Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.109 n.7-8 Pretoria Jan. 2013
RESEARCH LETTER
Cellular and molecular effects of electromagnetic radiation and sonic waves
Patrícia Froes MeyerI; Oscar Ariel RonzioII; Adenilson de Souza da FonsecaIII; Sebastião David Santos-FilhoIII; Mario Bernardo-FilhoIII, IV
IPostgraduate Program in Health Science, Rio Grande do Norte Federal University, Natal, Brazil
IIPhysical Agents Laboratory, Maimonides University, Buenos Aires, Argentina
IIIBiophysics and Biometry Department, Roberto Alcantara Gomes Biology Institute, Rio de Janeiro State University, Rio de Janeiro, Brazil
IVResearch Coordination, National Cancer Institute, Rio de Janeiro, Brazil
ABSTRACT
Electromagnetic radiation (in the form of pulsed magnetic fields, radiofrequency and intense pulsed light) and mechanical agents (such as sonic waves) have been used in physical therapy. The aim of this study was to assess the effects of low-intensity magnetic fields, sonic and radiofrequency waves, and intense pulsed light on the survival of Escherichia coli cultures and on the electrophoretic mobility of plasmid DNA. Exponentially growing E. coli AB1157 cultures and plasmid DNA samples were exposed to these physical agents and 0.9% NaCl (negative control) and SnCl2 (positive control) solutions. Aliquots of the cultures were diluted and spread onto a solidified rich medium. The colony-forming units were counted after overnight incubation and the survival fraction was calculated. Agarose gel electrophoresis was performed to visualise and quantify the plasmid topological forms. The results suggest that these agents do not alter the survival of E. coli cells or plasmid DNA electrophoresis mobility. Moreover, they do not protect against the lesive action of SnCl2. These physical agents therefore had no cytotoxic or genotoxic effects under the conditions studied.
Keywords: Escherichia coli; DNA; intense pulsed light; magnetic field; radiofrequency; stannous chloride
Introduction
Physical therapy devices used for the treatment of aesthetic disorders1,2 such as facial acne, can emit sonic and ultrasonic waves and electromagnetic radiation at an extremely low frequency as well as radiofrequency, light and infrared radiation.2,3 Low frequency pulsed electromagnetic fields (PEMF) could be used to treat diseases characterised by pain, inflammation and regeneration. Biological effects on the organs and body systems associated with the energies generated by these sources have been reported, but the findings remain inconsistent.4 Beneficial effects of electromagnetic fields on bone metabolism and hydroxyapatite osteointegration, suggesting osteogenesis stimulation, have been described.5
Some authors have suggested that audible sonic waves could interact with proteins, moving them to the lymphatic system, as in the bioresonance phenomenon.4 According to this phenomenon, proteins move to the lymphatic system as a result of the harmonics created by the sonic waves, thereby exiting the extracellular compartment.6
Effects associated with radiofrequency are related to heating of the tissues to 50 °C, at which cell death is induced by protein coagulation - an effect that could be useful in the treatment of tumours.7 Effects on the treatment of muscle and articular injuries have been reported with the use of radiofrequency. Radiofrequency has been used for aesthetic purposes and some authors have suggested a thermal action in deep tissues, promoting collagen denaturalisation and neocollagenogenesis.8,9
Intense pulsed light (IPL) sources have been used to treat abnormal scarring,10 burn sequelae, hyperchromia and benign vascular lesions.11,12
Stannous chloride (SnCl2) is the most widely used reducing agent in nuclear medicine for labelling cellular and molecular structures of biological interest. It is used with technetium-99m in single-photon emission computed tomography. However, it has been shown that SnCl2 is cytotoxic and genotoxic.13,14 In bacterial cultures and plasmid DNA, SnCl2 appears to induce damage through oxidative mechanisms related to free radical generation.15,16 Data from studies on Escherichia coli, deficient in DNA repair mechanisms, suggest that this chemical agent could induce lesions in DNA.14,16
Although extremely low-frequency electromagnetic fields, sonic, radiofrequency and IPL devices are used in therapeutic practice, there have been few studies on their biological effects. The aim of this study was therefore to evaluate the effect of these sources of energy on the survival of E. coli and on the electrophoretic mobility of plasmid DNA.
Materials and methods
Physical agent exposure
A PEMF device was used (700 Ohms, 110 V, 60 Hz) to generate a polarised electromagnetic field (north and south). Bacterial cultures and DNA samples were exposed at both magnetic poles (5 mT 30 min).
Sonic waves were generated by a Bioressonance® device configured at 3.3 KHz. Bacterial cultures and DNA samples were exposed for 20 min. The piezoelectric emitters were coupled to the samples with ultrasonic gel.
The PEMF and Bioressonance® devices are archetypes developed and tested by Oscar Ronzio, a physical therapist at Maimonides University in Buenos Aires, Argentina.
Radiofrequency, at a frequency of 550 KHz, was applied for 5 min using an electrical capacitive transference device (Tecatherap-VIP®, VIP Electromedicina®, Buenos Aires, Argentina). Bacterial cultures and DNA samples were placed between two equidistant (30 mm) rubber covers with carbon electrodes.
Bacterial cultures were exposed to one pulse (0.01 s, 3-7 J/cm2, 500-1200 nm) and DNA samples were exposed to one and two pulses (at the same specifications) of intense pulsed light using an IPL device (Radiance®, Tel Aviv, Israel).
Bacteria inactivation
Escherichia coli AB1157, a wild-type strain to repair DNA damage, was used in this study. Exponentially growing bacterial cultures in rich medium were centrifuged at 2000 rpm for 10 min and suspended in saline solution (0.9% NaCl). Samples of these suspensions (1.0 mL) were exposed to each physical agent. Unexposed samples treated with saline were used as a negative control and those incubated with SnCl2 (25 µg, 60 min) were used as a positive control. The cultures were diluted and spread on Petri dishes containing solidified rich medium. After overnight incubation at 37 °C, the colony-forming units were counted and the survival fractions were calculated by dividing the number of viable cells obtained per millilitre after treatment (with physical agents or SnCl2) by the number of viable cells before treatment.
Analysis of DNA mobility alterations
Samples of pBSK plasmids (200 ng) were obtained by the alkaline method17 and exposed to the physical agents as described. Unexposed plasmid samples treated with saline were used as a negative control and unexposed plasmid samples incubated with SnCl2 (200 µg, 40 min) were used as a positive control. Aliquots of each sample were then mixed with loading buffer (0.25% xylene cyanol, 0.25% bromophenol blue, 30% glycerol) and 0.8% agarose gel electrophoresis (8 V/cm) was performed in Tris-acetate-EDTA buffer (pH 8.0). Gels were then stained with ethidium bromide (0.5 µg/mL), and the DNA bands were visualised by fluorescence in an ultraviolet transilluminator system. The gel images were digitalised (Kodak Digital Science 1d, EDAS 120, Rochester, NY, USA) and the bands were semiquantified using the Gimp computer program. Plasmid conformations were quantified as either Form I supercoiled (a native conformation) or Form II open circle (resulting from a single-strand break).
Statistical analysis
Data are reported as means±SD of plasmid percentage forms. A oneway analysis of variance was performed to verify possible statistical differences. A rigorous statistical post-test (Bonferroni) was chosen to identify the p-value (p<0.05 as lesser significant level) and to compare each treated group with the control group (0.9% NaCl). InStat Graphpad software was used to perform statistical analyses (GraphPad InStat version 3.00 for Windows 95, GraphPad Software, San Diego, California, USA).
Results
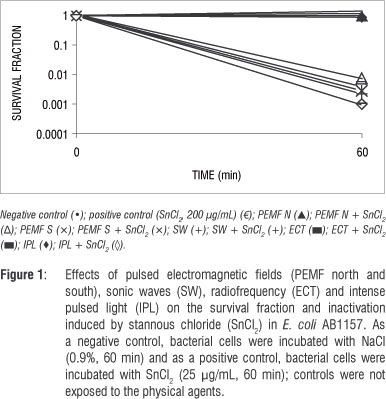
Figure 1 shows the survival fractions of E. coli AB1157 cultures treated with SnCl2 in the presence or absence of PEMF, sonic waves, radiofrequency and IPL. The data show no alteration in the survival fraction. Moreover, no protective effect from the physical agents against SnCl2 action in E. coli cultures was found.
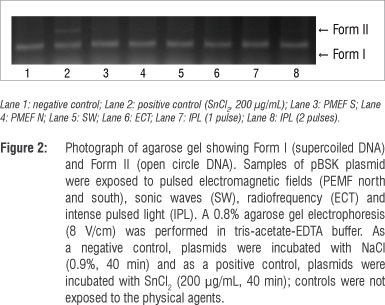
The electrophoretic profile of pBSK plasmid DNA under different experimental conditions is shown in Figure 2. In Lane 1, the plasmid DNA alone is found mostly as a supercoil form (Form I). Lane 2 shows the efficient cleavage of the SnCl2-induced plasmid DNA, illustrated by the formation of the open circular form (Form II). Lanes 3 to 5 show the electrophoretic profiles of plasmid DNA submitted to physical agents, suggesting no modifications in plasmid topology when compared with the control (Lane 1). Lanes 6 to 8 show that the physical agents did not alter the SnCl2 action on the electrophoretic mobility of the plasmid DNA.
Discussion
There is little information about the biological effects of sonic and radiofrequency waves, low intensity magnetic fields and IPL that have been used in therapeutic practice.18 Doubts related to safety persist owing to the lack of a scientific explanation for their mechanisms of action, as well as for the characterisation of parameters that may or may not have a harmful effect. The correct use of a physical agent is important, both for protecting public health and for ensuring exposure levels that result in the desired biological effects.19
The success of treating ulcers using PEMF appears to be as a result of fibroblast production stimulation and bactericide effects.20 Capponi and Ronzio4 suggest that PEMF is contraindicated in the presence of fungal and viral infections, because of its cell proliferation effects. Magnetostatic bacteria are extremely affected by PEMF; however, our data indicate that PEMF did not alter E. coli cell survival (Figure 1).
Studies using PEMP at a low frequency have reported nerve repair,21,22 increased osteogenesis,5 reduced hyperthrombocythemia and hyperfibrinogenemia,23 improvement in tropical ulcers24 and DNA synthesis.4 Kulishova et al.25 showed the efficacy of general magnetotherapy in conservative therapy of uterine myoma in women of reproductive age.
Our data also indicate that PEMF could not induce DNA strand breaks, at least when the technique involving DNA electrophoresis mobility in agarose gel was used (Figures 2 and 3). However, epidemiological data have suggested that PEMF may be a risk factor for breast cancer in humans.26
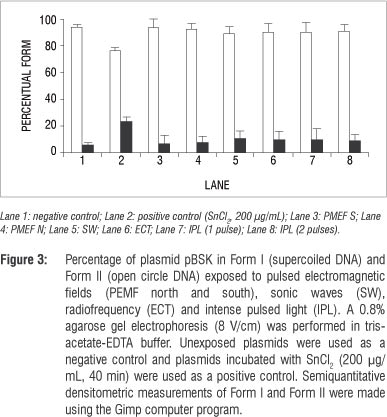
Radiofrequency could have both thermal and non-thermal effects. Ubeda27 reported cytotoxic effects in neuroblastoma and hepatocarcinoma cells in the non-thermal modality that controls the cancer's development. Despite Ubeda's findings, we found no effects of radiofrequency on E. coli cultures (Figure 1) and plasmid DNA (Figures 2 and 3). Ley-Valle28 described undesirable effects on the central nervous system when using 2-MHz radiofrequency.
Positive effects of sonic waves, that is increased collagen synthesis, have been described when used to treat scarring in humans.4 However, harmful effects were reported by Lennart29 who used ultrasonic (1 MHz) and high-intensity (>30 W/cm2) waves. Araújo et al.30 showed that 3 MHz, 3 W/cm2 ultrasound waves, in stationary and continuous application, stimulated venous thromboembolism and increased the number of lymphocytes. It has been described that these waves affect protozoans, inactivate viruses, destroy red blood cells and bacteria and hinder fungal multiplication.28 However, in this study, using 3.3-KHz sonic waves, no effects on bacterial cultures (Figure 1) or plasmid DNA (Figures 2 and 3) were found. In addition, our data suggest that sonic waves could not protect E. coli cells against the cytotoxic effect of SnCl2 or increase the action of this reducing agent.
Intense pulsed light systems are high-intensity light sources that emit polychromatic and non-coherent light, allowing great variability in the selection of individual aesthetic skin treatments31 such as facial rejuvenation,32 or for the treatment of skin diseases such as erythrosis.33 Isaac et al.12 and Perez Rivera et al.10 described the results of treating stains and benign vascular lesions (haemangiomas). Patients treated with IPL at 420-950 nm showed a return to baseline of their facial acne.34 Acne pathogenesis is believed to involve sebaceous follicular hyperplasia, hyperkeratinisation, Propionibacterium acne proliferation, inflammation and immune reactions. It has been suggested that IPL may decrease sebaceous gland size, pilosebaceous inflammation and Propionibacterium species populations,35 but the results obtained in our study, under the test conditions, indicate no effect of IPL on E. coli cells (Figure1) or plasmid DNA (Figures 2 and 3). Furthermore, no protective effect on the E. coli culture against the toxic effect of SnCl2 (Figure 1) was found.
In conclusion, at least under the conditions used in this investigation, as well as for the techniques used, our data suggest that low-frequency pulsated electromagnetic fields, audible sonic waves, radiofrequency and IPL do not have important biological effects. Moreover, these sources of energy do not modify the cytotoxic and genotoxic effects of SnCl2 on E. coli cells and on plasmid DNA.
Acknowledgements
This work received financial support from Universidade Federal do Rio Grande no Norte, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Universidade do Estado do Rio de Janeiro and Universidad Maimonides.
Authors' contributions
M.B.F. was the project leader; P.F.M., A.S.F. and O.A.R. performed most of the experiments; S.D.S.F. made conceptual contributions; A.S.F performed the plasmid experiments; and S.D.S.F., M.B.F. and P.F.M. wrote the manuscript.
References
1. Chang SE, Ahn SJ, Rhee DY, Choi JH, Moon KC, Suh HS et al. Treatment of facial acne papules and pustules in Korean patients using an intense pulsed light device equipped with a 530- to 750-nm filter. Dermatol Surg. 2007;33:676-679. http://dx.doi.org/10.1111/j.1524-4725.2007.33142.x
2. Heinrich H. Assessment of non-sinusoidal, pulsed, or intermittent exposure to low frequency electric and magnetic fields. Health Phys. 2007;92:541-546. http://dx.doi.org/10.1097/01.HP.0000262628.29600.b4
3. Johns LD. Nonthermal effects of therapeutic ultrasound: The frequency resonance hypothesis. J Athl Train. 2003;37:293-299.
4. Capponi R, Ronzio O. Avances en Fisioterapia [Advances in physiotherapy]. Buenos Aires: Agentes Físicos; 2006. Spanish. [ Links ]
5. Giordano N, Battisti E, Cerasi S, Fortunato M, Santacroce C, Rigato M. Effect of electromagnetic fields on bone mineral density and biochemical markers of bone turnover in osteoporosis: A single-blind, randomized pilot study. Curr Terap Res. 2001;62:155-161.
6. Meyer PF, Lustosa ACG, Morais JM, Carvalho MGF, Cavalcante JL, Ronzio AO. In vivo effects of low frequency sonic waves in soft tissue repair process. Physical Therapy Brazil. 2010;4:277-283.
7. Gallego VD, Martin IJP, Aguado JM, Garau PC, Bosquet SM, Gimeno AV, et al. Radiofrequency ablation as an alternative treatment for organ confined renal tumor. Actas Urol Esp. 2010;34:860-865. http://dx.doi.org/10.1016/S2173-5786(10)70214-6
8. Arnoczky SP, Aksan A. Thermal modification of connective tissue: Basic science considerations and clinical impressions. J Am Acad Orthop Surg. 2000;8:305-313.
9. Christine C, Dierickx MD. The role of deep heating for noninvasive skin rejuvenation. Lasers Surg Med. 2006;38:799-807. http://dx.doi.org/10.1002/lsm.20446
10. Perez Rivera F, Fridmanis M, Balbi L, Correa A, Goñi S, Gaglio P Treating benign vascular lesion cutaneous thoraxccervicofacial for intense pulsed light. Rev Argent Dermatol. 2002;83:14-22.
11. Sacks T, Barcaui C. Laser e luz pulsada de alta energia: indugáo e tratamento de reações alérgicas relacionadas a tatuagens [Laser and intense pulsed light: Induction and treatment of allergic reactions related to tattoos]. An Bras Dermato. 2004;79:709-714. Portuguese.
12. Isaac C, Salles AG, Soares MFD, Camargo CP Ferreira MC. Efeitos da luz intensa pulsada em seqüelas cicatriciais hipercrômicas pós-queimadura [Effects of intense pulsed light in hyperchromic scarring after burns]. Rev Soc Bras Cir Plást. 2006;21:175-179. Portuguese.
13. Pungartnik C, Viau C, Picada J, Caldeira-de-Araujo A, Henriques JA, Brendel M. Genotoxicity of stannous chloride in yeast and bacteria. Mutat Res. 2005;583:146-157. http://dx.doi.org/10.1016/j.mrgentox.2005.03.003
14. Almeida MC, Soares SF, Abreu PR, Jesus LM, Brito LC, Bernardo-Filho M. Protective effect of an aqueous extract of Harpagophytum procumbens upon Escherichia coli strains submitted to the lethal action of stannous chloride. Cell Mol Biol (Noisy-le-grand). 2007;53(suppl):OL923-927.
15. Dantas FJ, De Mattos JC, Moraes MO, Viana ME, Lage CA, Cabral-Neto JB, et al. Genotoxic effects of stannous chloride (SnCl2) in K562 cell line. Food Chem Toxicol. 2002;40:1493-1498. http://dx.doi.org/10.1016/S0278-6915(02)00087-X
16. El-Demerdash FM, Yousef MI, Zoheir MA. Stannous chloride induces alterations in enzyme activities, lipid peroxidation and histopathology in male rabbit: Antioxidant role of vitamin C. Food Chem Toxicol. 2005;43:1743-1752. http://dx.doi.org/10.1016/j.fct.2005.05.017
17. Sambroock J, Fritsch EF, Maniatis T. Extraction and purification of plasmid DNA. In: Sambroock J, Fritsch EF, Maniatis T, editors. Molecular cloning. A laboratory manual. New York: Cold Spring Harbour Laboratory Press; 1989. [ Links ]
18. Meyer PF, Santos-Filho SD, Ronzio AO, Bonelli L, Fonseca AS, Costa ICC, et al. Consequences of the magnetic field, sonic and radiofrequency waves and intense pulsed light on the labeling of blood constituents with technetium-99m. Braz Arch Biol Technol. 2007;50(spe):117-122.
19. Meyer PF, Cavalcante JL, Ronzio AO, Silva RMV, Medeiros ML, Patricio RA, et al. Efeitos das Ondas Sönicas de Baixa Frequência no Fibro Edema Gelóide: Estudo de Caso [Effects of sonic waves of low frequency on fiber edema geloid: A case study]. Rev Bras Terap Saúde. 2011;1:31-36. Portuguese.
20. Gupta A, Taly AB, Srivastava A, Kumar S, Thyloth M. Efficacy of pulsed electromagnetic field therapy in healing of pressure ulcers: A randomized control trial. Neurol India. 2009;57:622-626. http://dx.doi.org/10.4103/0028-3886.57820
21. Graak V, Chaudhary S, Bal BS, Sandhu JS. Evaluation of the efficacy of pulsed electromagnetic field in the management of patients with diabetic polyneuropathy. Int J Diabetes Dev Ctries. 2009;29:56-61. http://dx.doi.org/10.4103/0973-3930.53121
22. Baptista AF, Goes BT, Menezes D, Gomes FC, Zugaib J, Stipursky J, et al. PEMF fails to enhance nerve regeneration after sciatic nerve crush lesion. J Peripher Nerv Syst. 2009;14:285-293. http://dx.doi.org/10.1111/j.1529-8027.2009.00240.x
23. Ciejka E, Goraca A, Michalska M, Kostka B. The effect of low magnetic field on select parameters of blood coagulation. Pol Merkur Lekarski. 2005;19:148-155.
24. Sieron A, Cieslar G. Application of variable magnetic fields in medicine -15 years experience. Wiad Lek. 2003;56:434-441.
25. Kulishova TV, Tabashnikova NA, Akker LV. Efficacy of general magnetotherapy in conservative therapy of uterine myoma in women of reproductive age. Vopr Kurortol Fizioter Lech Fiz Kult. 2005;1:26-28.
26. Loberg LI, Engdahl WR, Gauger JR, McCormick DL. Cell viability and growth in a battery of human breast cancer cell lines exposed to 60 Hz magnetic fields. Radiat Res. 2000;153:725-728. http://dx.doi.org/10.1667/0033-7587(2000)153[0725:CVAGIA]2.0.CO;2
27. Ubeda A, Hernandez-Bule ML, Trillo MA, Martinez Matilla J, Monteiro T, Leal J. Effects on primary human lymphocytes of in vitro exposure to CRET signals in a thermal condition. Phys Med Bio. 2001;38:1-12.
28. Ley-Valle A. Non-invasive intracranial hyperthermia using the capacitive electric transfer - CET: Intratumoral and cerebral thermometry results. Neurosurgery. 2003;14:41-45.
29. Lennart J. Nonthermal effects of therapeutic ultrasound: The frequency resonance hypothesis. J Athl Train. 2002;37:293-299.
30. Araújo M, Baptista-Silva JCC, Gomes PO, Novo NF, Juliano Y Efeitos do ultra-som de baixa intensidade na veia auricular de coelhos [Effects of ultrasound of low frequency on the auricular vein of rabbits]. Acta Cir Bras. 2003;18:132-137. Portuguese. http://dx.doi.org/10.1590/S0102-86502003000100006
31. Raulin C, Greve B, Grema H. IPL technology: A review. Lasers Surg Med. 2003;32:78-87. http://dx.doi.org/10.1002/lsm.10145
32. Mezzana P, Valeriani M. Rejuvenation of the aging face using fractional hotothermolysis and intense pulsed light: A new technique. Acta Chir Plast. 2007;49:47-50.
33. Madonna Terracina FS, Curinga G, Mazzocchi M, Onesti MG, Scuderi N. Utilization of intense pulsed light in the treatment of face and neck erythrosis. Acta Chir Plast. 2007;49:51-54.
34. Santos MAV Belo VG, Santos G. Effectiveness of photodynamic therapy with topical 5-aminolevulinic acid and intense pulsed light versus intense pulsed light alone in the treatment of acne vulgaris. Comp Study Dermatol Surg. 2005;31:910-915. http://dx.doi.org/10.1111/j.1524-4725.2005.31804
35. Gold M, Biron JA, Boring M, Bridges TM, Bradshaw V. Treatment of moderate to severe inflammatory acne vulgaris: Photodynamic therapy with 5-aminolevulinic acid and a novel advanced fluorescence technology pulsed light source. J Drugs Dermatol. 2007;20:319-322.
 Correspondence:
Correspondence:
Sebastião David Santos-Filho
Departamento de Biofísica e Biometria
Instituto de Biologia Roberto Alcantara Gomes
Universidade do Estado do Rio de Janeiro
Avenida 28 de Setembro, 87, Vila Isabel
20551-030, Rio de Janeiro, Brazil
sdavidsfilho@gmail.com
Received: 09 Jan. 2013
Accepted: 04 Feb. 2013














