Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Science
On-line version ISSN 1996-7489
Print version ISSN 0038-2353
S. Afr. j. sci. vol.106 n.3-4 Pretoria Mar./Apr. 2010
RESEARCH ARTICLE
Salinity and temperature tolerance of the invasive freshwater gastropod Tarebia granifera
Nelson A.F. Miranda; Renzo Perissinotto; Christopher C. Appleton
School of Biological and Conservation Sciences, University of KwaZulu-Natal, Westville campus, South Africa
ABSTRACT
Invasive aquatic species, such as the gastropod Tarebia granifera, can cause ecological disturbances and potentially reduce biodiversity by displacing indigenous invertebrates. In South Africa, T. granifera was first recorded in an estuarine environment in the St Lucia Estuary. Its tolerance to salinity and temperature was investigated through the experimental manipulation of these factors. T. granifera can tolerate temperatures between 0 ºC and 47.5 ºC, allowing it to survive high temperature extremes. The species may also survive cold snaps and invade higher altitude areas. More remarkably, this snail survives high salinity for a relatively long time, as LS50 (lethal salinity for 50% of the population) was reached at 30 psu over 65-75 days. However, higher salinity adversely affected the T. granifera population. Snails acclimated to freshwater conditions and suddenly transferred to 30 psu experienced 100% mortality within 48 h. Snail activity also declined with increasing salinity. T. granifera's environmental tolerance and parthenogenetic characteristics are the keys to successful introduction and establishment. Therefore, the management of T. granifera may prove difficult in the short to medium term. The present findings constitute a contribution to the knowledge of biological invasions in Africa and to the understanding of estuarine invasions by T. granifera.
Keywords: alien species; aquatic mollusc; lethal tolerance; South Africa; St Lucia Estuary
INTRODUCTION
Invasive species have been widely reported as having a negative impact on the ecology and economy of a region.1 In South Africa, knowledge of aquatic invasive species has increased over the past two decades. So far, the focus has been on prominent invasive species, such as the water hyacinth Eichhornia crassipes,2 fish such as bass and trout,3 as well as invertebrates such as the mussel Mytilus galloprovincialis,4 the crab Carcinus maenas4 and the snail Physa acuta.5 However, there is a lack of studies on the ecological consequences of invasive aquatic invertebrates in freshwater and estuarine systems. These consequences may include biotic homogenisation, competitive exclusion of indigenous species and changes to the properties of the invaded habitat.4,6,7 Successful animal invaders progress beyond the introduction and establishment stages, spread in new habitats and finally have tangible ecological impacts. Management efforts should focus on preventing introduction, but, in many cases, the invader is not noticed until it is already established. Ecophysiological knowledge about the invading species is essential in considering whether, and how, to implement control measures.
Tarebia granifera is a freshwater prosobranch gastropod (family: Thiaridae), originally from South-East Asia.8,9 The species has spread rapidly in recent years across a number of countries throughout tropical and subtropical areas of the World.5,9,10,11,12 T. granifera may have been introduced into KwaZulu-Natal, South Africa, via the aquarium trade in the early 1990s.11 Itis present in an increasing number of fresh and brackish water bodies of KwaZulu-Natal and Mpumalanga provinces,5 including the Kruger National Park.13 The first record of T. granifera in an estuarine environment was in South Africa at the St Lucia Estuary. It was found in December 2005 in the Nkazama Stream (a freshwater environment) which flows into the eastern shore of the South Lake, 1.2 km north of Catalina Bay (Figure 1). The St Lucia estuarine system is part of the iSimangaliso (formerly known as the Greater St Lucia) Wetland Park. This is a World Heritage Site and a Ramsar Wetland of International Importance. Recently, St Lucia has been experiencing severe freshwater starvation. The mouth closed in June 2002 and this 'closed-mouth state' persisted until March 2007, leading to variable salinity and water temperature conditions.14 Under normal conditions, the St Lucia estuarine system has a surface area of around 350 km2 and an average depth of 0.9 m.15 This high surface area to volume ratio is conducive to great evaporative water losses, causing some areas to become hypersaline (salinity greater than 40 psu). However, other areas have developed fresh or brackish conditions due to the input of fresh water from rivers, streams or seepage from sand dune aquifers.14 The loss of water from the system has also meant a drop in water levels and the formation of large areas of very shallow depth (< 10 cm), which are quickly heated by the sun to reach temperatures over 45 ºC in summer.

In January 2007, a dense population (over 5 000 individuals per square metre) of T. granifera was found in a very shallow freshwater seepage zone just south of the Old Jetty in Catalina Bay and, in February 2007, it was found in similarly high densities at embayments as far south as Brodie's Crossing (Figure 1). Salinity conditions in these embayments were brackish rather than fresh, but this did not prevent T. granifera from invading.
Salinity and temperature are among the main factors influencing growth, reproduction, survival and distribution patterns of aquatic molluscs.16,17 A number of studies have assessed the effects of temperature and salinity on invasive aquatic invertebrates.18,19,20 T. granifera is of African and global interest due to its invasive ability and the potential impact on indigenous benthic communities.21 This study investigated the lethal tolerance limits of T. granifera to salinity and temperature through the experimental manipulation of these factors. It provides the first experimental data on the responses of this invader to key physicochemical factors in the saline/brackish environments of estuaries. The results reported here will contribute to an understanding of the threat that this snail poses, and possibly also to its management in Africa and elsewhere.
MATERIALS AND METHODS
Study site
Catalina Bay is located on a limestone flat on the eastern shore of St Lucia's South Lake (Figure 1). The fringing vegetation is mainly composed of Cyperus laevigatus, Juncus kraussii and Salicornia spp. The water in the bay is very shallow ( 0.5 m) and can reach a temperature of around 50 ºC during summer. From the beginning of 2005 until the breach of the St Lucia mouth in March 2007, the average salinity (± s.d.) in this area was 16 ± 9 psu, a reflection of the influence of the seepage of fresh water from dune systems located to the east of Catalina Bay. Several additional freshwater seepage zones were identified south of the Old Jetty at Catalina Bay. After the breach event, a large volume of sea water entered the system, causing an overall increase in water levels and salinity. During the open phase the average salinity in the bay rose to 30 ± 3 psu.
0.5 m) and can reach a temperature of around 50 ºC during summer. From the beginning of 2005 until the breach of the St Lucia mouth in March 2007, the average salinity (± s.d.) in this area was 16 ± 9 psu, a reflection of the influence of the seepage of fresh water from dune systems located to the east of Catalina Bay. Several additional freshwater seepage zones were identified south of the Old Jetty at Catalina Bay. After the breach event, a large volume of sea water entered the system, causing an overall increase in water levels and salinity. During the open phase the average salinity in the bay rose to 30 ± 3 psu.
Temperature tolerance
T. granifera snails (mean shell height ± s.d. = 12.5 ± 2.5 mm) were collected from Catalina Bay (Figure 1) on 11 April 2007 (salinity  13 psu), transported to the laboratory, sorted into groups of 20 and put into transparent plastic bottles (900 mL). These were filled with fresh water (
13 psu), transported to the laboratory, sorted into groups of 20 and put into transparent plastic bottles (900 mL). These were filled with fresh water ( 0 psu), placed in a controlled-environment room at 20 ± 1 ºC with a photoperiod of 12 h of light followed by 12 h of darkness. Snails were left to acclimate in these conditions for 48 h prior to the experiment and fed ad libitum on naturally occurring benthic microalgae. Suspensions of microalgae were obtained by scooping the upper few millimetres of sediment and re-suspending them through stirring, and then harvesting the supernatant. Temperature-controlled, aerated water baths were used to achieve the following treatments: 0 ºC, 5 ºC, 10 ºC, 20 ºC, 30 ºC, 40 ºC, 45 ºC, 47.5 ºC and 50 ºC. Bottles reached test temperatures within 30 min. A control water bath was placed outside and its temperature was not controlled. Six replicates (900-mL bottles with 20 live individuals each) were used per water bath. Mortality, defined as a lack of response to a mechanical stimulus (gentle prodding with a fine brush), was recorded after exposure times of 2 h, 4 h, 8 h, 16 h and 32 h. At 0 ºC, the water in some bottles froze due to their close proximity to the water bath's cooling element. Although these treatments were not included in the data analysis, the snails retrieved from such bottles were thawed and kept at a recovering temperature of 20 ºC for 12 h, after which mortality was tested. A second stock of snails was collected from Catalina Bay on 18 July 2007 (salinity
0 psu), placed in a controlled-environment room at 20 ± 1 ºC with a photoperiod of 12 h of light followed by 12 h of darkness. Snails were left to acclimate in these conditions for 48 h prior to the experiment and fed ad libitum on naturally occurring benthic microalgae. Suspensions of microalgae were obtained by scooping the upper few millimetres of sediment and re-suspending them through stirring, and then harvesting the supernatant. Temperature-controlled, aerated water baths were used to achieve the following treatments: 0 ºC, 5 ºC, 10 ºC, 20 ºC, 30 ºC, 40 ºC, 45 ºC, 47.5 ºC and 50 ºC. Bottles reached test temperatures within 30 min. A control water bath was placed outside and its temperature was not controlled. Six replicates (900-mL bottles with 20 live individuals each) were used per water bath. Mortality, defined as a lack of response to a mechanical stimulus (gentle prodding with a fine brush), was recorded after exposure times of 2 h, 4 h, 8 h, 16 h and 32 h. At 0 ºC, the water in some bottles froze due to their close proximity to the water bath's cooling element. Although these treatments were not included in the data analysis, the snails retrieved from such bottles were thawed and kept at a recovering temperature of 20 ºC for 12 h, after which mortality was tested. A second stock of snails was collected from Catalina Bay on 18 July 2007 (salinity  28 psu) and this procedure was repeated at 30 ± 2 psu (diluted, sterilised sea water was used) with a freshwater control treatment. Results were expressed as per cent survival.22
28 psu) and this procedure was repeated at 30 ± 2 psu (diluted, sterilised sea water was used) with a freshwater control treatment. Results were expressed as per cent survival.22
Salinity tolerance
The experimental procedure for salinity tolerance was similar to the above, using stock animals collected in July 2007. Six replicates (900-mL bottles with 20 live individuals each) were subjected to each of the following salinities: 0 ± 0.5 psu, 10 ± 2 psu, 20 ± 2 psu, 30 ± 2 psu and 40 ± 2 psu. The 40 ± 2 psu concentration was prepared by the addition of natural sea salt and measured using a water logger (YSI 6920, YSI Incorporated, Yellow Springs, OH, USA). Mortality was monitored over a period of 85 days. Dead individuals were removed immediately. T. granifera is ovoviviparous and has a brood pouch from which juveniles (shell height  1 mm) are born. Hence the presence of live juveniles in a bottle was recorded as birth. Water was changed every 5-10 days and food renewed weekly. The activity of snails in each bottle was monitored and classified according to four progressive criteria: actively moving, not moving (foot anchored), head out of shell (foot not anchored) and quiescent with operculum closed. The proportions of snails in each bottle conforming to these criteria were recorded every 10 days. These data were used to calculate the distribution of snail activity for each salinity treatment over the total exposure time up to 85 days. A 'salinity shock experiment' was also conducted, in which 12 replicates were acclimated to fresh water for a week. Six replicates were then transferred directly to 30 ± 2 psu, with the remainder serving as control. Mortality was recorded after 48 h.
1 mm) are born. Hence the presence of live juveniles in a bottle was recorded as birth. Water was changed every 5-10 days and food renewed weekly. The activity of snails in each bottle was monitored and classified according to four progressive criteria: actively moving, not moving (foot anchored), head out of shell (foot not anchored) and quiescent with operculum closed. The proportions of snails in each bottle conforming to these criteria were recorded every 10 days. These data were used to calculate the distribution of snail activity for each salinity treatment over the total exposure time up to 85 days. A 'salinity shock experiment' was also conducted, in which 12 replicates were acclimated to fresh water for a week. Six replicates were then transferred directly to 30 ± 2 psu, with the remainder serving as control. Mortality was recorded after 48 h.
Data analysis
Data were arcsine-transformed, following a one-sample Kolmogorov-Smirnov normality test.23 Repeated measures analyses of variance (RM-ANOVA) were used where each 900-mL bottle containing 20 live individuals was the unit of replication. Temperature tolerance data were tested using a three-way RM-ANOVA (dependent variable: survival; fixed factors: salinity, temperature, exposure time). Salinity tolerance data were tested using a two-way RM-ANOVA (dependent variable: survival; fixed factors: salinity and exposure time). Tukey post-hoc analyses were done on each of these two data sets. Differences between control and experimental treatments of the 'salinity shock experiment' were tested using a two-tailed independent-samples t-test. Homogeneity and normality of residuals were tested by a Levene's test and a one-sample Kolmogorov-Smirnov test, respectively. The statistical program SPSS-15 was used in all analyses.
RESULTS
Temperature tolerance
Survival in the control and the 0 ºC, 5 ºC, 10 ºC, 20 ºC, 30 ºC and 40 ºC treatments, at both 0 psu and 30 psu, exceeded 75% for 32 h (Figure 2). Snails died if the water in the bottles froze. Time to LT50 at 45 ºC was 8 h - 16 h at 0 psu and 16 h - 32 h at 30 psu (Figure 2). Mortality of 100% occurred within 2 h of exposure to 45.7 ºC and 50 ºC at both 0 psu and 30 psu (Figure 2). Thus, the thermal tolerance range of T. granifera was determined to be between 0 ºC and 47.5 ºC. A three-way RM-ANOVA showed no significant difference in survival between temperature treatments (Table 1). A Tukey post-hoc analysis showed, firstly, a significant difference in survival between the 45 ºC treatment at 0 psu and 30 psu and all other treatments (p < 0.05). Secondly, there were no significant differences between the 0 ºC, 5 ºC, 10 ºC, 20 ºC, 30 ºC and 40 ºC treatments (p > 0.05) and between the 47.5 ºC and 50 ºC treatments (p > 0.05) and, thirdly, the 47.5 ºC and 50 ºC treatments were significantly different from all others (p < 0.05).

Salinity tolerance
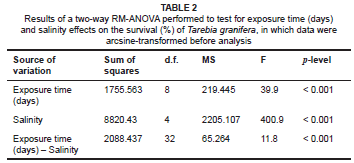
Throughout the 85 days, survival remained above 75% at 0 psu, 10 psu and 20 psu (Figures 3a-c) and several individuals gave birth under these conditions. Time to LS50 at 30 psu was 65-75 days (Figure 3d) but 15-25 days at 40 psu (Figure 3e). No births were recorded during either treatment. A two-way RM-ANOVA showed a significant difference in survival between salinity treatments (Table 2). A Tukey post-hoc analysis showed, firstly, no significant differences in terms of survival between the 0 psu, 10 psu and 20 psu treatments (p > 0.05) and, secondly, the 30 psu and the 40 psu treatments were significantly different from all other treatments (p < 0.05).
The monitoring of snail activity revealed that T. granifera spent more time in its shell with the operculum closed at high compared to low salinity. At 0 psu, 71% of snails were actively moving, at 10 psu 52%, at 20 psu 33%, at 30 psu 13% and at 40 psu only 2% (Figures 3a-e). At 0 psu, 5% of snails were quiescent, at 10 psu 6%, at 20 psu 16%, at 30 psu 71% and at 40 psu 96% (Figures 3a-e).
In the 'salinity shock experiment', LS50 at 30 psu was reached quickly, with 100% mortality after 48 h, while the control was significantly different because all snails remained alive (independent-samples t-test: t = 96.831, d.f. = 10, p < 0.001).
DISCUSSION
Range of environmental tolerance
T. granifera appears to have a wider temperature tolerance than previously proposed in the literature,24 but its relatively high levels of tolerance are typical of tropical species.25 The significance of its survival at temperatures lower than 10 ºC is that it may be able to survive cold snaps, as long as the temperature does not drop below zero. It may also indicate that T. granifera is able to invade areas at higher altitudes that have colder climates, although this has not happened yet in South Africa.21 This study did not focus on sub-lethal effects of temperature on T. granifera. However, it is likely that this species has an optimum temperature range for physiological activities around 30 ºC,since we observed physical activity to peak at that temperature (snails moved very fast in the bottles) and decrease in lower and higher temperatures (snails tended to retreat into their shells). If physical activity is taken to be a proxy for physiological activity, the results are in agreement with those found by Masilamoni et al.18 for the bivalve Brachidontes striatulus, although T. granifera has wider lethal temperature limits.18 The size of the snail was not taken into account, but mortality may be assumed to be independent of body size in this study.18 T. granifera seems to respond in similar ways to temperature under both high and low salinity, which means that further investigation is required to understand its osmoregulatory ability.
It is noteworthy that T. granifera was able to survive salinities from 30 psu to 40 psu for just under a month, after which time the snails died (Figures 3d and 3e). Salinity tolerance of freshwater molluscs is thought to be limited only to very dilute and stable brackish conditions,26,27,28 but T. granifera survived and gave birth to live juveniles at salinities of up to 20 psu. The behavioural response of reducing physical activity, retreating into the shell and closing the operculum is thought to be associated with a reduction in physiological activity and is the strategy used in an attempt to survive until favourable conditions are re-established. When T. granifera is under stress, its impact on the environment should be reduced. Thus, T. granifera may have a greater impact on freshwater than on brackish environments, a pattern already observed in the invasive bivalve Dreissena polymorpha.20 Unlike most other freshwater invasive invertebrates in South Africa, T. granifera has a brood pouch, which provides some measure of protection to developing stages before birth. Further studies should focus on the effects of salinity and temperature on reproduction. There is also a need for studies on T. granifera's ecological impact on freshwater and brackish environments, and the pre-adaptation of this species to a higher salinity environment is of importance as it contributes to the invasion process.
Expected ecosystem impact of T. granifera
Previous studies indicate that if T. granifera proliferates it may displace other invertebrates, thereby causing ecological disturbances and a possible reduction in biodiversity.5,10,29,30,31 However, no ecological studies have been done on this species in South Africa and the mechanisms of impact are not understood.21 The T. granifera population in the St Lucia Estuary has become well established but high salinity certainly poses an obstacle to its spread and subsequent impact. After a six-month period of open-mouth conditions, the St Lucia mouth closed again in August 2007. The large quantities of sea salt that entered the system caused an increase in salinity over the ensuing months, despite the input of rain water.32 In November 2007, high mortality was becoming evident in the T. granifera population at Catalina Bay (salinity  31 psu). This observation supports the results of the salinity tolerance experiment discussed earlier (Figure 3d). By March 2008, most of the population had died and many empty shells littered the shore as water levels dropped. In June 2008, at a recorded average salinity of 40 ± 2 psu, no living specimens were found in the study area. In April 2009, however, it was discovered that a portion of the population had remained in the adjacent freshwater seepage zones together with the indigenous salt-sensitive biota.14,33 Other disturbing findings made in the seepage zones a few months later (August 2009) were populations of Lymnaea columella and Aplexa marmorata, both invasive freshwater snail species from the Americas. How these invasive species were introduced and what sort of impact they are having is currently unknown. What is certain is that freshwater seepage zones in coastal areas are particularly vulnerable to invasion and may provide the opportunity for T. granifera populations to gradually adapt to higher salinity as they undergo a diffusion dispersal process.29
31 psu). This observation supports the results of the salinity tolerance experiment discussed earlier (Figure 3d). By March 2008, most of the population had died and many empty shells littered the shore as water levels dropped. In June 2008, at a recorded average salinity of 40 ± 2 psu, no living specimens were found in the study area. In April 2009, however, it was discovered that a portion of the population had remained in the adjacent freshwater seepage zones together with the indigenous salt-sensitive biota.14,33 Other disturbing findings made in the seepage zones a few months later (August 2009) were populations of Lymnaea columella and Aplexa marmorata, both invasive freshwater snail species from the Americas. How these invasive species were introduced and what sort of impact they are having is currently unknown. What is certain is that freshwater seepage zones in coastal areas are particularly vulnerable to invasion and may provide the opportunity for T. granifera populations to gradually adapt to higher salinity as they undergo a diffusion dispersal process.29
Prospects for the control of T. granifera
In order to completely purge T. granifera from an environment, 100% of the population has to be eliminated because of its parthenogenetic characteristic. The use of molluscicides in the St Lucia Estuary is not feasible at any stage, given that juveniles within the brood pouch may not be killed (personal observation) and non-target species, such as the indigenous snails Haminoea natalensis and Assiminea sp., which often dominate macrobenthic communities, may be adversely affected.31,34 Another option would be the introduction of a biological control agent, but this has its own widely reported risks.35,36,37,38 During this study, T. granifera showed a drop in physical activity as salinity increased (Figure 4) and, at salinities higher than 30 psu, snails were obviously under stress and died within a few weeks (Figure 3). The 'salinity shock experiment' showed that T. granifera was very vulnerable to sudden salinity increases. These findings suggest that invasions by this species could be controlled in temporarily open/closed estuaries by artificially breaching the mouth. However, such an action (effecting large-scale changes in order to manage a single species) may have unintended and negative consequences, as discussed in the growing literature on adaptive management.39,40,41,42
In summary, T. granifera can be regarded as a global invader of tropical and subtropical regions.43 The present findings suggest that tropical and subtropical freshwater and estuarine systems located in lowlands (altitude lower than 500 m) are particularly at risk in Africa, especially where the aquarium trade operates.21 However, so far there have been no reports of this species having spread northwards from South Africa. Nevertheless, situations similar to those recorded in the St Lucia Estuary already occur elsewhere in South Africa: populations of T. granifera are being discovered with increasing frequency in other areas of the iSimangaliso Park, such as Kosi Bay and Lake Sibaya (Kyle R 2008, personal communication, July 20; Taylor R 2008, personal communication, February 26), and other estuaries in KwaZulu-Natal, such as Nhlabane and Manzimtoti (Forbes AT 2008, personal communication, August 7; MacKay F 2009, personal communication, June 24). Similar discoveries are likely to be made in nearby Mozambique.21
ACKNOWLEDGEMENTS
We dedicate this article to the memory of Mr Amos Myeza, Technical Assistant at Ezemvelo KZN Wildlife EcoAdvice since 1976. We thank the iSimangaliso Park Authority and Ezemvelo KZN Wildlife for supporting this project. Special thanks go to R. Taylor, C. Fox and A. Myeza for their invaluable assistance. We also thank three anonymous reviewers for their valuable comments and suggestions. Funding was provided by the National Research Foundation, South Africa, Marine and Coastal Management (DEAT-MCM) and the World Wide Fund for Nature (WWF).
REFERENCES
1. Puth LM, Post DM. Studying invasion: Have we missed the boat? Ecol Lett. 2005;8:715-721. [ Links ]
2. Cilliers CJ. Biological control of water hyacinth, Eichhornia crassipes (Pontederiaceae), in South Africa. Agric Ecosyst Env. 1991;37:207-217. [ Links ]
3. Moosa V. An environmental perspective on invasive alien/aquatic invaders. In: Preston G, Brown G, Van Wyk E, editors. Proceedings of the South Africa/USA Bi-National Commission Conference on Best Management Practices for Preventing and Controlling Invasive Alien Species; 2000 Feb 22-24; Kirstenbosch Botanical Gardens, Cape Town, South Africa. Cape Town: Working for Water Programme; 2000. p. 8-10. [ Links ]
4. Griffiths CL, Hockey PAR, Van Erkom Schurink C, Le Roux PJ. Marine invasive aliens on South African shores: Implications for community structure and trophic functioning. S Afr J Mar Sci. 1992;12:713-722. [ Links ]
5. Appleton CC. Alien and invasive freshwater Gastropoda in South Africa. Afr J Aquat Sci. 2003;28:69-81. [ Links ]
6. Cambray JA. Impact on indigenous species biodiversity caused by globalisation of alien recreational freshwater fisheries. Hydrobiologia. 2003;500:217-230. [ Links ]
7. Mills EL, Casselman JM, Dermott R, et al. Lake Ontario: Food web dynamics in a changing ecosystem (1970-2000). Can J Fish Aquat Sci. 2003;60:471-490. [ Links ]
8. Pointier JP. [Freshwater malacological fauna of Guadeloupe Island (French Antilles)]. Bull Mus Hist Nat. 1974;235:905-933. French. [ Links ]
9. Chen K. A preliminary study on the reproductive ecology of the freshwater snail Tarebia granifera (Lamarck, 1822) (Prosobranchia: Thiaridae) in Jinlun River, south-eastern Taiwan. MSc thesis, Institute of Marine Biology, Nation Sun Yat-Sen University, Taiwan; 2003. [ Links ]
10. Abbott RT. A study of an intermediate snail host (Thiara granifera) of the oriental lung fluke (Paragonimus). Proc US Nat Mus. 1952;102:71-116. [ Links ]
11. Appleton CC, Nadasan DS. First report of Tarebia granifera (Lamarck, 1816) (Gastropoda: Thiaridae). J Mollus Stud. 2002;68:399-401. [ Links ]
12. Ben-Ami F. First report of the invasive freshwater snail Tarebia granifera (Lamarck, 1816) (Gastropoda: Thiaridae) from Israel. Nautilus. 2006;120:156-161. [ Links ]
13. De Kock KN, Wolmarans CT. Distribution and habitats of the alien invader freshwater snail Physa acuta in South Africa. Water SA. 2007;33:717-722. [ Links ]
14. Taylor R, Kelbe B, Haldorsen S, Botha GA. Ground-water dependent ecology of the shoreline of the subtropical Lake St. Lucia. Environ Geol. 2005;49:586-600. [ Links ]
15. Taylor R, Adams JB, Haldorsen S. Primary habitats of the St Lucia System, South Africa, and their responses to mouth management. Afr J Aquat Sci. 2006;31:31-41. [ Links ]
16. Kinne O. The effects of temperature and salinity on marine and brackish water animals: II. Salinity and temperature salinity combinations. Oceanogr Mar Biol. 1964;2:281-339. [ Links ]
17. Wiederholm AM. Distribution of Pomatoschistus minutus and P. microps (Gobiidae, Pisces) in the Bothnian Sea: Importance of salinity and temperature. Mem Soc Fauna Flora Fennica. 1987;63:56-62. [ Links ]
18. Masilamoni JG, Nandakumar K, Jesudoss KS, Azariah J, Satapathy KK, Nair KVK. Influence of temperature on the physiological responses of the bivalve Brachiodontes striatulus and its significance in fouling control. Mar Environ Res. 2002;53:51-63. [ Links ]
19. Thiyagarajan V, Qian PY. Effect of temperature, salinity and delayed attachment on development of the solitary ascidian Styela plicata (Lesueur). J Exp Biol Ecol. 2003;290:133-146. [ Links ]
20. Lauringson V, Mälton E, Kotta J, Kangur K, Orav-Kotta H, Kotta I. Environmental factors influencing the biodeposition of the suspension feeding bivalve Dreissena polymorpha (Pallas): Comparison of brackish and freshwater populations. Estuar Coast Shelf Sci. 2007;75:459-467. [ Links ]
21. Appleton CC, Forbes AT, Demetriades NT. The occurrence, bionomics and potential impacts of the invasive freshwater snail Tarebia granifera (Lamarck, 1822) (Gastropoda: Thiaridae) in South Africa. Zool Med Leiden. 2009;83:525-536. [ Links ]
22. Stephan CE. Methods for calculating an LC50. Paper presented at: The First Annual Symposium on Aquatic Toxicology and Hazard Evaluation. Mayer FL, Hamelink JL, editors. Proceedings of the 1st Annual Symposium on Aquatic Toxicology and Hazard Evaluation; 1976 Oct 25-26; Memphis, TN, USA. ASTM STP 634; 1976. p. 65-84. [ Links ]
23. Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River: Prentice Hall; 1999. [ Links ]
24. Chaniotis BN, Butler JM, Ferguson FF, Jobin WR. Thermal limits, desiccation tolerance, and humidity reactions of Thiara (Tarebia) granifera (Gastropoda: Thiaridae), host of the Asiatic lung fluke disease. Caribb J Sci. 1980;16:91-93. [ Links ]
25. Stillman JH, Somero GN. A comparative analysis of the upper thermal tolerance limits of eastern pacific porcelain crabs, genus Petrolisthes: Influences of latitude, vertical zonation, acclimation and phylogeny. Physiol Biochem Zool. 2000;73:200-208. [ Links ]
26. Copeland BJ, Tenore KR, Horton DB. Oligohaline regime. In: Odum HT, Copeland BJ, McMahon EA, editors. Coastal ecological systems of the United States vol. ii. Washington DC: The Conservation Foundation, 1974; p. 315-357. [ Links ]
27. Donnelly FA, Appleton CC, Schutte CHJ. The influence of salinity on certain aspects of the biology of Bulinus (Physopsis) africanus. Int J Parasitol. 1983;13:539-545. [ Links ]
28. Jordan PJ, Deaton LE. Osmotic regulation and salinity tolerance in the freshwater snail Pomacea bridgesi and the freshwater clam Lampsilis teres. Comp Biochem Physiol A. 1999;122:199-205. [ Links ]
29. Pointier JP, Samadi S, Jarne P, Delay B. Introduction and spread of Thiara granifera (Lamarck, 1822) in Martinique, French West Indies. Biodivers Conserv. 1998;7:1277-1290. [ Links ]
30. Canete R, Yong M, Sanchez J, Wong L, Gutierrez A. Population dynamics of intermediate snail hosts of Fasciola hepatica and some environmental factors in San Juan y Martinez municipality, Cuba. Mem I Oswaldo Cruz. 2004;99:257-262. [ Links ]
31. Pillay D, Perissinotto R. The benthic macrofauna of the St Lucia Estuary during the 2005 drought year. Estuar Coast Shelf Sci. 2007;77:35-46. [ Links ]
32. Bate GC, Taylor RH. Sediment salt-load in the St Lucia Estuary during the severe drought of 2002-2006. Environ Geol. 2007, doi: 10.1007/s00254-007-1057-3. [ Links ]
33. Vrdoljak SM, Hart RC. Groundwater seeps as potentially important refugia for freshwater fishes on the Eastern Shores of Lake St Lucia, KwaZulu-Natal, South Africa. Afr J Aquat Sci. 2007;32:125-132. [ Links ]
34. McCullough FS, Grayal P, Duncan J, Christie JD. Molluscicides in schistosomiasis control. Bull World Health Organ. 1980;58:681-689. [ Links ]
35. Howarth FG. Environmental impacts of classical biological control. Annu Rev Entomol. 1991;36:485-509. [ Links ]
36. Simberloff D, Stiling P. How risky is biological control? Ecology. 1996;77:1965-1974. [ Links ]
37. Hoddle MS. Restoring balance: Using exotic species to control invasive exotic species. Conserv Biol. 2004;18:38-49. [ Links ]
38. Louda SM, Stiling P. The double-edged sword of biological control in conservation and restoration. Conserv Biol. 2004;18:50-53. [ Links ]
39. Norton BG. Sustainability: A philosophy of adaptive ecosystem management. Chicago: University of Chicago Press; 2005. [ Links ]
40. Buckley YM. The role of research for integrated management of invasive species, invaded landscapes and communities. J Appl Ecol. 2008;45:397-402. [ Links ]
41. Evans JM, Wilkie AC, Burkhardt J. Adaptive management of non-native species: Moving beyond the 'either-or' through experimental pluralism. J Agric Environ Ethics. 2008;21:521-539. [ Links ]
42. Simberloff D. Moving beyond strawmen and artificial dichotomies: Adaptive management when an endangered species uses an invasive one. J Agric Environ Ethics. 2009;22:73-80. [ Links ]
43. Pointier JP, Facon B, Jarne P, David P. Thiarid snails, invading gastropods of tropical freshwater habitats. Xenophora. 2003;104:14-20. [ Links ]
 Correspondence to:
Correspondence to:
Nelson Miranda
Postal address:
School of Biological and Conservation Sciences, University of KwaZulu-Natal
Westville campus, Private Bag X54001, Durban 4000, South Africa
email: 204507499@ukzn.ac.za
Received: 17 July 2009
Accepted: 01 Feb. 2010
Published: 23 Apr. 2010
This article is available at: http://www.sajs.co.za














