Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Science
versión On-line ISSN 1996-7489
versión impresa ISSN 0038-2353
S. Afr. j. sci. vol.106 no.9-10 Pretoria sep./oct. 2010
RESEARCH ARTICLE
Isolation and identification of iron ore-solubilising fungus
Rasheed AdelekeI; Eugene CloeteII; Damase KhasaIII
IDepartment of Microbiology and Plant Pathology, University of Pretoria, South Africa
IIDepartment of Microbiology, Stellenbosch University, South Africa
IIICentre d'étude de la forêt, Universite Laval, Canada
ABSTRACT
Potential mineral-solubilising fungi were successfully isolated from the surfaces of iron ore minerals. Four isolates were obtained and identified by molecular and phylogenetic methods as close relatives of three different genera, namely Penicillium (for isolate FO), Alternaria (for isolates SFC2 and KFC1) and Epicoccum (for isolate SFC2B). The use of tricalcium phosphate (Ca3(PO4)2) in phosphate-solubilising experiments confirmed isolate FO as the only phosphate solubiliser among the isolated fungi. The bioleaching capabilities of both the fungus and its spent liquid medium were tested and compared using two types of iron ore materials, conglomerate and shale, from the Sishen Iron Ore Mine as sources of potassium (K) and phosphorus (P). The spent liquid medium removed more K (a maximum of 32.94% removal, from conglomerate), than the fungus (a maximum of 21.36% removal, from shale). However, the fungus removed more P (a maximum of 58.33% removal, from conglomerate) than the spent liquid medium (a maximum of 29.25% removal, from conglomerate). The results also indicated a potential relationship between the removal of K or P and the production of organic acids by the fungus. A high production of gluconic acid could be related to the ability of the fungus to reduce K and P. Acetic, citric and maleic acids were also produced by the fungus, but in lower quantities. In addition, particle size and iron ore type were also shown to have significant effects on the removal of potassium and phosphorus from the iron ore minerals. We therefore conclude that the spent liquid medium from the fungal isolate FO can potentially be used for biobeneficiation of iron ore minerals.
Keywords: biohydrometallurgy; fungi; iron ore; organic acids; particle size
INTRODUCTION
One of the consequences of global technological advancement is the fast depletion rate of valuable minerals, which are becoming increasingly difficult to find in their pure forms. For example, in iron ore materials, nutrients such as potassium (K) and phosphorus (P), which are naturally beneficial to living organisms, can be a hazard when they occur in excess (for example, when potassium oxide [K2O] exceeds 0.24% and P exceeds 0.03%).1,2,3,4 The hazardous nature of their presence arises because they interfere in the operation of the blast furnace, eventually reducing the strength and ductility of the iron ore materials. Therefore, iron ore minerals containing low concentrations of these elements are prized. There is therefore greater interest in technologies that use microorganisms to mobilise or remove unwanted nutrients from valuable minerals. Such technologies, collectively referred to as biohydrometallurgy, are positively acknowledged for their environmental and economic advantages5,6 and could be utilised in extraction and purification of different minerals during and after mining operations.2,7,8
A common characteristic of potential microbial agents for the mobilisation of nutrients from minerals is the production of metabolites that contain organic acids, which could aid the solubilisation of hard and complex mineral materials.9,10,11 These organic acids are low-molecular weight carbon compounds that are capable of forming complexes with various minerals.9,12,13 The actions of these organic acids can occur in two different ways. The first method involves direct mineral attack by the metal-complexing organic acid anions as well as protons.9 Because of the high chelation constants of [Al (C2O4)3]3-, 2. 0 × 1016, and [Fe (C2O4)3]3-, 3. 9 × 1016, mineral sites containing aluminium and iron are easy targets for attack by organic acid anions.4,14 Secondly, there is a structural similarity between protons from organic acids and K from minerals such as muscovite - both are monovalent - but the size of the protons (0. 32 × 10-10 m) is much smaller than that of K (2.03 × 10-10 m)14,15 and so they are able to replace interlayer K, which is found in some minerals such as the iron ores investigated in this study.
Therefore, an important screening process for bioleaching microbes involves the direct or indirect evaluation of the ability of the microbes to produce organic acids. Investigators are beginning to acknowledge the importance of organic acids during the utilisation of indigenous microorganisms to leach elements from minerals. Delvasto et al.4 used Burkholderia caribensis FeGL03, isolated from iron ore samples, to reduce the P content of the iron ore by up to 20%. In their investigation, high production of gluconic acid by the bacterium was linked to its phosphate-solubilising ability. In another study, Williams16 used citric acid produced by Aspergillusniger to reduce the K in Sishen iron ore by 17.65%, but there was no reduction of P.
In this study, using an indirect approach, fungal isolates indigenous to iron ore were tested for their phosphate-solubilising activities as indicators of their organic acid production.10,11 The potential of the fungus and that of the spent liquid medium (used to grow the fungus) to remove K and P from iron ore samples were evaluated and compared.
MATERIALS AND METHODS
Two different types of iron ore samples, namely conglomerate (KGT) and shale (SK), were obtained from Sishen Iron Ore Mine in the Northern Cape Province of South Africa. The inductively-coupled plasma (ICP) analyses of four samples of each of these iron ore types revealed that the conglomerate originally contained an average of 0.81% K2O and 0.14% P, whereas shale had an average of 0.42% K2O and 0.09% P. In addition, ICP revealed the chemical composition, based on an average of the four samples, of shale to be: SiO2 (32.7%), Al2O3 (3.84%) and Fe2O3 (63.1%) with trace amounts of TiO2, CaO, MgO, NaCr2O3, NiO, V2O5 and ZrO2. That for the conglomerate was: SiO2 (5.01%), Al2O3 (3.61%) and Fe2O3 (90.20%) with trace amounts of TiO2, CaO, MgO, Na2O, MnO, Cr2O3, NiO, V2O5 and ZrO2.
The iron ore materials were milled and separated into two different particle size ranges, namely 0.22 mm - 0.84 mm (particle size A) and 0.1 mm - 0.21 mm (particle size B), by sieving. Separation according to size was followed by treatment of the iron ore materials with 0.1 M hydrochloric acid (HCl) for 24 h to remove exchangeable bases. The samples were then washed repeatedly with distilled water and adjusted to a pH of 4. The iron ore samples were then shaken at 100 rpm for 24 h and dried at 60 ºC. The treated samples were analysed again using ICP to assess any possible change in their K and P contents. Thereafter, the changes obtained were negligible when compared to the initial K and P contents of the iron ore minerals. The dried samples were used in the leaching experiment as sources of K and P.
Preparation of media and isolation of fungi from iron ore samples
Two common fungal growth media were used for the initial isolation of the fungi, namely potato dextrose agar (PDA) (Biolab, Wadeville, Johannesburg, South Africa) and modified Melin-Norkrans (MMN) medium.17 The fungal isolation process was carried out under sterile conditions and involved the addition of 250 mL deionised water to 100 g iron ore materials. The mixture was shaken for 24 h at 60 rpm using an orbital incubator (SI 50, Stuart Scientific, Stone, Staffordshire, UK) at room temperature. Thereafter, 10 mL of the homogenised mixture was vortexed and 50 µL of the vortexed mixture was inoculated onto pre-prepared plates of PDA and MMN agar. All the plates were incubated at 37 ºC for 5 days. To obtain a pure culture of the isolated fungi, mycelia fragments were scraped off the surface of the growth medium and suspended in 1 mL deionised water inside 1.5-mL tubes. The suspension was vortexed to separate the clustered mycelia. A 50 µL aliquot of each suspension was spread onto new plates of MMN agar and PDA with the aid of an autoclaved glass spreader. After 5 days, distinct growing mycelia of the fungi were sub-inoculated onto new plates to obtain pure cultures of the fungi. This method enhanced the purity of the isolates by encouraging growth from individual hyphae. The pure cultures obtained were then transferred onto phosphate-solubilising medium (PSM) by inoculation at the centre of the agar plate. The composition of the PSM was: 0.10 g/L (NH4)2SO4, 0.25 g/L MgSO4·7H2O, 5.00 g/L MgCl2·6H2O, 0.20 g/L KCl, 2.50 g/L Ca3(PO4)2, 10 g/L C6H12O6 (glucose) and 20 g/L agar.18 After 10 days of incubation at 37 ºC, halos forming around the areas of growth of the fungi indicated the phosphate-solubilising ability of these fungi. Fungi that tested positive by this method were then identified using the molecular methods described next.
Molecular identification of the isolates
Genomic DNA extraction was carried out using the Zymo Research Fungal/Bacterial DNA Kit™ (Orange, CA, USA) according to the manufacurer's instructions. DNA extraction was followed by a polymerase chain reaction (PCR) to amplify the internal transcribed spacer (ITS) regions of the isolated fungi. The PCR was performed by an MJ Mini thermal cycler (Bio-Rad, Hercules, CA, USA) using a 50-µL reaction mix that consisted of 0.5 µM each of both forward (ITS1F, 5'-CTTGGTCATTTAGAGGAAGTAA-3'; at a temperature of 49.7 ºC) and reverse (ITS4, 5'-CCTCCGCTTATTGATATGC-3'; at a temperature of 52.1 ºC) primers,19,20 2 µL of the DNA template and 25 µL of Fermentas Master Mix (2X; Vilnius, Lithuania) that contained Taq polymerase, dNTPs, buffer and MgCl2. The cycling conditions included an initial denaturing cycle of 3 min at 94 ºC, followed by 30 denaturing cycles of 1 min at 94 ºC, an annealing cycle of 30 s at 50 ºC and DNA elongation for 2 min at 72 ºC. There was a final elongation period of 8 min at 72 ºC. The PCR products were separated electrophoretically on a 1% agarose gel and visualised by ethidium bromide-UV fluorescence to determine the size of the amplified bands. Cleaning of the PCR product was done using the PROMEGA Wizard SV Gel and PCR purification kit (Sunnyvale, CA, USA) before resuspension in 30 µL of nuclease-free double-distilled water. The cleaned PCR product was sent to the Inqaba Biotechnical Industries (Pty) Ltd Sequencing Facility, Pretoria, South Africa. Forward and reverse sequences of the ITS regions obtained were aligned using BioEdit software.21 Thereafter, the homologous sequences were compared using the basic local alignment search tool (BLAST) on the National Center for Biotechnology Information website to obtain the nearest identical organisms based on the percentage similarity. Four of the many identical sequences for each fungus were selected from GenBank for phylogenetic analysis.
Phylogenetic analyses
Phylogenetic analyses were carried out using Mega 4 software22. The neighbour-joining (NJ) method23 was used to infer the evolutionary history of the isolates and the bootstrap consensus tree was inferred from 1000 replicates. There were 463 positions in the final dataset and Aspergillus niger was used as the outgroup.
Fungal treatment
Fungi that produced visible halos on the PSM were selected for the leaching experiment. The experiment involved the direct use of the fungi as well as the spent liquid medium that was used to grow the fungi. Three plugs of phosphate-solubilising fungi (PSF) taken from the edge of the growing medium were inoculated onto a phosphate-solubilising broth (PSB) that consisted of iron ore materials used to substitute the Ca3(PO4)2. Then 5 g of the iron ore material was added to 50 mL of the medium that was shaken at 100 rpm and incubated at 37 ºC for 10 days. The control contained water and the iron ore sample with no fungus (CT).
Spent liquid medium treatment
Phosphate-solubilising fungi were grown on PSB by inoculating three plugs (each with a diameter of 4 mm) of the fungal culture onto the PSB. The incubation period was 10 days at 37 ºC. In a study done by Williams,16 the quantity of organic acid (in this case, citric acid produced by A. niger) reached a maximum on Day 4 of the incubation period. Considering an average of 4 to 5 days to attain sufficient organic acid production and another 5 days for the organic acids to act on the iron ore minerals, a 10-day incubation period was selected. The same conditions were used for the control experiments.
Incubation was followed by separation of the fungus and the spent liquid medium by filtration using 0.2-µm uniflow syringe filters (Whatman, Maidstone, UK). The liquid portion - the spent liquid medium - was then used for the leaching process, for which 5 g of autoclaved iron ore materials were added to 50 mL of the spent liquid medium. The mixture was then incubated without shaking for 10 days, at a temperature of 45 ºC to discourage the growth of fungal spores. Because our intention was to eventually develop a method that could be used for heap leaching, shaking of the flask was excluded.
Harvesting
For both fungal and spent liquid medium treatments, harvesting was done using filtration through filter paper 0.45 µm in size. Iron ore samples collected were then washed with HCl and later rinsed with deionised water. Liquid parts from both treatments were preserved at -40 ºC for subsequent high-performance liquid chromatography (HPLC).
Organic acid detection
HPLC was used to identify the organic acids released during both fungal and spent liquid medium treatments. The method described by Sheng et al.24 was used to analyse for four different organic acids, namely gluconic, acetic, citric and maleic acids.
Statistical analyses
SAS software version 9.2 25 was used for the statistical analyses. A three-way analysis of variance (ANOVA) model was adjusted to the data; the three factors were: (1) treatment (three levels: fungal [F], spent liquid medium [M] or control [CT]); (2) iron ore type (two levels: conglomerate [KB] or shale [SB]); and (3) particle size (two levels: A or B). For all other variables (organic acids), a three-way ANOVA model was also adjusted to the data for three factors: (1) treatment (two levels: fungal [F] or spent liquid medium [M]), (2) iron ore type (two levels: conglomerate [KB] or shale [SB]) and (3) particle size (two levels: A or B). All variables were log-transformed in order to satisfy the assumptions of the model (i.e. normality and homogeneity of variances). The normality assumption was verified by the Shapiro-Wilk's statistic, while the homogeneity of variance was verified visually with the residual plots. Following a significant effect of any source of variation, multiple comparisons were done to ascertain the significant difference.
RESULTS
The isolates were obtained and identified through molecular and phylogenetic approaches as shown in Figure 1. The sequences obtained have been deposited in GenBank and accession numbers allocated (Figure 1). The ITS phylogenetic analyses of the four isolates and their closest relatives obtained from GenBank supported three major lineages in NJ analysis. The genera identified with this process were Penicillium (FO), Alternaria (SFC2 and KFC1) and Epicoccum (SFC2B) (Figure 1). The P solubilisation experiment indicated that isolate FO (GU187961) was the only isolate capable of solubilising Ca3(PO4)2, therefore, it was the only fungus among the four used in the biobeneficiation experiment.
Both treatment (fungal or spent liquid medium) and particle size had a significant effect on K removal, but iron ore type did not (Table 1). However, all three factors (treatment, particle size and iron ore type) significantly affected the removal of P (Table 1). The greatest quantity of K2O removed (32.94%) was from particle size A of conglomerate by treatment with spent liquid medium (Figure 2), whereas the greatest quantity of P removed (58.33%) was from particle size B of shale by fungal treatment (Figure 3). The fungal isolate FO was able to remove more K from the smaller particle size B than the larger particle size A of KGT, but there was no significant difference in P removal between particle sizes of both iron ore types. In comparison, the spent liquid medium was able to remove more of both K and P from the larger particle size A of both iron ore types (Figures 2 and 3).
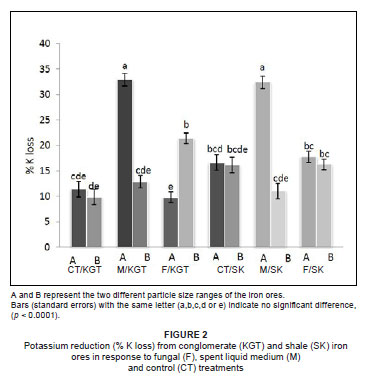
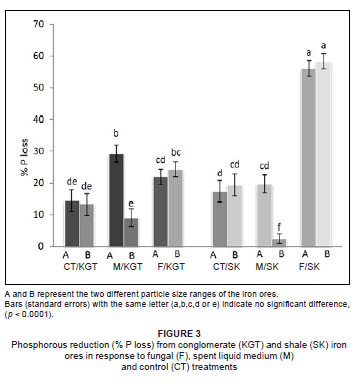
Among the organic acids, gluconic acid appeared to be the most important in the removal of K and P because the quantity produced by the fungal isolate FO (GU187961) was between 4-fold and 200-fold greater than those of the other organic acids (maleic, acetic and citric acids). The quantity of maleic acid produced was the second greatest, while quantities of both acetic acid and citric acid released by the fungus were generally low (Table 2).
DISCUSSION
There are two commonly used methods for the isolation, characterisation and utilisation of mineral-associated microbes in biohydrometallurgical processes. The first method is the direct enrichment culture method where the mineral is added to a defined medium for the purpose of isolation and leaching of the mineral.26 In this situation, the associated microbes are expected to multiply and participate in the leaching of the mineral.27,28 The problem with this method is the inability to immediately identify the specific organism responsible for the leaching when, or if, it occurs. The other method involves direct isolation from the surface of the mineral and subsequent utilisation of the isolates for leaching processes.29 This method allows the immediate identification of the organism responsible for the leaching process. The disadvantage of this method is the inability to obtain information about possible heterotrophic leaching that can occur from more than one microbe. However, both methods have been used successfully to investigate biohydrometallurgical processes.26,29 This study utilised the second method, which allowed the initial isolation of four different fungal isolates, namely FO, SFC2, SFC2B and KFC1. The three fungal genera identified in this study have been isolated previously from other minerals and mining sites.30,31,32 For example, Rezza et al.27 were able to isolate three different fungi from spodumene, one of which was Penicllium purpurogenum. In addition, in the present study, we have been able to show differences in the leaching abilities between the direct use of fungi and the use of spent liquid medium.
The production of organic acids has been established in both agromining and biomining industries as essential for the natural dissolution of complex mineral materials by microorganisms.24,27,33 Therefore, an indirect screening approach involving in vitro P solubilisation by the fungi was used as an indicator of organic acid production. Delvasto et al.34 screened isolated microbes for their abilities to dissolve insoluble forms of P in an in vitro experiment. A. niger obtained from this process was then used for leaching of iron ore minerals. In another study on the removal of potassium and silicon, Sheng et al.24 screened isolates obtained from silicate minerals for their solublisation potentials and used one of them, Bacillus globisporus Q12, for solubilisation experiments.
The influence of particle size in the use of fungal treatment can be explained by the fact that there is a greater surface area of minerals exposed to microbial activity when smaller particles are used.35 However, in the present study, the same phenomenon cannot be used to explain the leaching that occurred during treatment with the spent liquid medium because of the influence of another factor, namely shaking of the flasks was excluded in order to simulate heap leaching. Franz et al.36 suggested that shaking of the flasks during leaching is essential for the production of organic acid and proper aeration. It can therefore be suggested that a lack of good aeration from not shaking the flasks probably affected mixing of the spent liquid medium iron ore minerals.
Leaching occurring from treatment with the spent liquid medium probably could be linked to the organic acids detected in the spent liquid medium. Some of the organic acids, particularly gluconic acid, detected in this study have been reported previously as essential in both weathering and bioleaching experiments. For example, Sheng et al.24 reported that the ability of B. globisporus Q12 in the solubilisation of silicate minerals was ascribed to the production of both gluconic and acetic acids. Although the quantities of the other organic acids detected were small, their contribution to the leaching process, as well as those of other metabolites contained in the spent liquid medium cannot be entirely ruled out. For instance, acetic and citric acids often have been reported to influence the leaching of non-sulphudic minerals.5,4,34
The underlying objective of this study was not to compare the rate of leaching by the fungus and that of the spent liquid medium, but to ascertain the possibility of using the spent liquid medium as an alternative to direct use of the fungus, because of the surface-attachment problem that usually is encountered with the direct use of the fungus.37 Therefore, the different concentrations of organic acids that were present at the beginning of both fungal and spent liquid medium treatments were not considered as 'relevant' to achieving the aim of the study. However, considering these initial organic acid concentrations, the high removal of K by the spent liquid medium treatment can partially be attributed to the initial higher concentrations of organic acids in this treatment.36
Utilisation of P by the fungus appears to be driving the concentration gradient for removal and resulted in a high level of P removal from the iron ore. This scenario, associated with the physical presence of the microbe, is sometimes needed for bioleaching to occur effectively, especially in situations in which the organic acid is not the only factor involved. It is therefore plausible that, in addition to the fungal production of organic acids, scavenging was used as a feeding mechanism by the fungus to remove P from the iron ore.4,37,38
Although the amounts required to be removed (70.19% of K2O and 78.57% of P in conglomerate, and 43.17% of K2O and 66.67% of P in shale) in order to meet the commercial standard needed for exportation of these minerals were not attained, this study has highlighted the importance of factors such as iron ore type and particle size in the bioleaching processes. It should also be noted that optimisation of the methods adopted in this study, especially harvesting at intervals, is necessary to increase the potential viability of this method in biohydrometallurgy. In addition, the effect of particle size should be interpreted with caution - apart from the aeration effect already mentioned, there could also be a grinding effect on the mineral solubilisation. In a study conducted by Shrihari et al. ,39 it was discovered that the grinding of minerals could help create specific sites for microbial attachment that subsequently affect dissolution of minerals.
Successful development of a biobeneficiation method for iron ore entails many steps. Identification of potential microbes is a major part of the developmental process. Factors such as difficult adaptation of non-indigenous microbes, biofilm formation and lack of cheap carbon sources have hindered the development of this technology.4,5 Our study, to the best of our knowledge, is the first to use fungus isolated from Sishen iron ore for bioleaching. A major challenge to the further development of biobeneficiation is the use of sterile conditions; presently, maintenance of sterile conditions is expensive for the iron ore industry, because of the low price of iron ore. Although the spent liquid medium treatment also involved the use of sterile techniques, we have shown that the number of steps needed to produce a leaching solution with the optimum quantity of organic acids can be reduced.
ACKNOWLEDGEMENTS
We would like to thank Kumba Iron Ore Ltd for financial support and material resources. We are also grateful for the contribution of Mr Gaetan Daigle to the statistical analyses and to Heinrich Geyer and Alicia van der Merwe for supplying some of the chemicals.
REFERENCES
1. Parks EJ, Olson GJ, Brinckman FE, Baldi F. Characterization by high performance liquid chromatography (HPLC) of the solubilization of phosphorus in iron ore by a fungus. J Ind Microbiol Biot. 1990;5(2):183-189. [ Links ]
2. Yusfin Y, Chernousov P, Garten V, Karpov Y, Petelin A. The role of alkalis and conserving resources in blast-furnace smelting. Metallurgist. 1999;43(2):54-58. [ Links ]
3. Williams P, Cloete T. Microbial community study of the iron ore concentrate of the Sishen Iron Ore Mine, South Africa. World J Microb Biot. 2008;24(11):2531-2538. [ Links ]
4. Delvasto P, Ballester A, Muñoz JA, et al. Mobilization of phosphorus from iron ore by the bacterium Burkholderia caribensis FeGL03. Minerals Eng. 2009;22(1):1-9. [ Links ]
5. Jain N, Sharma D. Biohydrometallurgy for nonsulfidic minerals - a review. Geomicrobiol J. 2004;21:135-144. [ Links ]
6. Rawlings D. Characteristics and adaptability of iron- and sulfur-oxidizing microorganisms used for the recovery of metals from minerals and their concentrates. Microb Cell Fact. 2005;4(1):13. [ Links ]
7. Davies J, Moon JT, Traice FB. Alkalis in the blast furnace. Ironmaking Steelmaking. 1978;5(4):151-161. [ Links ]
8. Elkasabgy T. Effect of alkalis on reduction behavior of acid iron ore pellets. Transactions ISIJ. 1984;24:612-621. [ Links ]
9. Gadd GM. Fungal production of citric and oxalic acid: Importance in metal speciation, physiology and biogeochemical processes. Adv Microb Physiol. 1999;41:47-92. [ Links ]
10. Lin T, Huang H, Shen F, Young C. The protons of gluconic acid are the major factor responsible for the dissolution of tricalcium phosphate by Burkholderia cepacia CC-Al74. Bioresour Technol. 2006;97(7):957-960. [ Links ]
11. Xiao C, Chi R, He H, Zhang W. Characterization of tricalcium phosphate solubilization by Stenotrophomonas maltophilia YC isolated from phosphate mines. Journal of Central South University of Technology. 2009;16(4):581-587. [ Links ]
12. Paris F, Bonnaud P, Ranger J, Lapeyrie F. In vitro weathering of phlogopite by ectomycorrhizal fungi. Plant Soil. 1995;177(2):191-201. [ Links ]
13. Goldstein A, Lester T, Brown J. Research on the metabolic engineering of the direct oxidation pathway for extraction of phosphate from ore has generated preliminary evidence for PQQ biosynthesis in Escherichia coli as well as a possible role for the highly conserved region of quinoprotein dehydrogenases. Biochim Biophys Acta. 2003;1647(1-2):266-271. [ Links ]
14. Yuan L, Huang J, Li X, Christie P. Biological mobilization of potassium from clay minerals by ectomycorrhizal fungi and Eucalypt seedling roots. Plant Soil. 2004;262(1):351-361. [ Links ]
15. Lapeyrie F, Chilvers GA, Bhem CA. Oxalic acid synthesis by the mycorrhizal fungus Paxillus involutus (Batsch. Ex Fr.) Fr. New Phytol. 1987;106(1):139-146. [ Links ]
16. Williams PJ. The use of Aspergillus niger for the removal of potassium and phosphorous from the iron ore of the Sishen iron ore mine, South Africa. PhD Thesis, Pretoria, University of Pretoria, 2008. [ Links ]
17. Mehta S, Nautiyal CS. An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr Microbiol. 2001;43(1):51-56. [ Links ]
18. Marx DH. The influence of ectotrophic mycorrhizal fungi on the resistance of pine root to pathogenic infections. I. Antagonism of mycorrhizal fungi to root pathogenic fungi and soil bacteria. Phytopathology. 1969;59:153-163. [ Links ]
19. White TJ, Bruns T, Lee S, Taylor JW. Amplification and direct sequencing of fungal ribosomal genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: A guide to methods and applications. New York: Academic Press Inc, 1990; p.315-322. [ Links ]
20. Gardes M, Bruns TD. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol Ecol. 1993;2(2):113-118. [ Links ]
21. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp. 1999;41:95-98. [ Links ]
22. Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular evolutionary genetics analysis (MEGA) software; 2007. [ Links ]
23. Saitou N, Nei. M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406-425. [ Links ]
24. Sheng XF, Zhao F, He LY, Qiu G, Chen L. Isolation and characterization of silicate mineral-solubilizing Bacillus globisporus Q12 from the surfaces of weathered feldspar. Can J Microbiol. 2008;54(5):1064-1068. [ Links ]
25. SAS software, version 9.2, 2009 (SAS Institute, Inc., Cary, NC). [ Links ]
26. Goebel BM, Stackebrandt E. Cultural and phylogenetic analysis of mixed microbial populations found in natural and commercial bioleaching environments. Appl Environ Microbiol. 1994;60(5):1614-1621. [ Links ]
27. Rezza I, Salinas E, Calvente V, Benuzzi D, Sanz de Tosetti MI. Extraction of lithium from spodumene by bioleaching. Lett Appl Microbiol. 1997;25(5):172-176. [ Links ]
28. Rezza I, Salinas E, Elorza M, Sanz de Tosetti M, Donati E. Mechanisms involved in bioleaching of an aluminosilicate by heterotrophic microorganisms. Process Biochem. 2001;36(6):495-500. [ Links ]
29. Delvasto P, Valverde A, Ballester A, et al. Diversity and activity of phosphate bioleaching bacteria from a high-phosphorus iron ore. Hydrometallurgy. 2008;92(3-4):124-129. [ Links ]
30. Burford EP, Kierans M, Gadd GM. Geomycology: Fungi in mineral substrata. Mycologist. 2003;17(3):98-107. [ Links ]
31. Qui G, Liu X, Zhou H. Microbial community structure and function in sulfide ore bioleaching systems. T Nonferr Metal Soc. 2008;18(6):1295-1301. [ Links ]
32. Sabat J, Gupta N. Iron ore solubilization by Penicillium restrictum: Effect of carbon source and incubation days. Am-Euras J Agron. 2009;2(1):43-44. [ Links ]
33. Van Schöll L, Hoffland E, Van Breemen N. Organic anion exudation by ectomycorrhizal fungi and Pinus sylvestris in response to nutrient deficiencies. New Phytol. 2006;170(1):153-163. [ Links ]
34. Delvasto P, Ballester A, Muñoz JA, González F, Blázquez ML, García-Balboa C. Exploring the possibilities of biological beneficiation of iron-ores: The phosphorus problem. Proceedings of the 15th Steelmaking Conference, 5th Ironmaking Conference & 1st Environment and Recycling Symposium IAS (CD-ROM); 2005 Nov 7-10; Buenos Aires, Argentina. Argentinean Steelmaking Institute (IAS); 2005. [ Links ]
35. Modak JM, Vasan SS, Natarajan KA. Calcium removal from bauxite using Paenibacillus polymyxa. In: Kawatra SK, Natarajan KA, editors. Mineral biotechnology: microbial aspects of mineral beneficiation, metal extraction, and environmental control. United States of America: Society for mining, metallurgy, and exploration, 2001; p. 13-25. [ Links ]
36. Franz A, Burgstaller W, Schinner F. Leaching with Penicillium simplicissimum: Influence of metals and buffers on proton extrusion and citric acid production. Appl Environ Microbiol. 1991;57(3):769-774. [ Links ]
37. Adeleke RA, Cloete E, Bertrand A, Khasa DP. Mobilisation of potassium and phosphorus from iron ore by ectomycorrhizal fungi. World J Microb Biot. 2010; doi:10.1007/s11274-010-0372-0. [ Links ]
38. Banfield JF, Barker WW, Welch SA, Taunton A. Biological impact on mineral dissolution: Application of the lichen model to understanding mineral weathering in the rhizosphere. Proc Natl Acad Sci U S A. 1999;96(7):3404-3411. [ Links ]
39. Shrihari JJM, Kumar R, Gandhi KS. Dissolution of particles of pyrite mineral by direct attachment of Thiobacillus ferrooxidans. Hydrometallurgy. 1995;38(2):175–187. [ Links ]
 Correspondence to:
Correspondence to:
Rasheed Adeleke
Postal address: Department of Microbiology and Plant Pathology University of Pretoria New Agriculture building
Lunnon Road
Hillcrest 0083, South Africa
email: r_adeleke@yahoo.com
Received: 20 Jan. 2010
Accepted: 27 July 2010
Published: 30 Sept. 2010
This article is available at: http://www.sajs.co.za
© 2010. The Authors. Licensee: Open Journals Publishing. This work is licensed under the Creative Commons Attribution License.














