spanish population. Parasitol Research. 2007;100:1205-1211. http://dx.doi.org/10.1007/s00436-006-0390-x [ Links ]
40. Hultmann L, Rustad T. Iced storage of Atlantic salmon (Salmo salar) - Effects on endogenous enzymes and their impact on muscle proteins and texture. Food Chem. 2004;87:31-41. http://dx.doi.org/10.1016/j.foodchem.2003.10.013 [ Links ]
 Correspondence:
Correspondence:
Suné Henning
Department of Food Technology,
Cape Peninsula University of Technology, Symphony Way
Bellville 7535, South Africa
Email: hennings@cput.ac.za
Received: 31 Aug. 2012
Revised: 20 May 2013
Accepted: 29 Jul. 2013














