Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Science
versão On-line ISSN 1996-7489
versão impressa ISSN 0038-2353
S. Afr. j. sci. vol.108 no.9-10 Pretoria Jan. 2012
SRESEARCH ARTICLE
Heavy metal contamination of vegetables cultivated in home gardens in the Eastern Cape
Callistus Bvenura; Anthony Jide Afolayan
Phytomedicine Research Centre, University of Fort Hare, Alice, South Africa
ABSTRACT
The accumulation of some essential (copper, manganese and zinc) and toxic metals (lead and cadmium) in cultivated vegetables - Brassica oleracea (cabbage), Daucus carota (carrot), Allium cepa (onion), Spinacia oleracea (spinach) and Solanum lycopersicum (tomato) - was examined. The vegetables were locally cultivated in home gardens in Alice, a small town in the Eastern Cape Province of South Africa. Samples of these vegetables were randomly collected from residential areas, dried, digested and analysed for the heavy metals using inductively coupled plasma optical emission spectrometry. The concentrations of heavy metals in the vegetables were in the range of 0.01 mg/kg - 1.12 mg/kg dry weight for cadmium, 0.92 mg/kg - 9.29 mg/kg for copper, 0.04 mg/kg - 373.38 mg/kg for manganese and 4.27 mg/kg - 89.88 mg/kg for zinc. Lead was undetectable in all the samples. Results of analysis of soils from the area revealed that cadmium in soil was in the range of 0.01 mg/kg - 0.08 mg/kg, copper levels were 4.95 mg/kg - 7.66 mg/kg, lead levels were 5.15 mg/kg - 14.01 mg/kg and zinc levels were 15.58 mg/kg - 53.01 mg/kg. The concentration of manganese was the highest of all the metals, ranging between 377.61 mg/kg and 499.68 mg/kg, at all three residential sites. Although the concentrations in soils and vegetables of the critical heavy metals, such as lead and cadmium, may not pose a threat (according to FAO/WHO standards), the concentration of manganese was very high in spinach and soils, whilst that of zinc exceeded safe levels in spinach, onions and tomatoes. However, neither the soils nor the vegetables were consistently found to pose a risk to human health.
Introduction
The ever-growing global concern over the release and subsequent deposition of heavy metals in soils cannot be overemphasised. In 1990, Alloway1 propounded that anthropogenic activities, such as mining, agriculture and industry, tend to release heavy metals into the soil, water and atmosphere; hence the release of heavy metals has often been linked with soils in urban areas, usually near industrial sites and agricultural lands. While justification for research has been fuelled by well-known sources of contamination, like industrial sites, automobiles in big towns and cities and phosphate fertilisers in agricultural lands, little is known regarding heavy metal contamination in home gardens, especially in rural areas and small towns. Less attention has been focused on the possible accumulation of heavy metals in small home gardens where most families in rural areas and small towns cultivate various crops to sell to supplement their incomes, as well as to eat to fortify their diets. Both organic and inorganic fertilisers, as well as agrochemicals, are applied in some of these gardens.
In 1987, Zurera et al.2 proposed that a possible source of heavy metals in home gardens was residual irrigation water. In 1996, Mortvedt3 suggested that organic and inorganic fertilisers were a source of heavy metals and, more recently, Kabata-Pendias4 suggested agrochemicals. Some authors have suggested that paints containing heavy metals are often washed off the walls by rain and are thus an added source of heavy metals in gardens.5 Heavy metals may also be present in livestock feeds and in water as trace elements for the maintenance of health or as growth promoters. The majority of the heavy metals in feeds consumed by livestock is excreted in the faeces or urine and will be present in manure that is subsequently applied to the land, leading to the uptake of heavy metals by plants, through various processes.6
Alice is a small town in a predominantly rural area in South Africa. Preliminary surveys have shown that some inhabitants of Alice, who have home gardens, improve the fertility of their gardens by adding compost or animal manure obtained from cattle, goats, chicken and pigs, whilst others apply inorganic fertilisers. Some homes also have cattle kraals and pigsties near their gardens, thereby providing a ready and steady source of manure. Agrochemicals are also occasionally used in some gardens to combat pests and diseases. Some of the homes and gardens are located close to roads. Automobiles can also be a source of heavy metal contamination.
The possibility of heavy metal contamination in vegetables in home gardens in Alice, a small town in a predominantly rural area in South Africa, was investigated. The heavy metals investigated were: cadmium (Cd), copper (Cu), lead (Pb), manganese (Mn) and zinc (Zn). Although some of these are essential, all five of these heavy metals are toxic to humans, even at relatively low levels.7,8,9,10,11,12,13 The vegetables analysed were cabbage (Daucus carota), carrots (Daucus carota), onions (Allium cepa), tomatoes (Solanum lycopersicum) and spinach (Spinacia oleracea); these vegetables were selected because they are cultivated in most of the gardens in Alice. Leafy vegetables reportedly accumulate higher amounts of heavy metals because they absorb these metals into their leaves.14 To assess the potential of the heavy metals to pose a health hazard, the absolute amounts of each heavy metal in the vegetables and soils were compared to the amount of the heavy metal known by FAO15 and India16 to cause negative effects on human health.17
Materials and methods
Study area
Alice is a small town located at 32°42'S, 26°50'E in the Eastern Cape Province of South Africa. The town is about 20 km to the east of Fort Beaufort in the Nkonkobe Municipality. Ntselamanzi, Golf Course, Happy Rest and the University of Fort Hare are residential areas that surround the business centre of Alice. These areas are located on the upper hilly slopes where the soils are shallow and of low cropping potential. Van Averbeke and Marais18 found that the parent material of Alice consists mainly of mudstone and dolerite, which are made up of fine sand and silt but contain significant amounts of clay. According to Van Averbeke and Marais18, the sand fraction in the lower slopes consists of quartz and lesser amounts of plagioclase rock fragments, iron and manganese oxides. The area is generally semi-arid, as observed by Marais and Brutsch19, with a mean annual rainfall of approximately 575 mm. In summer, mean daily temperatures are approximately 22.5 °C during the day and 18.0 °C at night, whilst in winter, temperatures are about 13.6 °C during the day and 10.3 °C at night.
Sample collection and preparation
The procedure for sample collection and preparation described by Okalebo et al.20 was followed. Five types of vegetables were bought from home gardens in three residential areas in Alice: Ntselamanzi, Golf Course and Happy Rest. Each vegetable was collected from four different gardens per sampling site. The vegetables were harvested from each corner of the plots and also from the middle of the plots. Table 1 shows the number of samples that were collected from each of the three sites. The number of samples of each vegetable collected depended on the availability of the vegetable per sampling site. Samples from each site were combined to make a composite sample of each vegetable per site. The vegetables were washed first with tap water and then with deionised water to remove any possible foliar contaminants, such as pesticides, fertilisers, dust or mud. The vegetables were then cut into small pieces using a stainless steel knife and oven dried to remove moisture. Oven drying was performed in a dust-free, forced-draft oven at 65 °C to a constant mass. The dried tissue was stored in a moisture-free atmosphere prior to further processing. The samples were then ground using a ceramic mortar and pestle to reduce the dried material to a suitable size for digestion and analysis.
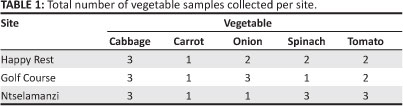
Soil samples were collected from the same locations as the vegetables. Soil samples from the same site were mixed to form a composite sample; these samples were air dried to remove moisture, after which they were sieved through a 2-mm sieve.
Digestion and analysis
The method described by Okalebo et al.20 was used for the digestion of plant material. Selenium powder, sulphuric acid and salicylic acid were the reagents used for digestion. The finely ground material was divided into samples of 0.3 g, which were placed in dry, clean digestion tubes. A volume of 2.5 mL of the digestion mixture was added to each tube and allowed to react at room temperature for 2 h. The tubes were heated in a block digester at 110 °C for 60 min. After removal from the digester, the tubes were allowed to cool and three successive portions of 1 mL hydrogen peroxide were added, allowing at least 10 s between additions because of the volatility of the reaction. The tubes were returned to the block digester at a temperature of 330 °C and were removed from the block digester when the digest was colourless. The tubes were allowed to cool to room temperature before their contents were transferred to 50-mL volumetric flasks and deionised water was added to attain volumes of 50 mL. Standards were prepared for all the elements. The samples were then analysed for the various elements by an inductively coupled plasmaZ optical emission spectrometer (ICP OES; Varian 710-ES series, SMM Instruments, Cape Town, South Africa).
A mass of 5 g of sieved soil was transferred to clean 250-mL plastic bottles fitted with air-tight screw caps and 50 mL of 1% EDTA solution was added. The suspension was mixed using a reciprocating shaker for 1 h and filtered using Whatman filter paper number 542, after which the sample was analysed for heavy metals using the ICP OES.
Data conversion
The heavy metal contents obtained from the ICP OES analysis in mg/L were converted into mg/kg using Temminghoff and Houba's21 formula:
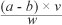 where a is the concentration of the heavy metal in the sample (mg/L); b is the concentration of the heavy metal in the blank (mg/L); ν is the total volume of digest (mL) and w is the weight of the plant material (g).
where a is the concentration of the heavy metal in the sample (mg/L); b is the concentration of the heavy metal in the blank (mg/L); ν is the total volume of digest (mL) and w is the weight of the plant material (g).
Statistical analysis
Where applicable, the data were subjected to statistical analysis using MINITAB Release 12.22 A one-way analysis of variance was used to compare concentrations of heavy metals between vegetable types. Means were compared using Duncan's multiple range test. The means were treated as significantly different atp < 0.05.
Results and discussion
Heavy metals in soils
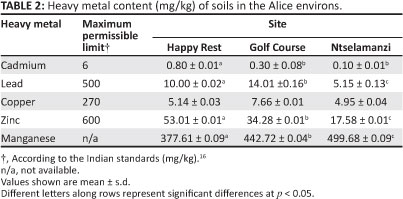
The mean values of extractable Cu, Cd, Mn, Pb and Zn in the soils were variable (Table 2). Of the five heavy metals, the concentration of Mn was the highest, followed by Zn, Pb, Cu and Cd. All concentrations were found to be below the maximum levels permitted for soils in India.16
Manganese
The mean concentration of Mn in soil in all the areas was 440 mg/kg. This concentration is within the range generally accepted globally, that is, between 20 mg/kg and 10 000 mg/kg and is below the global average for soil of 1000 mg/kg.14
Lead
The mean concentration of Pb in the soil was 9.72 mg/kg. This concentration was higher than values reported for Saudi Arabia,23 where a range of between 0.11 mg/kg and 2.59 mg/kg was reported, but lower than that in the Pearl River Delta of China24 where values of up to 40.00 mg/kg have been reported. Pb is present in undisturbed soils at levels ranging from less than 1 mg/kg to well over 10% in ore materials.1
Cadmium
The mean concentration of Cd in the soil was 0.40 mg/kg. The Cd composition of the soil in Alice is similar to that reported by others around the world. A study done in Harare, Zimbabwe25 revealed that while Cd ranged between 0.50 mg/kg and 2.90 mg/kg, concentrations of the element still remained below the FAO/WHO15 maximum permissible limits. In Saudi Arabia,23 it was observed that Cd ranged between 0.05 mg/kg and 0.87 mg/kg - values which are not very different from those obtained in Alice. In the Pearl River Delta in South China,24 values of Cd in soil ranged from 0.34 mg/kg in paddy soils to 0.58 mg/kg in crop soils. The global average concentration of Cd in unpolluted soils is between 0.01 mg/kg and 2.00 mg/kg, as reported by Adriano13.
Zinc
The mean concentration of Zn in the soil was 34.95 mg/kg. This concentration compares well with values reported from other countries, such as Zimbabwe25 and South China,24 but is higher than those reported from Saudi Arabia23 and India.26 The concentration of Zn in unpolluted soils globally is expected to be between 10 mg/kg and 300 mg/kg, while the mean concentration is expected to be about 40 mg/kg.13
Copper
The mean concentration of Cu in the soil was 5.91 mg/kg. The Cu content of soils obtained from Alice is similar to those in Saudi Arabia23 and India,26 but is much lower than those reported from the Pearl River Delta in South China.24 In western Delhi, India,27 it was observed that the Cu content of soils that were treated with sewage water ranged from 0.49 mg/kg to 32.30 mg/kg - a range which is higher than that obtained for Alice. The average Cu content of soils in the USA is 25 mg/kg and that in Canada is 22 mg/kg, whilst, in general, arable soils contain between 1 mg/kg and 50 mg/kg of Cu.1
Origin of heavy metals in the soil
The parent material of soils in Alice consists of igneous rocks which are rich in iron (Fe) and Mn oxides,18 which may be a major contributing factor in the abundance of Mn in the soil. The area is also in a semi-arid region and Mn is known to be abundant in such regions.4 Pb in the Alice environs is assumed to be largely from geological origins, as well as from some minor contaminants which include leaded paint that peels off walls and is washed into gardens when it rains and exhaust fumes from automobiles. Although most soils are expected to contain less than 1 mg/kg of Cd, those contaminated by anthropogenic activities or those developed on parent materials with enormously high contents of the metal, will contain greater concentrations.1,13 In this study, the low concentrations of Cd corresponded with those obtained from the vegetable samples harvested from the same soils. It can therefore be concluded that the concentration of Cd in the soils can be attributed largely to the parent material of the soil. By comparing the expected concentration of Zn in unpolluted soils with that obtained in the soils in Alice, one can conclude that the Zn in the soils in Alice is most likely of geological origin, although organic waste, especially from livestock, phosphate fertilisers and other minor contaminants, may also have contributed. The low levels of Cu in Alice home gardens can be largely attributed to the parent material of the soil and, to a lesser extent, the use of pesticides, herbicides, organic and inorganic fertilisers, and crop residues.
Heavy metals in vegetables
The total concentrations of heavy metals in the cabbage, carrot, onion, spinach and tomato samples from Happy Rest, Golf Course and Ntselamanzi are shown in Table 3. The results pertaining to Pb are omitted because Pb was below the instrumental detection limit in all vegetable samples from all three sites. All the heavy metals found in cabbage were below both the Indian16 and FAO/WHO15 maximum permissible limits. However, because no permissible limits were available for Mn, levels suggested by Kabata-Pendias4 were used for Mn and it was found that cabbage obtained from Happy Rest, as well as spinach harvested from Happy Rest and Ntselamanzi, contained phytotoxic levels of Mn. The levels of Cd in carrots, spinach and tomatoes were lower than the Indian16 maximum permissible limits, but exceeded the FAO/WHO15 maximum permissible limits in carrots from all three sites, spinach from Ntselamanzi and tomatoes from Happy Rest and Golf Course. Concentrations of Zn found in onions, spinach and tomatoes from Happy Rest and Ntselamanzi were above both the Indian16 and FAO/WHO15 maximum permissible limits. Cu levels in all the vegetables were below both the Indian16 and FAO/WHO15 maximum permissible limits.
Cabbage
The mean concentrations of heavy metals in cabbage decreased in the order of Mn (28.85 mg/kg) > Zn (27.38 mg/kg) > Cu (0.62 mg/kg) > Cd (0.24 mg/kg) > Pb (below limit of detection). In Poland, heavy metals absorbed by cabbage decreased in the following order: Mn > Zn > Pb > Cu > Cd.28 These results are similar to those obtained in this study, namely, a low accumulation of Cd and Cu and a high accumulation of Mn and Zn.
Carrots
The mean concentrations of heavy metals in carrots from all three sites decreased in the order of: Mn (12.32 mg/kg) > Zn (9.94 mg/kg) > Cu (3.08 mg/kg) > Cd (0.96 mg/kg) > Pb (below detection limit). Alexander et al.29 reported in 2006 that carrots absorbed some selected heavy metals in the order: Zn > Pb > Cu > Cd. The order of Zn, Cu and Cd reported by them is as for that observed in this study. The concentration of Cd in carrots was consistently high in all three sites, compared with the concentrations of Cd in the other vegetables. However, the highest mean concentration of Cd was found in spinach.
Spinach
The mean concentrations of heavy metals in spinach decreased in the order of: Mn (108.03 mg/kg) > Zn (46.86 mg/kg) > Cu (6.87 mg/kg) > Cd (0.53 mg/kg) > Pb (below limit of detection). An order similar to the one observed in Alice was observed in the United Kingdom29: Intawongse and Dean30 reported that the uptake of Cd, Cu, Mn and Zn by plants corresponded to an increasing level of soil contamination, while the uptake of Pb was low. They further reported that spinach accumulated a high content of Mn and Zn, but low levels of Cu and Pb; these results are not dissimilar to those obtained in this study. In studies conducted in the peri-urban area of Delhi in India, Singh and Kumar26 found that Cu in spinach ranged from 7 mg/kg to 50 mg/kg, Zn from 51 mg/kg to 282 mg/kg, Pb from 1.4 mg/kg to 9.0 mg/kg and Cd from 1.7 mg/kg to 9.2 mg/kg. Studies conducted in Kano State, Nigeria by Lawal and Audu31 revealed that levels of Cu, Pb and Zn in spinach that was irrigated with wastewater were below the maximum permissible limits of the National Agency for Food and Drug Administration and Control of Nigeria. Kudirat and Funmilayo32 also found that levels of Cd and Zn in a leafy vegetable sold in 10 markets in Lagos, Nigeria were below the maximum permissible limits according to Nigerian standards.
Onions
Onions generally accumulated the lowest concentrations of the heavy metals analysed. The descending order of mean concentrations of heavy metals in onions was: Zn (61.97 mg/kg) > Mn (27.09 mg/kg) > Cu (8.70 mg/kg) > Cd (0.20 mg/kg) > Pb (below detection limit). The concentrations of heavy metals in vegetables from Alice were higher than those obtained in Saudi Arabia except for Cd.23 However, the results were lower than those obtained in Turkey in 2005 by Demirezen and Aksoy33. Alexander et al.29 observed the following order of magnitude in heavy metal content in onions: Zn > Cu > Pb > Cd. Audu and Lawal34 recorded concentrations in onions of 0.53 mg/kg - 0.95 mg/kg for Pb, 1.45 mg/kg - 1.98 mg/kg for Mn, 4.20 mg/kg - 7.50 mg/kg for Cu and 10.33 mg/kg - 18.89 mg/kg for Zn. Their results are similar to those obtained from Alice for Cu and Pb, although their concentrations of Zn and Mn were much lower.
Tomatoes
The mean concentrations of heavy metals in tomatoes cultivated in Alice decreased in the order of: Zn (28.82 mg/kg) > Mn (16.02 mg/kg) > Cu (7.46 mg/kg) > Cd (0.31 mg/kg) > Pb (below limit of detection). Research in Nigeria showed that Zn, Cu and Pb levels in tomatoes were all below the FAO maximum permissible limits15; these levels, however, were lower than those obtained in this study for tomatoes grown in Alice. In a study conducted in the rural areas of Turkey, by Demirezen and Aksoy33, the Cd, Pb, Cu and Zn levels in tomatoes were all higher than those detected in Alice: the concentration of Zn was 115.97 mg/kg whilst that of Cu was 37.50 mg/kg. The values obtained in the Turkish study were also much higher than those reported in Brazil by Dias et al.35, in which the concentration of Zn was between 38.60 mg/kg and 42.20 mg/kg, Cu was between 12.80 mg/kg and 19.00 mg/kg and Pb was between 12.00 mg/kg and 16.60 mg/kg. In Saudi Arabia,23 the concentrations of heavy metals in tomatoes were 0.77 mg/kg for Cd, 4.47 mg/kg for Cu, 7.39 mg/kg for Mn, 2.59 mg/kg for Pb and 9.60 mg/kg for Zn. The results of heavy metal contamination in tomatoes in Alice are generally similar to those obtained in Brazil35 and Saudi Arabia.23
Origin of heavy metals in vegetables
Finster et al.36 discovered that the concentration of Pb was higher in the roots than in the shoots of cabbage growing on lead-contaminated garden soils. A maximum of 612 mg/kg was recorded in the soils, yet the concentration of Pb in the shoots of the cabbage was less than 10 mg/kg. Adriano13 observed that a high percentage of the Pb deposited on the surface of vegetation can be removed by washing the vegetation with water. He further noted that as much as 80% or more Pb was washed off from grass by water. Removing the outer layers of the cabbage leaves and washing the remaining edible parts prior to analysis for heavy metal content could have removed aerosol Pb, thereby leaving only that which was absorbed by the roots - which in this case was minimal and therefore undetectable. However, it is common practice in households to remove several outer layers of cabbage leaves and to clean the remaining edible parts prior to cooking for hygiene reasons. It is therefore unlikely that cabbage from home gardens that is eaten in these households would contain aerosol Pb. In addition, Wild37 has proposed that Pb in the soil is not absorbed by vegetables because it is not readily soluble in soils.
In a Canadian study, John38 observed that the shoots of Brassica spp. accumulated more Cd than did carrots, but less than that absorbed by spinach. In another study, Koeppe39 discovered that carrot roots contained at least twice the Cd content found in shoots of corn, soybeans and other agronomic crops, which could explain why carrots in Alice consistently showed higher levels of Cd than the other vegetables.
High levels of Mn (between 46.66 mg/kg and 165.06 mg/kg) observed in spinach and cabbage reveal a high uptake of Mn, beyond toxic levels, by these plants,13 although these levels may not necessarily be toxic to humans. Results of soil analysis show high levels of Mn, ranging between 377.61 mg/kg and 499.68 mg/kg, which are comparable with the high concentrations found in spinach. Mn usually accumulates on top soils as a result of its fixation by organic matter. It may thus be expected that during the summer season, the relatively high decomposition rate of organic matter is likely to release Mn into the soil for possible uptake by plants.40 Mn is also very abundant in the Alice area as a result of the geological origins of the soil.18 Furthermore, South Africa possesses the world's largest known Mn ores.41 In general, where there was a high concentration of Mn in the soil in Alice, there was a tendency to have a greater accumulation of Mn in the vegetables; this trend was specifically apparent for Mn in spinach.
The high concentrations of Zn and Cu in soils in Brazil, reported by Dias et al.35 were attributed to agricultural products that are added to the soil. In Alice, although Zn was above the WHO/FAO15 maximum permissible limits in onions and spinach from Ntselamanzi and in tomatoes from Happy Rest, the concentration of Zn in the soil was lower than the maximum permissible limit. Golf Course is a relatively newly constructed area compared with Ntselamanzi and Happy Rest, suggesting that a continuous deposition of Zn for many years, from materials such as roofing material, from organic and inorganic fertilisers, and from residual irrigation waters, in these suburbs may have contributed to the consistently higher amount of Zn in the soil, which is in turn available for uptake by the plants. Generally, results from analysis of the vegetables in comparison with the soils, show that the uptake of heavy metals by the vegetables corresponds to increasing levels of soil contamination.
Conclusions
Results of this study suggest that there may not be a significant threat posed by heavy metal contamination of vegetables and soils in the home gardens of Alice. The contributions of automobiles in the area which could aid in the deposition of heavy metals, residual waters that are used for irrigation and other sources of heavy metals such as leaded paint, animal manure, compost, inorganic fertilisers, pesticides and herbicides, do not pose a real threat with respect to the contamination of the vegetables and soils by heavy metals. The mean concentrations of heavy metals in the soils are too low to warrant a build-up to toxic levels because their application is mostly moderate in volume. However, no matter how low the levels of heavy metals in the environment are, their presence is not desirable. The challenge is to determine the minimum amount of each source of contamination that will result in the accumulation of heavy metals in soils beyond acceptable limits. High levels of Zn and Mn in some of the vegetable and soil samples may be linked largely to the parent material of the soils in the area, therefore making their absorption by the plants inevitable. However, some vegetables will not readily absorb some elements no matter how abundant they are in the soil whilst some elements may also inhibit the uptake of other elements.
Acknowledgements
This research was funded by the Government of Zimbabwe Presidential Scholarship programme in collaboration with the Govan Mbeki Research and Development Centre of the University of Fort Hare.
Competing interests
We declare that we have no financial or personal relationships which may have inappropriately influenced us in writing this paper.
Authors' contributions
C.B. collected the vegetable and soil samples from the three sites, performed all the experiments, analysed the data and wrote the manuscript. A.J.A. was the project leader and supervised the study, proofread the manuscript and sourced the funding.
References
1. Alloway BJ. Heavy metals in soils. 1st ed. London: Blackie and Son Limited; 1990. [ Links ]
2. Zurera G, Estrada B, Rincon F, Pozo R. Lead and cadmium contamination levels in edible vegetables. Bull Environ Contam Toxicol. 1987;38:805-812. http://dx.doi.org/10.1007/BF01616705 [ Links ]
3. Mortvedt JJ. Heavy metal contaminants in inorganic fertilizers. Fert Res. 1996;43:55-61. http://dx.doi.org/10.1007/BF00747683 [ Links ]
4. Kabata-Pendias A. Trace elements in soils and plants. 3rd ed. Boca Racon, FL: CRC Press LLC; 2001. [ Links ]
5. Romieu I, Palazuelos RE, Avila MH, et al. Sources of lead exposure in Mexico City. Environ Health Perspect. 1994;102(4):384-389. http://dx.doi.org/10.1289/ehp.94102384 [ Links ]
6. Nicholson FA, Smith SR, Alloway BJ, Smith-Carlton C, Chambers BJ. An inventory of heavy metals inputs to agricultural soils in England and Wales. Sci Total Environ. 2003;311(1-3):205-219. http://dx.doi.org/10.1016/S0048-9697(03)00139-6 [ Links ]
7. Mejare M, Bulow L. Metal-binding proteins and peptides in bioremediation and phytoremediation of heavy metals. Trends Biotechnol. 2001;19(2):67-73. http://dx.doi.org/10.1016/S0167-7799(00)01534-1 [ Links ]
8. Okoye COB, Ugwu JN. Impact of environmental cadmium, lead, copper and zinc on quality of goat meat in Nigeria. Ethiopia Bull Chem Soc. 2010;24(1):133-138. [ Links ]
9. Bell RW, Dell B. Micronutrients for sustainable food, feed, fiber and bioenergy production. 1st ed. Paris: International Fertilizer Industry Association; 2008. [ Links ]
10. Gaetke LM, Chow KC. Copper toxicity, oxidative stress and antioxidant nutrients. Toxicology. 2003;189:141-163. http://dx.doi.org/10.1016/S0300-483X(03)00159-8 [ Links ]
11. Aschner LJ, Aschner M. Nutritional aspects of manganese homeostasis. Mol Asp Med. 2005;26(4-5):353-362. http://dx.doi.org/10.1016/j.mam.2005.07.003 [ Links ]
12. Lenntech Water Treatment Purification. Manganese [homepage on the Internet]. No date [accessed 2010 June]. Available from: http://www.lenntech.com/periodic/elements/mn.htm [ Links ]
13. Adriano DC. Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metals. 2nd ed. New York: Springer Verlag; 2001. [ Links ]
14. Sharma VK, Kansal BD. Heavy metal contamination of soils and plants with sewage irrigation. Pollution. 1986;4:86-91. [ Links ]
15. Codex Alimentarius. Maximum levels for cadmium in cereals, pulses and legumes: Joint FAO/WHO standards, CAC/GL 39. c2001 [cited 2009 June]. Available from: http://img.21food.cn/img/biaozhun/20100729/180/11294167.pdf [ Links ]
16. Awashthi SK. Prevention of Food Adulteration Act no 37 of 1954. Central and state rules as amended for 1999. 3rd ed. New Delhi: Ashoka Law House; 2000. [ Links ]
17. Sinha S, Gupta AK, Bhatt K, Pandey K, Rai UN, Singh KP. Distribution of metals in the edible plants grown at Jajmau, Kanpur (India) receiving treated tannery wastewater: Relation with physico-chemical properties of the soil. Environ Monit Assess. 2006;115:1-22. http://dx.doi.org/10.1007/s10661-006-5036-z [ Links ]
18. Van Averbeke W, Marais JN. An evaluation of Ciskeian ecotopes for rainfed cropping. Final Report. Alice: Agricultural and Rural Development Research Institute, University of Fort Hare; 1991. [ Links ]
19. Marais JN, Brutsch MO. The Ehlers system of assessing the suitability of temperature regime of a region for crop production. Paper presented at SASHS. Proceedings of SASHS; 1994 Jan 21-25; Nelspruit, South Africa. [ Links ]
20. Okalebo JR, Gathua KW, Woomer PL. Laboratory methods of soil and plant analysis: A working manual. 2nd ed. Nairobi: TSBF-CIAT and SACRED Africa; 2002. [ Links ]
21. Temminghoff EJM, Houba VJG. Plant analysis procedures. 2nd ed. Dordrecht: Kluwer Academic Publishers; 2004. [ Links ]
22. MINITAB. Version WINSV12.11. State College, PA: MINITAB Inc.; 1998. [ Links ]
23. Mohamed AE, Rashed MN, Mofty A. Assessment of essential and toxic elements in some kinds of vegetables. Ecotoxicol Environ Saf. 2003;55:251-260. http://dx.doi.org/10.1016/S0147-6513(03)00026-5 [ Links ]
24. Wong SC, Li XD, Zhang G, Qi SH, Min YS. Heavy metals in agricultural soils of the Pearl River Delta, South China. Environ Pollut. 2002;119(1):33-44. http://dx.doi.org/10.1016/S0269-7491(01)00325-6 [ Links ]
25. Mapanda F, Mangwayama EN, Nyamangara J, Giller KE. The effect of longterm irrigation using wastewater on heavy metal contents of soils under vegetables in Harare, Zimbabwe. Agric Ecosyst Environ. 2005;107:151-165. http://dx.doi.org/10.1016/j.agee.2004.11.005 [ Links ]
26. Singh S, Kumar M. Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ Monit Assess. 2006;120:79-91. http://dx.doi.org/10.1007/s10661-005-9050-3 [ Links ]
27. Rattan RK, Datta SP, Chhonkar PK, Suribabu K, Singh AK. Long-term impact of irrigation with sewage effluents on heavy metal content in soils, crops and groundwater: A case study. Agric Ecosys Environ. 2005;109:310-322. http://dx.doi.org/10.1016/j.agee.2005.02.025 [ Links ]
28. Ciura J, Poniedzialek M, Sekara A, Jedrszczyk E. The possibility of using crops as metal phytoremediants. Polish J Environ Stud. 2005;14(1):17-22. [ Links ]
29. Alexander PD, Alloway BJ, Dourado AM. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut. 2006;144(3):736-745. http://dx.doi.org/10.1016/j.envpol.2006.03.001 [ Links ]
30. Intawongse M, Dean JR. Uptake of heavy metals by vegetable plants grown on contaminated soil and their bioavailability in the human gastrointestinal tract. Food Addit Contam. 2006;23(1):36-48. http://dx.doi.org/10.1080/02652030500387554 [ Links ]
31. Lawal AO, Audu AA. Analysis of heavy metals found in vegetables from cultivated irrigated gardens in the Kano metropolis, Nigeria. J Environ Chem Ecotoxicol. 2011;3(6):142-148. [ Links ]
32. Kudirat LM, Funmilayo DV. Heavy metal levels in vegetables from selected markets in Lagos, Nigeria. Affr J Food Sci Technol. 2011;2(1):18-21. [ Links ]
33. Demirezen D, Aksoy A. Heavy metal levels in vegetables in Turkey are within safe limits for copper, zinc, neon and exceeded for cadmium and lead. J Food Qual. 2006;29:252-265. http://dx.doi.org/10.1111/j.1745-4557.2006.00072.x [ Links ]
34. Audu AA, Lawal AO. Variation in metal contents of plants in vegetable garden sites in Kano Metropolis. J Appl Sci Environ Manage. 2006;10:105-109. [ Links ]
35. Dias CMF, Souza CMM, Monnerat PH. Distribution of heavy metals in agricultural soils from a rural area in Rio de Janeiro state, Brazil [homepage on the Internet]. No date [cited 2010 March]. Available from: http://www.cprm.gov.br/pgagem/Manuscripts/diasc.htm [ Links ]
36. Finster ME, Gray KA, Binns HJ. Lead levels of edibles grown in contaminated residential soils: A field survey. Sci Total Environ. 2003;320(2-3):245-257. http://dx.doi.org/10.1016/j.scitotenv.2003.08.009 [ Links ]
37. Wild A. Soils and the environment. An introduction. Cambridge: Cambridge University Press; 1993. [ Links ]
38. John MK. Cadmium uptake by eight food crops as influenced by various soil levels of cadmium. Environ Pollut. 1973;4(1):7-15. http://dx.doi.org/10.1016/0013-9327(73)90026-8 [ Links ]
39. Koeppe DE. The uptake, distribution and effect of cadmium and lead in plants. Sci Total Environ. 1977;7(3):197-206. http://dx.doi.org/10.1016/0048-9697(77)90043-2 [ Links ]
40. McGrath SP, Chang AC, Page AL, Wilter E. Land application of sewage sludge: Scientific perspectives of heavy metal loading limits in Europe and the United States. Environ Rev. 1994;2:108-118. http://dx.doi.org/10.1139/a94-006 [ Links ]
41. Barcza NA, Featherstone RA, Finn CWP. Recent developments in the ferro-alloy field in South Africa [document on the Internet]. c1982 [cited 2010 June]. Available from: http://www.mintek.co.za/Pyromet/Files/1982Barcza.pdf [ Links ]
 Correspondence to:
Correspondence to:
Anthony Afolayan
Private Bag X1314
Alice 5700, South Africa
Email: aafolayan@ufh.ac.za
Received: 08 Apr. 2011
Accepted: 18 Mar. 2012
Published: 07 Sept. 2012
© 2012. The Authors. Licensee: AOSIS OpenJournals. This work is licensed under the Creative Commons Attribution License.














